Class 4 Medicines Defect Information: Orifarm UK Ltd, Buccolam 10mg Oromucosal solution, EL (23)A/18
Orifarm UK Ltd has informed the MHRA that the Patient Information Leaflet (PIL) in one batch of Buccolam 10mg Oromucosal solution has missing information regarding having to break the seal on the inner container before use.
MDR number
MDR 109-05/23
Company name
Orifarm UK Limited
Product name
Buccolam 10mg Oromucosal solution
PL number
PLPI 45985/0272
| Batch No | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| 620903600 | 08/2024 | 4 pre-filled oral syringe | 11/04/2023 |
Active Pharmaceutical Ingredient: midazolam hydrochloride
Brief description of the problem
Orifarm UK Ltd has informed the MHRA that the Patient Information Leaflet (PIL) in one batch of Buccolam 10mg Oromucosal solution has missing information regarding having to break the seal on the inner container before use. All other sections of the PIL are unaffected.
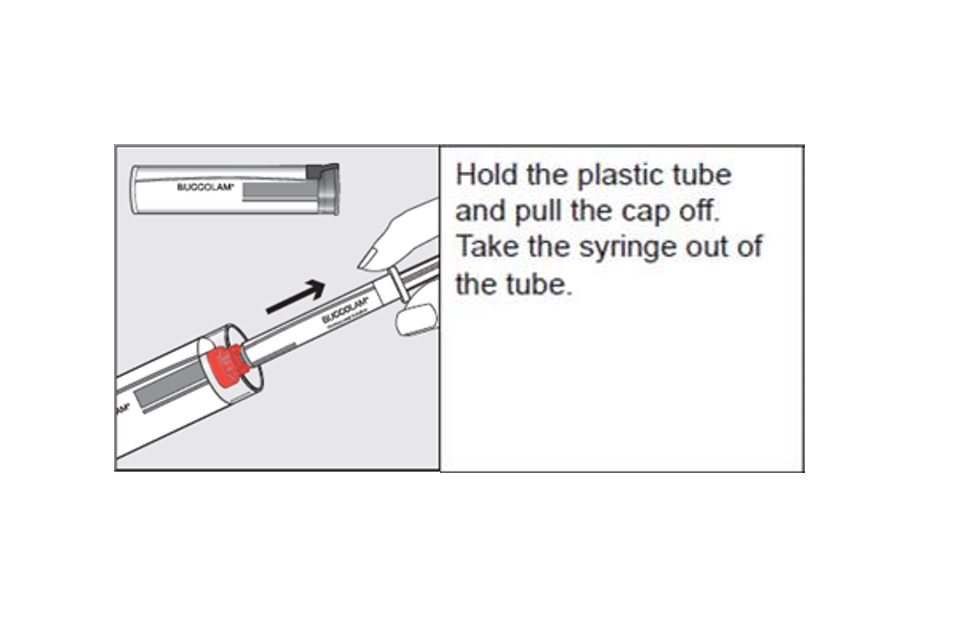
The information in step 1 of the PIL should include text indicating to break the seal at one end:
Step 1 (incorrect PIL)
Step 1 (correct PIL)
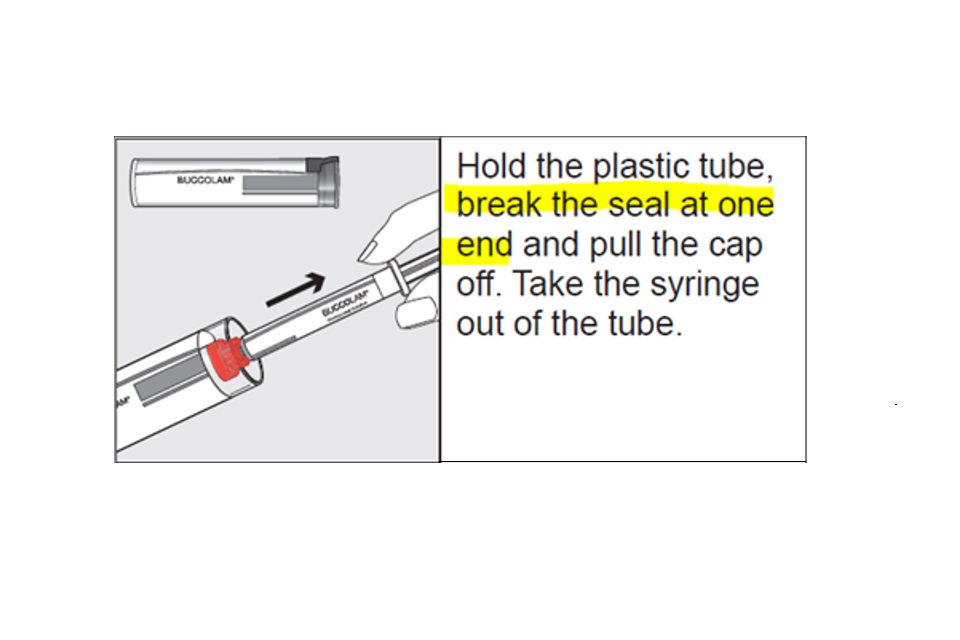
Advice for healthcare professionals
Healthcare professionals are advised to inform patients that there is an error in the PIL. The information regarding breaking the seal on the inner container before use as per ‘Step 1 (correct PIL)’ as shown in the images in this notification, should be followed.
The Marketing Authorisation Holder can supply copies of the correct PIL on request, so that any affected packs remaining in the dispensary can be supplemented with the correct information. Future copies of the PIL will reflect the correct information.
Advice for patients
There is missing information in the Patient Information Leaflet that accompanies this batch of Buccolam 10 mg Oromucosal solution. The information regarding breaking the seal on the inner container before use as per ‘Step 1 (correct PIL)’, as shown in the images in this notification, should be followed. The medicine itself is not affected and patients do not need to take any action.
If you have concerns about a medicine you may be using, please contact your healthcare professional. Patients who experience adverse reactions or have any questions about the medication should seek medical attention. Any suspected adverse reactions should be reported via the MHRA Yellow Card scheme.
Further Information
For more information, medical or supply enquiries, please contact 01923 204333, or email uk_enquiries@orifarm.com
Recipients of this Medicines Notification should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists, dispensing general practitioners, hospital pharmacists, general practitioners, dental practices, dentists, nurses, neurologists, neurology specialist nurses, epilepsy specialist nurses and community pharmacists for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download documents