Class 4 Medicines Defect Information: Lucis Pharma Ltd, Oxycodone Hydrochloride 10mg/ml oral solution, EL (22)A/49
Lucis Pharma Ltd has informed the MHRA that there is a typographical error with text on the rear side of the outer packaging for Oxycodone Hydrochloride 10mg/ml Oral Solution.
MDR number
MDR 176-11/22
Company name
Lucis Pharma Ltd
Product name
Oxycodone Hydrochloride 10mg/ml oral solution, PL 42176/0015
| Batch number | Expiry date | Pack size | First distributed |
|---|---|---|---|
| 21050004 | November 2023 | 120mL Bottle | 15/07/2021 |
| 22080005 | February 2025 | 120mL Bottle | 13/10/2022 |
Active Pharmaceutical Ingredient: Oxycodone Hydrochloride
Brief description of the problem
Lucis Pharma Ltd has informed the MHRA that there is a typographical error with text on the rear side of the outer packaging for Oxycodone Hydrochloride 10mg/ml Oral Solution.
The text incorrectly states that: ‘Each 1ml of oxycodone hydrochloride oral solution contains 1mg of oxycodone hydrochloride.’
The correct text should state: ‘Each 1ml of oxycodone hydrochloride oral solution contains 10mg of oxycodone hydrochloride.’
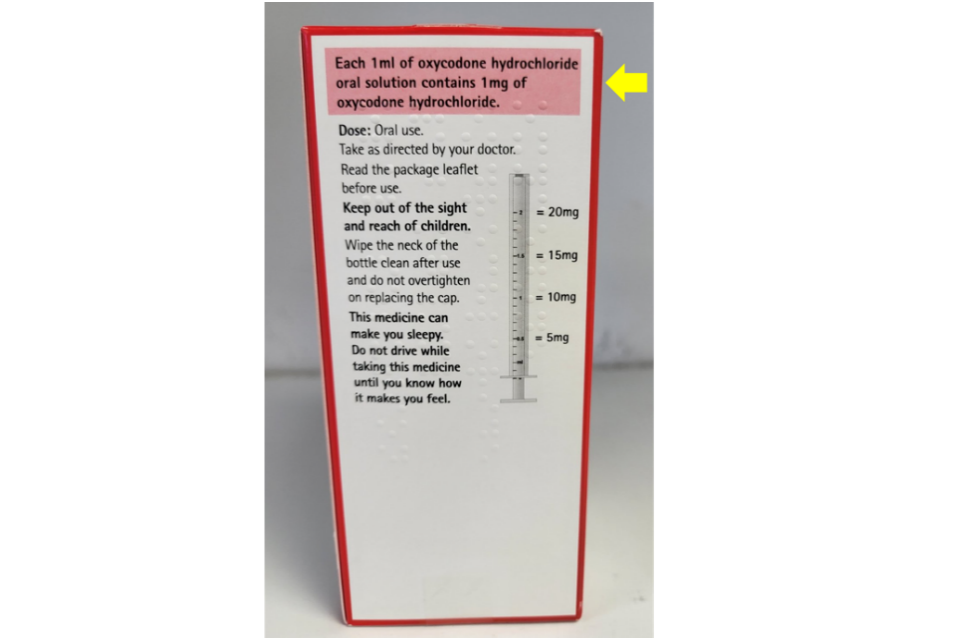
The strength of the product is printed correctly on all other sides of the outer packaging, including the label on the bottle.
Advice for healthcare professionals
Healthcare professionals should note that there is no risk to product quality and efficacy, therefore the affected batches are not being recalled.
Healthcare professionals should exercise caution when dispensing or supplying this product. Please refer to the correct information stated on the bottle label and in the Patient Information Leaflet inserted in the pack.
Advice for patients
This notification relates to a typographical error on the outer packaging of the product. The medicine itself is not affected and patients do not need to take any action.
Further Information
For medical information and stock control queries please contact: enquiries@lucispharma.co.uk.
Recipients of this Medicines Notification should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download document