Adrenaline (Epinephrine) Injection 1:10,000 1mg/10ml, Amiodarone Injection 30mg/ml, Ephedrine Hydrochloride Injection 3mg/ml - misalignment of syringe label
(Aurum Pharmaceuticals Limited (trading as Martindale Pharma)) Pre-filled syringes from affected batches should be used with caution.– class 3 action within 5 days. (EL 13(A)13)
29 April 2013
Class 4 medicines defect information
Caution in use
Distribute to pharmacy level
MDR 62-03/13
Product details
Aurum Pharmaceuticals Limited (trading as Martindale Pharma)
Adrenaline 1mg/10ml solution for injection in a pre-filled syringe PL 12064/0006
Amiodarone 30mg/ml solution for injection in a pre-filled syringe PL 12064/0047
Ephedrine Hydrochloride 3mg/ml solution for injection in a pre-filled syringe PL 12064/0043
| Product name | Batch number range from and to inclusive | Expiry date range from and to inclusive | First distributed | |||
|---|---|---|---|---|---|---|
| Adrenaline 1mg/10ml | From 5000879 to 5000964 | From 05/2014 to 07/2014 | 06 Feb 2013 | |||
| Amiodarone 30mg/ml | From 5000610 to 5000870 | From 02/2014 to 11/2014 | 28 Nov 2012 | |||
| Ephedrine Hydrochloride 3mg/ml | From 5000452 to 5000846 | From 09/2013 to 10/2014 | 22 Dec 2011 |
Aurum Pharmaceuticals Limited (trading as Martindale Pharma) has informed us that there is a misalignment of the syringe label of the above pre-filled syringe batches. The syringe label includes the graduated volume markings.
Pre-filled syringes from the affected batches should be used with caution.
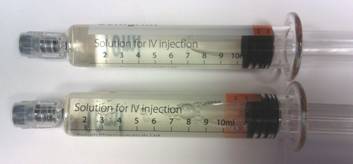
An image of two syringes. One of them with a misaligned label.
The syringe in the top position has the label in the correct position.
The syringe in the lower position has the label applied too close to the end of the barrel and should be used with caution.
The implication of this misalignment is that incorrect dosing could occur if the entire contents of the syringe are not used. For example if a dose was titrated between the intermediate and completely empty position.
The fill-volume and total dose in the syringe is correct. It may appear that there is over-fill of the syringe. Do not discard what appears to be excess before administration.
This issue has been corrected by the manufacturer however this issue may be present within the market for a period of time.
Recipients of this drug alert should bring it to the attention of relevant contacts by copy of this letter. Local area teams are asked to forward this to relevant clinics, general practitioners and community pharmacists for information.