Human papillomavirus (HPV) immunisation programme - first-year safety review
The human papillomavirus (HPV) immunisation programme is now entering its second year and this article summarises the safety experience to date.
Article date: October 2009
The human papillomavirus (HPV) immunisation programme is now entering its second year. The programme has so far been a success and the latest uptake levels continue to be very encouraging. At least 1.4 million doses of Cervarix HPV vaccine have now been administered across the UK. More information on the programme can be found at www.immunisation.nhs.uk.
This level of exposure in the UK is an important milestone in understanding the overall safety profile of the vaccine during routine use. This article summarises the safety experience to date.
- The total number and nature of suspected side effects reported via the Yellow Card Scheme during the first year of the Cervarix vaccination programme has been very much as expected
- Most reports have related to the signs and symptoms of recognised side effects, which are generally not serious
- No new risks have been identified, and the balance of risks and benefits remains positive
- As with all vaccines, the MHRA and Commission on Human Medicines (CHM) will continue to monitor closely the safety of Cervarix during continued use in the UK
MHRA pharmacovigilance strategy
The MHRA continuously monitors the safety of all vaccines and medicines used in the UK, including Cervarix HPV vaccine (as well as Gardasil HPV vaccine, which is not used in the routine immunisation programme).
We receive reports of suspected side effects via the Yellow Card Scheme. It is important to bear in mind that a report of a suspected adverse reaction does not necessarily mean that it has been caused by Cervarix. Reports may represent true side effects, or they may be due to underlying illness and therefore purely coincidental events that would have occurred anyway in the absence of vaccination.
As part of the Cervarix pharmacovigilance strategy, at the start of the immunisation programme we wrote to healthcare professionals involved to encourage use of the Yellow Card Scheme to report suspected side effects. The Scheme is also open to members of the public. We review all new reports of suspected side effects on a daily basis, using an epidemiological approach to detect potential safety signals. Our ongoing analysis of the safety of Cervarix is published every week at www.mhra.gov.uk/hpvvaccine.
Cervarix: current safety profile
As at the end of July 2009, the MHRA had received 2195 Yellow Cards, including 4830 adverse-reaction terms, in association with Cervarix (including reports in which the brand of HPV vaccine was not stated by the reporter). Most Yellow Card reports have been submitted by nurses (mainly school nurses).
Number of reports received between April 2008 and July 2009:
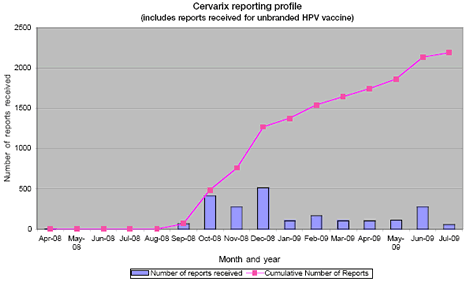
Image of graph showing Cervarix reporting profile.
Range of Yellow Card reports received based on patient age:
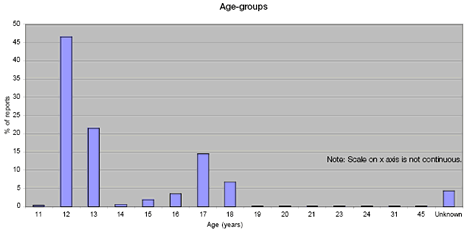
Image of graph showing age of patients reporting.
As expected, most reports were for girls age 12–13 years and 17–18 years—the age-groups targeted in the first year of the immunisation programme.
Type of adverse reactions reported to the Yellow Card Scheme:
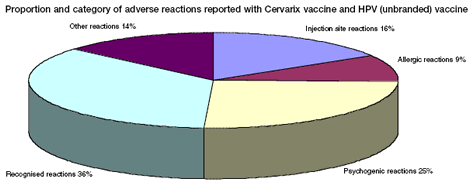
Psychogenic events include vasovagal syncope, faints, panic attacks, and associated symptoms. These can occur with any injection procedure, not only vaccination, and can be common in adolescents. Such events can be associated with a wide range of temporary signs and symptoms, including: loss of consciousness; vision disturbance; injury; limb jerking (often misinterpreted as a seizure or convulsion); limb numbness or tingling; and difficulty in breathing or hyperventilation. These are due to fear or anticipation of the needle injection and are not side effects of Cervarix vaccine as such. More information on the specific adverse reactions reported can be found at www.mhra.gov.uk/hpvvaccine.
The ten most commonly reported suspected adverse reactions up to the end of July, 2009 were:
- Dizziness: 468
- Headache: 433
- Nausea: 422
- Pain in extremity: 248
- Syncope: 199
- Vomiting: 178
- Malaise: 161
- Fatigue: 133
- Pyrexia: 106
- Rash: 96
Potential safety issues assessed
Chronic Fatigue Syndrome and associated conditions
Based on case reports of chronic fatigue syndrome (CFS) and associated conditions, the MHRA sought the advice of CHM on whether the available evidence provided any indication that Cervarix may cause these conditions.
CFS is a fairly common condition that occurs naturally among the population of girls vaccinated. CHM noted that as more than 1 million doses of Cervarix have been given in the UK, at least 60 cases of CFS would have been expected already by chance alone. Accounting for various levels of possible under-reporting to the Yellow Card Scheme, the 13 reports of possible chronic-fatigue-like symptoms (including cases reported in the UK media) do not indicate that Cervarix is causing any excess of CFS cases.
CHM also noted that there are insufficient clinical details to determine if any of the reported cases met the case definition of CFS as defined by the National Institute for Health and Clinical Excellence, and there was a lack of consistency in the clinical pattern between cases. Even if such cases were eventually confirmed to be CFS, CHM advised that the evidence does not support a causal link with Cervarix vaccine because many more cases would have been expected to occur by chance among the number of girls immunised so far. This issue will remain under review by the MHRA.
Guillain-Barré syndrome and facial palsy
One case of Guillain-Barré syndrome and one case of facial palsy have been reported via the Yellow Card Scheme. Our analysis of the reporting rate of such conditions compared with the expected incidence among adolescent girls indicates that the vaccine is not associated with a risk of these conditions.
Summary
The number and nature of suspected adverse reactions received so far is very much in line with what we expected to receive at this time. CHM reviewed these data on Sept 18, 2009 and agreed that following substantial usage no new or serious risks have been identified during use of Cervarix in the UK, and that the balance of benefits and risks remains positive. As with all vaccines, the MHRA and CHM will continue to closely monitor the safety of Cervarix during continued use in the UK.
Have you reported a Yellow Card for the HPV vaccine?
As the start of the second year of the Cervarix immunisation programme begins, it is essential that all three doses are given to ensure protection. Thank you to those who have sent us Yellow Cards for Cervarix. Please continue to help us monitor the safety of Cervarix by reporting any suspected adverse reactions associated with the vaccine via the Yellow Card Scheme, either online at www.yellowcard.gov.uk or by post (paper Yellow Cards are available at the back of the British National Formulary or call 0800 731 6789). Please also remind vaccinees and their carers that they too can also report suspected side effects via the Yellow Card Scheme.
Information for vaccinees and parents
In its August edition of Vaccine Update (www.immunisation.nhs.uk), the Department of Health reminded healthcare professionals of the importance of fully informing vaccinees/their carers about their immunisations and possible side effects. It stressed the need to provide verbal or written information, which may take the form of provision and discussion of the patient information leaflet (PIL). The MHRA fully endorses this advice. PILs are provided in the Cervarix vaccine packs and can also be viewed/downloaded via the electronic Medicines Compendium (http://emc.medicines.org.uk).
Article citation: Drug Safety Update Oct 2009, vol 3 issue 3: 5.