Natalizumab (Tysabri▼): progressive multifocal leukoencephalopathy—updated advice to support early detection
Perform a quantitative serum anti-JCV antibody test—including index value—to support risk stratification for progressive multifocal leukoencephalopathy. For high-risk patients, consider more frequent MRI screening.
Post-publication note - December 2024
The advice provided in this article is currently under review.
Updated risk estimates are available as a result of an EU review of natalizumab.
Advice for healthcare professionals:
Before starting natalizumab treatment
New advice:
- Perform a baseline quantitative serum anti-JCV antibody test—including the index value—to support risk stratification for progressive multifocal leukoencephalopathy (PML)
Reminder of previous advice:
- Before starting treatment, a recent (usually within 3 months) MRI should be available as a reference
- Counsel patients and carers on the risk of PML — an updated Treatment Initiation Form will be available in due course
- Advise patients and carers on symptoms to watch out for and to get medical advice urgently if they occur
During natalizumab treatment
New advice:
- Perform a quantitative serum anti-JCV antibody test—including the index value—every 6 months for the patients specified in the algorithm below (see figure 2)
- For high-risk patients (see below), consider the following extra precautions:
- more frequent MRI screening for PML, such as every 3–6 months using an abbreviated protocol (FLAIR, T2-weighted, and DW imaging): earlier detection of PML in asymptomatic patients may be associated with improved PML outcome
- If you suspect PML, extend the MRI protocol to include contrast-enhanced T1-weighted imaging and consider testing for JCV DNA in the cerebrospinal fluid using ultrasensitive polymerase chain reaction (PCR)
Reminder of previous advice (for all patients):
- If you suspect PML at any time, stop natalizumab treatment and investigate appropriately until PML has been excluded
- Perform a quantitative serum anti-JCV antibody test—including the index value—for any patient with unknown antibody index (all patients should be tested at least once)
- Perform a full cranial MRI scan at least yearly for the entire duration of treatment, to have up-to-date reference images
- Monitor patients for signs and symptoms or appearance of new neurological dysfunction (eg motor, cognitive, or psychiatric symptoms), bearing in mind that PML can present with features similar to multiple sclerosis
- Consider PML in the differential diagnosis of any patient presenting with neurological symptoms or new brain lesions in their MRI scan – cases of asymptomatic PML, diagnosed based on MRI scans and positive JCV DNA in the cerebrospinal fluid, have been reported
- After 2 years of treatment, remind patients of the risk of PML with natalizumab using the updated Treatment Continuation Form, which will be available in due course
After stopping natalizumab treatment:
New advice:
- Advise patients and carers to continue to watch out for signs and symptoms of PML for 6 months after the last dose—use the new Treatment Discontinuation Form, which will be available in due course, to aid this discussion
- Continue the same monitoring protocol for 6 months after the last dose, as PML has been reported during this time
Natalizumab (Tysabri) is a single disease-modifying therapy for adults with multiple sclerosis who have high disease activity despite treatment with beta-interferon, or who have rapidly evolving severe relapsing remitting disease.
Natalizumab is associated with a risk of PML— a rare, progressive, and demyelinating disease of the central nervous system that can be fatal. It is caused by activation of John Cunningham virus (JCV), which usually remains latent and typically only causes PML in immunocompromised patients.
Up to August 2015, there had been 582 reports worldwide from clinical practice of PML in patients receiving natalizumab. Up to 30 March 2016, we had received 33 Yellow Card reports of PML in patients receiving natalizumab in the UK. Evidence from these reports and several studies has led to new advice to reduce the risk of PML. This advice is summarised above, along with a reminder of the previous advice which still applies.
Clinically asymptomatic PML: importance of early detection
Recent analyses suggest that earlier detection of PML is associated with improved outcomes. Cases of asymptomatic PML, diagnosed based on MRI scans and positive JCV DNA in the cerebrospinal fluid, have been reported. PML which is clinically asymptomatic at diagnosis has more localised or unilobar lesions on MRI scans compared with symptomatic patients. Occasionally, particularly in patients with small lesions, exclusively grey matter involvement of PML has been observed on MRI scans.
Note that these analyses have important potential limitations, including lead time bias and length time bias. There was also information missing on MRI frequency in symptomatic PML cases, preventing comparison with PML cases asymptomatic at onset.[footnote 1]
Therefore the risk-proportionate MRI screening protocol for PML described in this article is recommended for patients receiving natalizumab. It is also important that patients do not have any signs or symptoms of PML before switching to other disease-modifying treatments (see other articles in this issue on dimethyl fumarate and fingolimod).
PML risk factors
The risk of PML in patients receiving natalizumab is already known to be higher in patients who:
- are serum anti-JCV antibody positive
- have had immunosuppressant therapy
- have been receiving natalizumab for a long time (especially for more than 2 years)
Recent data show that in patients who have not had immunosuppressant therapy and are serum anti-JCV antibody positive, the risk of PML rises with increased serum anti-JCV antibody index (see figure 1).
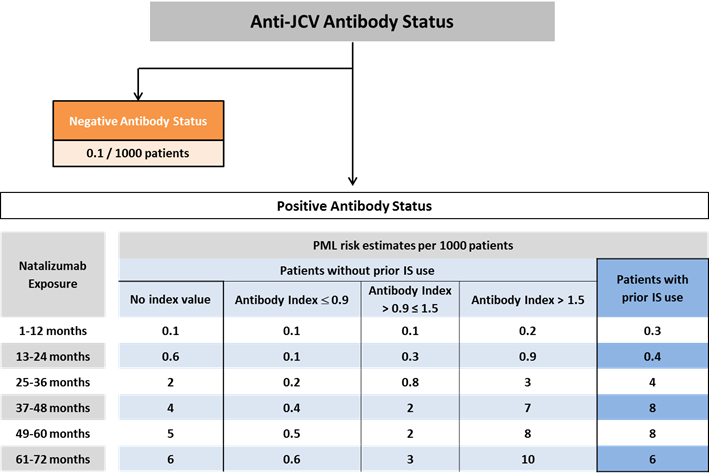
Image of diagram showing anti-JCV antibody status.
Figure 1: PML risk estimates in serum anti-JCV antibody positive patients were derived using Life Table method based on the pooled cohort of 21,696 patients who participated in the STRATIFY-2, TOP, TYGRIS, and STRATA studies. Further stratification of PML risk by serum anti-JCV antibody index interval for patients with no history of immunosuppressant use were derived from combining the overall yearly risk with the antibody index distribution. The risk of PML in serum anti-JCV antibody negative patients was estimated based on data from clinical practice from approximately 125,000 exposed patients.
Figure 1 shows that the risk of PML is low at index values ≤0.9, and increases substantially at index values >1.5 in patients who have been receiving natalizumab for more than 2 years.
Therefore, the following groups of patients have been defined as being at high risk of PML:
- those who have all three risk factors for PML (ie immunosuppressant therapy, serum anti-JCV antibody positive, and more than 2 years of natalizumab exposure)
- those who have not had immunosuppressant therapy but have a high serum anti-JCV antibody index and more than 2 years of natalizumab exposure
For these high-risk groups, consider the extra precautions listed in the ‘during natalizumab treatment’ section above, and in figure 2 below.
6-monthly serum anti-JCV antibody testing
Perform a quantitative serum anti-JCV antibody test—including the index value—every 6 months in the following patients (see algorithm below):
- those who test negative for serum anti-JCV antibodies
- those who have low serum anti-JCV antibody index values, less than 2 years of natalizumab exposure, and who have not had immunosuppressant therapy
It is important to test these patients every 6 months because their serum anti-JCV antibody status might fluctuate, they might develop a new JCV infection, or they might have had a false negative finding. Also, patients who had a low serum anti-JCV antibody index at baseline may change to a high serum anti-JCV antibody index during treatment.
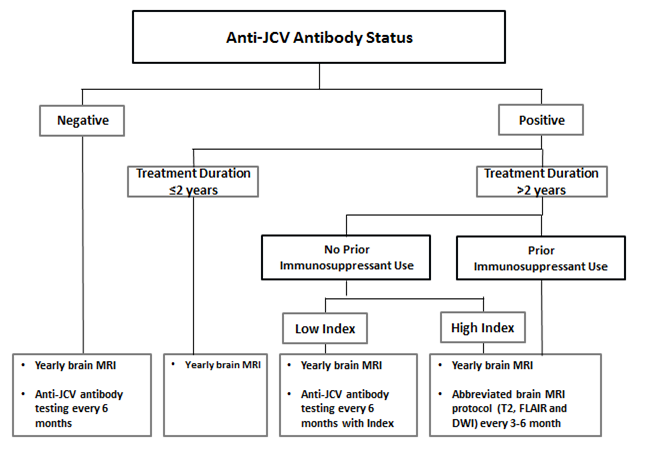
Image of diagram showing anti-JCV antibody status.
Figure 2: Algorithm of recommended patient monitoring (figure reproduced with permission from Biogen)
Risk of PML with other multiple sclerosis treatments
Other multiple sclerosis treatments—fingolimod (Gilenya) and dimethyl fumarate (Tecfidera)—have also been linked to a risk of PML.
Post-publication note – January 2018
On 30 January 2018, following a query from a reader, we corrected the second bullet point in the section “Advice for healthcare professionals: Before starting natalizumab treatment” to clarify that the baseline cranial MRI scan should be done before initiation of natalizumab treatment rather than within 3 months of treatment initiation. This amended version is consistent with advice in the March 2010 Drug Safety Update and the Summary of Product Characteristics for natalizumab as of January 2018.
Further information
in March 2016
European Medicines Agency announcement February 2016
Article citation: Drug Safety Update Vol 9 issue 9 April 2016: 2.
-
Dong-Si et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann Clin Transl Neurol 2014; 1: 755–64. ↩