Prednidale 5 mg Tablets - Product defect batch recall alert
Details of a Class I recall for Prednidale 5 mg Tablets.
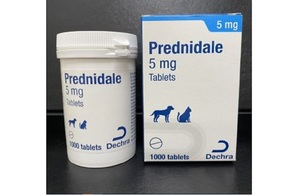
We wish to make veterinary surgeons aware that Dechra Ltd has issued a Class I recall for Prednidale 5 mg Tablets, batch 136037 with immediate effect.
The reason for the recall is potential mould contamination after opening of the primary container. The recall is for the above-mentioned batch only.
Dechra Veterinary Products is contacting wholesale dealers and veterinary surgeons to examine inventory immediately and quarantine products subject to this recall.
For further information regarding the recall, please contact UK Customer Services Team on 01939 211200 or Stephen Hines on 01756 791 311 email stephen.hines@dechra.com