Hearing implants merger could mean worse deal for NHS and patients
The merger of 2 leading providers of hearing implants could lead to higher prices for the NHS, and reduced quality and slower innovation for UK patients who rely on these life-changing devices.
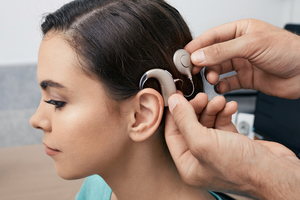
Someone getting hearing device fitted.
The Competition and Markets Authority (CMA) is concerned that Cochlear’s proposed purchase of Oticon Medical – the hearing implant division of Demant –would have a negative impact on patients who need hearing implants, the majority of whom access these through the NHS.
Cochlear and Demant are both global suppliers of cochlear implants and bone conduction solutions, which are types of hearing devices that are surgically implanted. These devices help improve hearing for people with mild to severe or total hearing loss but serve different patient needs depending on the cause of each individual’s hearing loss.
Following its Phase 1 investigation, the CMA found that the proposed deal would result in the merged businesses having a 90-100% share of the bone conduction solutions market in the UK. Should the deal go ahead as planned, the CMA is concerned that this would result in the elimination of the strongest competitor in this segment (Oticon Medical), which could lead to reduced innovation, higher prices or less choice for hospitals and their patients.
The CMA also investigated the impact of the merger on the supply of cochlear implants, where Cochlear has a very strong position. It found that, in the UK, Oticon Medical has only had a very small position in this segment, is not a significant rival of Cochlear and was unlikely to become a significant rival in the future. The CMA also found that the merged businesses would still face competition from 2 other providers in the UK, both of whom offer a stronger constraint on Cochlear than Oticon Medical. As such, the CMA’s competition concerns only relate to bone conduction solutions.
Demant has argued that it had decided to discontinue its hearing implants business in the UK and therefore will no longer compete with Cochlear even if the merger does not go ahead. However, the CMA found that Demant considered a range of options for the business and is not persuaded that Demant would exit the business without the merger.
Sorcha O’Carroll, CMA Senior Director of Mergers, said:
We’re concerned that this deal could lead to higher costs for the NHS and worse outcomes for patients who rely on life-changing hearing implants. The merger will wipe out one of the main suppliers and leave Cochlear with a near monopoly in the supply of bone conduction implants.
Healthy competition in the medical technology sector is central to continued innovation, more choice and improvements in patient treatments.
Cochlear and Demant now have until 13 December 2022 to address the CMA’s concerns through the offer of undertakings. If they are unable to address the CMA’s concerns, the deal will be referred for an in-depth Phase 2 investigation.
For more information, visit the Cochlear/Oticon Medical merger inquiry page.
Notes to editors
- All media enquiries should be directed to the CMA press office by email on press@cma.gov.uk, or by phone on 020 3738 6460.
- ‘Cochlear’ refers to Cochlear Limited and ‘Demant’ refers to Demant A/S.