Public perception of herbal medicines
In 2008 MHRA commissioned Ipsos MORI to research the public’s perception of herbal medicines, the findings may help doctors and pharmacists when considering how to advise patients.
Article date: March 2009
Many healthcare professionals often encounter patients who are taking herbal medicines. In 2008 MHRA commissioned Ipsos MORI to research the public’s perception of herbal medicines. The findings may help doctors and pharmacists when considering how to advise their patients.
35% of adults say they have ever taken herbal medicines (26% have done so in the past 2 years). 29% of adults have used an over-the-counter herbal medicine; 5% have used traditional Chinese medicine (TCM) supplied by a TCM practitioner, clinic, or shop; and 8% a herbal medicine supplied by other herbal or traditional practitioner.
Perceptions on safety issues
When asked what advice on potential risks or possible problems should be given to a friend of family member considering using herbal medicines 36% of adults questioned said they are unaware of any possible problems or risks to look out for. The risk most commonly identified unprompted was that some herbal medicines have side effects (12%); the risk of interactions with conventional medication was identified by 6%; no other single risk was identified by more than 6%.
Information and trust
Qualitative findings suggest that the public generally are not very discerning about who they would approach for advice on herbal medicines—anyone with an interest in the subject, be they friends, family, or shop assistant was trusted to give good advice:
- 17% and 9% of adults questioned have ever used a doctor or pharmacist, respectively, as a source of information about herbal medicines
- 15% and 13% of adults have ever used family or friends/colleagues/workmates, respectively, as source of information
- When asked which one source of advice they would most trust about herbal medicines, 44% said a doctor and 14% a pharmacist; the next highest were herbal/traditional medicine practitioner (8%) and family (5%)
- 4% of adults have ever used product information included with the packaging as a source of information about herbal medicines (compared with 27% for all medicines in an Ipsos MORI survey for MHRA in 2006)
Regulation
29% of British adults believe herbal medicines are currently regulated in the UK, whereas 31% believe they are not, and 30% do not know. 10% believe that some herbal medicines are regulated, and some are not.
TCMs
Users of TCMs are more likely (76%) than any other group of users or non-users to believe that natural means safe. They are also more likely (44%) than other groups of users to believe that products were regulated. The qualitative research suggests that TCM practitioners may be heavily trusted by some users, with some participants suggesting that TCM is suitable for more serious medical conditions.
Key findings from adult herbal medicines users in past 2 years:
“To what extent do you agree/disagree with the statement that…
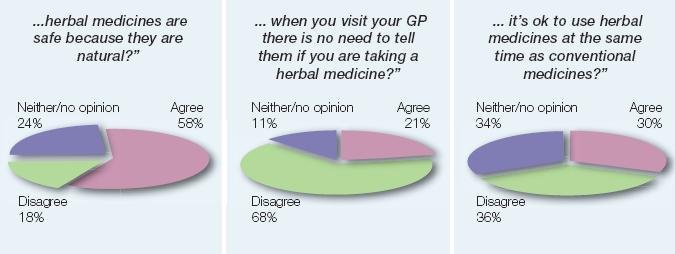
Image of pie graphs showing patient agreement/disagreement towards certain statements.
Advice for healthcare professionals from the Herbal Medicines Advisory Committee:
Patient vulnerability
Be aware that:
- Patients may be obtaining information from unreliable sources; they may be particularly vulnerable to some inexpert or unscrupulous operators in the unlicensed sector who may promote the belief that “natural” means “safe” to discourage scrutiny of the safety and quality of their products
- Some herbal medicine users, of TCMs in particular, may be taking unlicensed herbal medicines for serious medical conditions. The MHRA continues to find evidence of TCMs and other unlicensed herbal products illegally containing potent and toxic undeclared ingredients
Informed choice of products made to assured standards
- For patients who wish to use herbal medicines there is now a growing range of regulated herbal medicines under the traditional herbal registration (THR) scheme that meet assured standards of safety, quality, and patient information. Agreed minor indications are permitted on the basis of traditional use. These products can be identified by the THR number on the packaging. Alongside these are herbal medicines with a product licence (PL) which also meet assured standards
Advice available
- For THRs a copy of the Summary of Product Characteristics and patient leaflet is available on the MHRA website in Public Assessment Reports for herbal medicines
See also Herbal Medicines Advisory Committee
Reporting of adverse reactions
All medicines, including herbal remedies,have the potential to cause side effects. If a patient experiences an adverse reaction suspected to be associated with the use of a herbal medicine please submit a Yellow Card
Technical information about the research
General public qualitative research: four discussion groups were conducted in July 2008 at two locations (Stockport and Croydon). Two groups were conducted in each location, one with users, and one with non-users of herbal medicines.
General public quantitative research: Questions were placed on the Ipsos MORI Omnibus. A nationally representative quota sample of 2305 adults (aged 15 years or older) was interviewed in 197 sampling points throughout Great Britain. Interviews were arried out face-to-face in respondents’ homes. Fieldwork was conducted in September 2008. Data are weighted to match the profile of the Great Britain adult population.
Article citation: Drug Safety Update March 2009, vol 2 issue 8: 11.