Recall alert for Anesketin 100 mg/ml 5ml Solution for Injection
Product defect recall alert for Anesketin 100 mg/ml 5ml Solution for Injection for Dogs, Cats and Horses due to a discrepancy in processing settings.
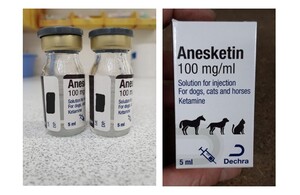
We wish to make wholesalers and veterinary surgeons aware that Dechra has initiated a Class II recall to Veterinary level with immediate effect for certain batches of Anesketin 100 mg/ml 5ml Solution for Injection for Dogs, Cats and Horses. Authorisation numbers Vm 16849/5002 and Vm 16849/3002, previously Vm 16849/4048 by Dechra.
The reason for the recall is potential leakage from the vials due to a discrepancy in processing settings. This recall is for these batches only:
| Batch Number | Expiry date |
|---|---|
| 144611 | 30/10/2025 |
| 149131 | 30/06/2026 |
| 150269 | 31/10/2026 |
Dechra is contacting wholesale dealers and veterinary surgeons to examine inventory immediately and quarantine products subject to this recall.
For further information regarding the recall please email Laura.Eveson@dechra.com or Tracey Smith tracey.smith@dechra.com. Alternatively call 01939 211200.