Class 4 Medicines Defect Information: Sandoz Ltd., Risperidone 1mg, 2mg, 3mg Tablets, EL(24)A/43
Sandoz Ltd. has informed the MHRA that there is missing safety information in the Patient Information Leaflet (PIL) and Summary of Product Characteristics (SmPC) for Risperidone 1mg, 2mg and 3mg and Tablets.
DMRC reference number
DMRC 32698072
Company name
Sandoz Ltd.
Risperidone 1mg Tablets, PL 04416/0662
SNOMED Code
38913811000001102
| Batch Number | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| NK4910 | Jul-2026 | 20 | 12-Feb-2024 |
| NK4909 | Aug-2026 | 60 | 17-Jan-2024 |
Active Pharmaceutical Ingredient: risperidone
Risperidone 2mg Tablets, PL 04416/0663
SNOMED Code
20891211000001105
| Batch Number | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| NL4679 | Oct-2026 | 60 | 12-Jan-2024 |
Active Pharmaceutical Ingredient: risperidone
Risperidone 3mg Tablets, PL 04416/0664
SNOMED Code
20894411000001106
| Batch Number | Expiry Date | Pack Size | First Distributed |
|---|---|---|---|
| MM5115 | Jul-2025 | 60 | 03-Mar-2024 |
| NE5508 | Mar-2026 | 60 | 09-Aug-2024 |
Active Pharmaceutical Ingredient: risperidone
Brief description of the problem
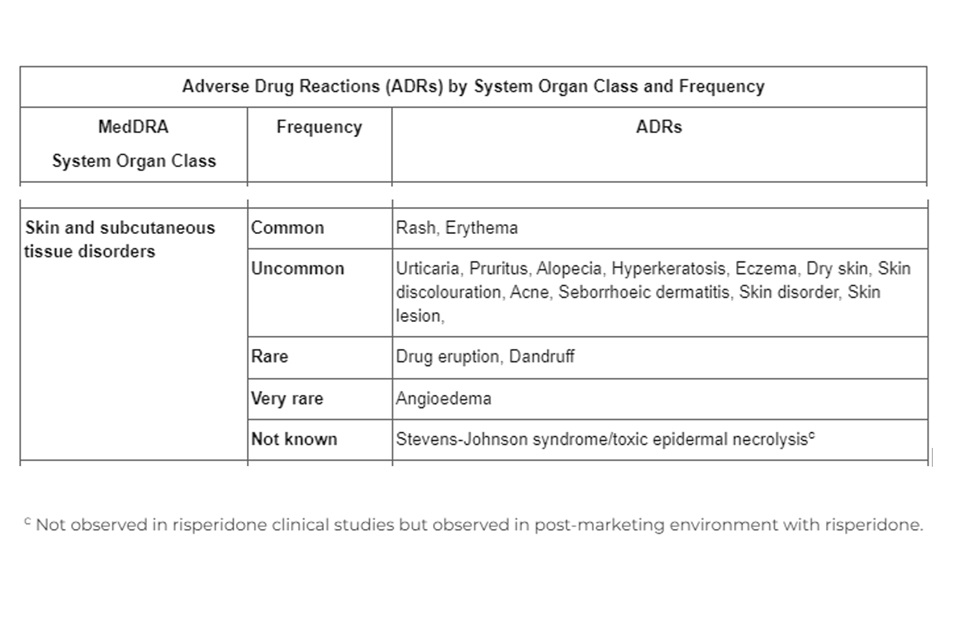
Sandoz Ltd. has informed the MHRA that there is missing safety information in the Patient Information Leaflet (PIL) and Summary of Product Characteristics (SmPC) for Risperidone 1mg, 2mg and 3mg and Tablets. The PIL and SmPC does not include the Adverse event of Stevens Johnson’s syndrome and toxic epidermal necrolysis in section 4.8 Adverse drug reactions for the MedDRA System Organ Class ‘Skin and subcutaneous tissue disorders’.
The missing information on the Risperidone 1mg, 2mg and 3mg Tablets PIL and SmPC is as follows:
Advice for healthcare professionals
There is no risk to product quality or impact to safety of the medicines listed in this notification because of this missing information. Healthcare professionals are advised to review the content of this notification, as it provides information that is missing from the current label and take this into account when prescribing. If the medicines listed in this notification are supplied or dispensed, ensure that patients are aware of the missing information displayed above. It is important to advise patients that if patients experience any of the above symptoms they should seek immediate medical advice.
Refer to updated SmPC as follows:
SmPC, Risperidone 1mg Tablets, PL 04416/0662
SmPC, Risperidone 2mg Tablets, PL 04416/0663
SmPC, Risperidone 3mg Tablets, PL 04416/0664
Sandoz Ltd. has confirmed that all future batches will contain the correct PIL. Upon request, Sandoz Ltd. will post hard copies of the updated PIL to pharmacies so that any remaining stock in the dispensary can be supplemented with the correct PIL information (see contact details below).
Advice for patients
Patients do not need to take any action. The information above is missing from the Patient Information Leaflet. The missing information, summarised in this notification, does not change or affect the quality of the product. Therefore you can safely continue your treatment. However, should you experience any adverse effects/side effects with the prescribed medication please contact your healthcare professional.
Patients who experience adverse reactions or have any questions about their medication should seek medical attention. Any suspected adverse reactions should also be reported via the MHRA Yellow Card scheme.
Further Information
For medical information queries, please contact: sandozgb@EU.propharmagroup.com, Telephone: +44 1276 698 101.
For stock control queries, please contact: sales.sandoz-gb@sandoz.com, Telephone: +44 1276 698607.
Recipients of this Medicines Notification should bring it to the attention of relevant contacts by copy of this notice. NHS regional teams are asked to forward this to community pharmacists and dispensing general practitioners for information.
Yours faithfully
Defective Medicines Report Centre
10 South Colonnade
Canary Wharf
London
E14 4PU
Telephone +44 (0)20 3080 6574
Download document