Horizon Scanning Case Study: Developing standards for Adeno-associated virus gene therapies
Horizon scanning at the MHRA leads to external funding being secured, helping our scientists work in a new area of standardisation that will support developers and manufacturers of AAV gene therapies
The Issue
Gene therapies are medicines that modify a person’s genes to prevent, treat or cure disease. These innovative approaches fall under the class of medicines called Advanced Therapy Medicinal Products or ATMPs (other ATMP are cell therapies). A gene therapy medicine is composed of a carrier or vector that helps transfer the gene or genetic material into cells. One area of innovation and growth currently is gene therapies based on Adeno-associated viral (AAV) vectors, which are non-replicative, non-pathogenic single-stranded DNA parvoviruses. However, this field can be challenged by analytical and manufacturing barriers.
Analytical methods to assess the presence of impurities, quality and concentration of a product have been developed, but these are performed without any common point of reference and different analytics measure different characteristics of the product, thereby limiting comparison between products, batches of product and sites of manufacture. Standardisation of AAV products would enable manufacturers to harmonise dosing and the quality of their products thereby increase patients’ confidence in these therapies and ultimately increase the safety of these potentially life transforming innovative medicines.
The change that is needed
In this case, horizon scanning identified a need for standardisation of AAV products to support developers and manufacturers of these therapies, and to enable patients to access these innovative products but to also ensure they are regulated appropriately and are safe and effective. Without a reference material to benchmark their products to it is difficult to establish the concentration (copies or particles per mL), and the particle empty/full ratio of products. To this end, AAV reference materials are required, that are available and used by all, to further advance the field.
What the MHRA did
To help with this issue, we searched the life sciences funding ecosystem for relevant funding opportunities to support the manufacture and characterisation of an AAV reference material. Together with colleagues at the UK Cell and Gene Therapy Catapult (CGTC), we secured funding from the Regulators’ Pioneer Fund (RPF), launched by the Department for Business, Energy and Industrial Strategy (BEIS), to support the 6-month project.
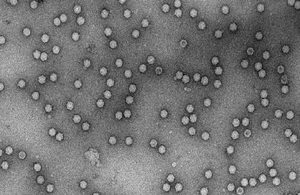
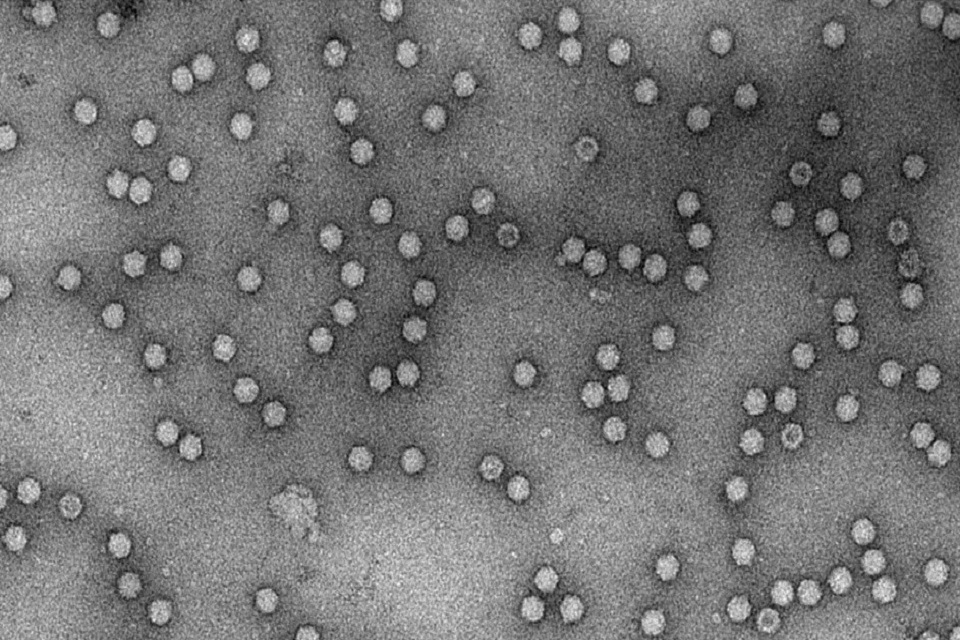
The CGTC produced and purified a batch of AAV2 material using their new state of the art bioreactors and facilities at Braintree, which we then analysed by Enzyme Linked Immunosorbent Assays (ELISA), electron microscopy and Polymerase Chain Reaction (PCR). We trialed freeze drying the material, which removes the need for a low temperature-controlled supply chain, so easing future distribution. The materials are now being evaluated by multiple external collaborators, from industrial stakeholders to academic laboratories, and its ‘fitness for use’ as a reference material is being assessed.
Outcomes
Through horizon scanning we identified the need for AAV reference materials to support future standardisation of AAV-based gene therapies, which will increase patient confidence in these therapies and the safety of these innovative medicines, and by securing external grant funding we are moving closer to meeting the objective of AVV standardisation. Through this work we have also developed strong collaborative working arrangements with the CGTC and a network of experts interested in AAV standardisation.
The Scientist Sarah Kempster, who led the work for the MHRA said: ‘Securing the funding from the Regulators Pioneers Fund was critical to making this project happen; it has enabled us to build a network of experts in the field which ensures the MHRA is at the cutting edge of new technologies in gene therapy analytics leading to better patient health’.
Electron micrograph of AAV viral particles
This project has been made possible by a grant from the £3.7 million Regulators’ Pioneer Fund launched by the Department for Business, Energy and Industrial Strategy (BEIS). The fund enables UK regulators and local authorities to help create a UK regulatory environment that unleashes innovation and makes the UK the best place to start and grow a business. The Cell and Gene Therapy Catapult is part of the Catapult Network, which supports businesses in transforming great ideas into valuable products and services. It is a network of world-leading technology and innovation centres established by Innovate UK.