Class 2 Medicines Recall: Nitrofurantoin CNX Therapeutics 100 mg Prolonged-Release capsules, EL(25)A/05
CNX Therapeutics is recalling the batches listed in this notification as a precautionary measure due to a small number of packs that contain an additional tablet of Nitrofurantoin. The registered product is a capsule containing powder and two yellow tablets.
DMRC reference number
DMRC-34425982
Medicine Details
Nitrofurantoin CNX Therapeutics 100 mg Prolonged-Release capsules
PL: 19635/0006
Active ingredient: nitrofurantoin
SNOMED code: 44125511000001105
Manufactured By
CNX Therapeutics Limited
Affected Lot Batch Numbers
| Batch Number | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| 24849001 | 31/07/2026 | 14 capsules | 13/12/2024 |
| 24849002 | 31/07/2026 | 14 capsules | 13/12/2024 |
Background
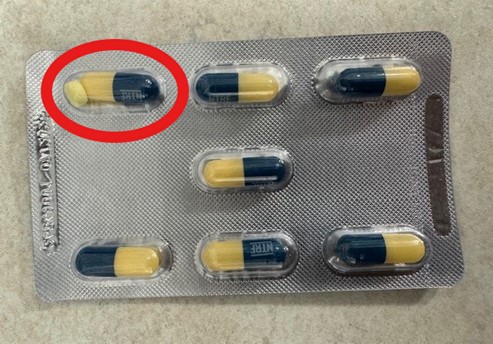
CNX Therapeutics is recalling the above batches as a precautionary measure due to a small number of packs that contain an additional tablet of Nitrofurantoin. The registered product is a capsule containing powder and two yellow tablets. A small percentage of blister pockets have been found to contain an additional yellow tablet alongside the capsule, these are from the manufacturing process and not from broken capsules.
Picture of the extra tablet in a blister of a nitrofurantoin capsule:
Advice for Healthcare Professionals
Stop supplying the above listed batches immediately. Quarantine all remaining stock and return it to your supplier using your supplier’s approved process.
Should a patient present a pack containing an extra tablet, the tablet may be removed and the patient assured that the capsule is safe to take stating the extra tablet did not come from the capsule.
Advice for Patients
If you find a pack containing an additional tablet, please take the medication to the dispensing pharmacy you obtained the medicine from. Patients may continue to take capsules from non-impacted packs as prescribed by your healthcare professional.
A small percentage of blister pockets have been found to contain an additional yellow tablet alongside the capsule, these are from the manufacturing process and not from broken capsules.
Patients that have taken the additional tablet with the capsule have ingested a higher dose of nitrofurantoin than was intended, however, the effects are understood and this should not have caused harm.
Patients who experience adverse reactions or have any questions about the medication should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Additional Information
For medical information enquiries please contact 0207 821 2840 or medinfo@cnx-therapeutics.com
For stock control enquiries please contact 0207 821 2840 or supplychain@cnx-therapeutics.com
Recipients of this Medicines Recall should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download document