Update to intravenous use of co-amoxiclav products - Adverse events
More information following our investigation into an increase of adverse event reports following the cascade use of intravenous co-amoxiclav (amoxicillin and clavulanic acid) products.
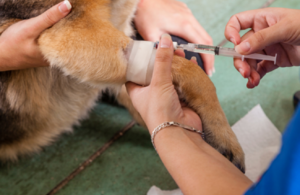
Since our initial notification on 9 August 2023, we have worked with the Marketing Authorisation Holder to investigate adverse event reports relating to the veterinary cascade use of human medicinal intravenous co-amoxiclav products in animals.
A significant proportion of the adverse event reports we have assessed this year to date have involved either batches MG2395 or MN7817 of Co-amoxiclav 500/100 mg Powder for Solution for Injection/Infusion.
Sandoz, the human product Marketing Authorisation Holder, performed an in-depth review and confirmed that they had not noted anything of concern relating to this product or batches.
They confirmed they had seen no increase in the number of adverse events received in humans, noted no quality issues, or any deviations from the standardised manufacturing process, distribution chain, or storage conditions during transportation.
Symptoms included in adverse event reports
Reports often include anaphylaxis/hypersensitivity signs with a rapid onset, usually within minutes of administration of the product. The most frequently reported signs are urticaria, allergic oedema (often facial/periocular), erythema and pruritus. This can also be accompanied by hypotension and tachycardia.
Report directly to VMD
We continue to urge anyone who is aware of an adverse event in an animal following administration of intravenous co-amoxiclav to report directly to us via our online reporting form Report a suspected problem with an animal medicine or microchip.
Please provide all relevant information, including the batch number.
The reporting of adverse events is critical to our ongoing monitoring activities in order to protect animal health, public health, and the environment.
Find out more about pharmacovigilance on www.vmdconnect.uk/adverse-events.
Any human adverse reactions to authorised human medicines should be reported to the Medicines and Healthcare Products Regulatory Agency (MHRA) (Report a problem with a medicine or medical device - GOV.UK (www.gov.uk)).
About the products
Authorised human products for intravenous administration containing co-amoxiclav are regularly used in animals via the veterinary medicines cascade.
Co-amoxiclav is used in the treatment of infection and is often used peri-operatively.