100 Days Mission: First Implementation Report - HTML
Updated 7 December 2021
1OO DAYS MISSION
First Implementation Report
Reducing the impact of future pandemics by making Diagnostics, Therapeutics and Vaccines available within 100 days
An independent report on progress to G7 Leaders from G7 Chief Scientific Advisers
2nd December 2021
Mobilising the 100 Days Mission
Foreword
G7 Leaders welcomed the 100 Days Mission (100DM) at the June Carbis Bay Summit, and committed to working together, between sectors and across national borders, to deliver this ambitious Apollo mission. Leaders spoke of the unpredictability of future health emergencies and emphasised the need to harness scientific innovation and public-private collaboration to develop an armamentarium of diagnostics, therapeutics and vaccines (DTVs) available within the first 100 days of a future pandemic threat being detected, consistent with our core principles around equitable access and high regulatory standards.
It’s on this commitment that we, as G7 countries and global science leaders, launched the 100DM and have since made early progress in mobilising the Mission, working with our G20 and wider international partners, the World Health Organization (WHO), the life sciences and biotechnology sectors, and international organisations. We have taken steps domestically and internationally to bolster political and scientific support for the 100DM, ensuring recommendations are reflected in our national policies and promulgated through public and private sector delivery plans. Innovation and collaboration have flourished in this environment and the results, although nascent, are particularly exciting.
I would like to take this opportunity to thank science colleagues and all 100DM implementation partners who have committed to this ambitious agenda and its concerted delivery over the next five years; the 100DM steering group members for their continued engagement; and lastly to Melinda French Gates and the wider Bill and Melinda Gates Foundation for their support in developing the 100DM report, and their focus on putting equity at the heart of how we prepare for future pandemics.
This independent advisory report from Chief Scientific Advisers (CSAs), the first progress update since the summit, is encouraging but it demonstrates that there’s a long way to go. This is, therefore, a call to action for us as international science leaders, with our policy, industry, academic and philanthropic partners, and with the full backing of the G7 leaders, to make every effort to deliver this ambitious Mission.
The report contains proposed 100DM roadmaps which provide clear milestones over the coming year and out to 2026 to galvanise collective international action in pursuit of the Mission. In the year ahead, working with our international partners, we recommend the following are pursued as priorities:
i) securing global agreement on the priority virus families against which we should accelerate the development of prototype libraries of DTVs;
ii) applying innovations in ‘programmable’ vaccine and therapeutic platform technologies (e.g. mRNA, viral vector) to COVID-19 variants and to wider endemic diseases, including to support technology transfer and strengthen global manufacturing capacity;
iii) improving international coordination on clinical trials to enable an efficient approach to testing of DTVs, and harmonised and streamlined regulatoryprocesses; and
iv) embedding a mission-driven approach to global implementation, including by establishing a 100DM Science and Technology Expert Group and annual G7 CSA reviews: incentivising collaboration, mobilising investment and championing mutually beneficial public-private partnerships.
Our collective ambition as G7 CSAs is for the 100DM to be a sustained endeavour, initiated through the UK’s presidency and carried forward across futurepresidencies, with the wider international community, until the Mission isachieved. As we prepare to hand over the baton, it’s important to remind ourselves that the work to prepare the world for a future pandemic must be inclusive. We will ensure equitable access to DTVs is at the heart of our approach, for the benefit of all.
The 100 Days Mission is ambitious, but achievable and essential.
Sir Patrick Vallance
Chapter 1
Introduction
The COVID-19 pandemic is the most devastating health and socioeconomic catastrophe of recent times and has caused untold damage. As of 24th November 2021 there were 257 million cumulative COVID-19 cases and 5.2 million COVID-19 related deaths worldwide.[footnote 1] In June 2020 the International Monetary Fund (IMF) estimated that the cumulative loss to the global economy over the years 2020 and 2021 are worth $12 trillion,[footnote 2] while in January this year The Economist estimated that forgone GDP over the same period is worth $10.3 trillion.[footnote 3]
As devastating as the current pandemic is, there is a reasonable likelihood that another serious pandemic could occur soon, possibly within the next decade.[footnote 4] The risk of a new pathogen emerging and becoming a threat is rising constantly as humanity expands into previously uninhabited regions and vulnerable populations.[footnote 5] We must, therefore, act now to strengthen our collective defences or risk being unprepared.
Seized by the gravity of the crisis, in February 2021 the UK Prime Minister, Boris Johnson, challenged G7 leaders to explore how to harness the unprecedented scientific innovation and public and private collaboration seen in the current crisis to reduce the time from discovery to deployment of diagnostics, therapeutics and vaccines (DTVs) in future health crises, building on the target set by the Coalition for Epidemic Preparedness Innovations (CEPI).[footnote 6]
Under the leadership of Sir Patrick Vallance, the pandemic preparedness partnership was established as an independent group of international experts to advise the UK’s G7 Presidency on how to develop safe, effective and affordable DTVs within the first 100 days of a pandemic.[footnote 7] In June 2021, CEOs of life sciences and biotechnology companies leading the efforts to develop COVID-19 DTVs backed the ambition of the 100 Days Mission (100DM) set out by the pandemic preparedness partnership.[footnote 8]
Two weeks later at the Carbis Bay Summit, G7 Leaders welcomed the 100DM, emphasising the importance of science-led innovation, and invited G7 Chief Scientific Advisers (CSAs) or their equivalents to review progress and report to leaders before the end of the year. Leaders recognised that achieving the Mission required continued and concerted collaboration between the public and private sectors, within and beyond the G7, and a ‘no regrets’ approach to implementation.
Leaders agreed that in the first 100 days from the World Health Organization (WHO) declaring a Public Health Emergency of International Concern (PHEIC), we should aim for accurate and approved rapid diagnostic tests, an initial regimen of therapeutics and vaccines ready to be produced at scale for global deployment, ensuring equitable access for the benefit of all:
Diagram 1: The Mission
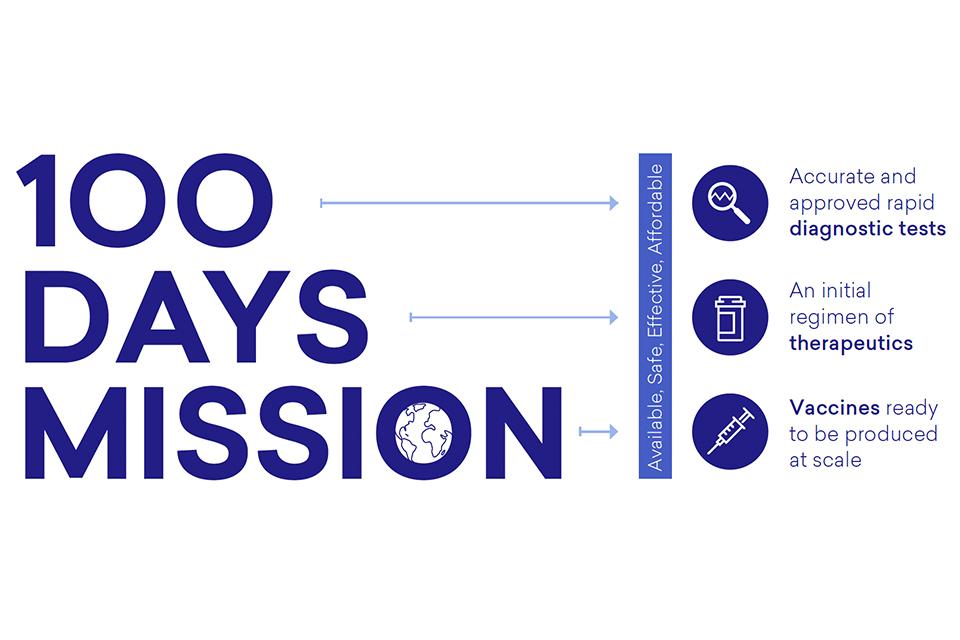
Diagram 1: The Mission
Approach to Implementation
The UK, as G7 President, has worked with G7 CSAs, the WHO and global 100DM implementing partners to mobilise delivery. We have taken a systematic approach to implementation, across three lines of engagement:
-
Broadening international political, institutional and scientific support for the Mission and its recommendations. Since the G7 summit, we have secured the support of the G20 to shorten the cycle for the development of safe and effective DTVs from 300 to 100 days following the identification of pandemic threats.[footnote 9] We continue to work closely with the WHO, in their strong normative, convening and coordination function across several recommendations. We are also working with low and lower-middle-income countries (LMICs) and wider international organisations to communicate and strengthen global cooperation across the 100DM recommendations and to advocate for science-based and equitable access to the solutions that arise.
-
Convening and mobilising organisations responsible for leading implementation of the specific 100DM recommendations, by setting clear expectations and timelines, aligning recommendations with existing policies, programmes and institutions wherever possible. For example, the UK Government CSA chaired a meeting of global life science and biotech industry leaders in November to review progress since the Life Sciences Event in June, develop the forward looking 100DM DTV roadmaps and agree key next steps for 2022.
-
Calling on G7 Governments to take concrete action domestically tobolster support for the 100DM by ensuring recommendations are considered in our national policies and taken forward in public and private sector delivery plans. For example in June, Japan embarked upon the “National Strategy for Vaccine Development and Manufacturing” as a national strategy for long-term continuous efforts to develop and manufacture vaccines for potential future pandemics. In September, the White House published the American Pandemic Preparedness Plan (AP3), enshrining the 100DM as a central tenet of its approach. Also in September, the European Union launched the European Health Emergency preparedness and Response Authority[footnote 10] (HERA) to improve the EU’s readiness for health emergencies, including by supporting research and innovation for the development of new medical countermeasures. Germany launched a new Centre for Pandemic Vaccines and Therapeutics (ZEPAI) in October, to serve as a centre for pandemic preparedness, coordination and response in the future.
Chapter 2 of this report captures progress to date in implementing the 100DM, chapter 3 presents the forward looking DTV roadmaps, chapter 4 sets out the cross-cutting enablers of the Mission, and chapter 5 outlines a proposed future governance framework to drive implementation.
Diagram 2[footnote 11]
What Could Have Been: Comparing the COVID-19 Timeline to a Proposed 100 Days Mission Timeline
COVID-19 Timeline
In the COVID-19 timeline, it took 336 days from the point at which WHO declared a Public Health Emergency of International Concern to attaining one diagnostic test, therapeutic and vaccine against COVID-19. In the 100 Days Mission timeline, it will take 100 days from the point at which WHO declares a Public Health Emergency of International Concern to attain an aramanantarium of diagnostics, therapeutics and vaccines against a future pandemic. The two timelines are represented below:
2014
-
-16 years
First clinical trial of an mRNA vaccine -
-5 years
WHO workshop on prioritisation of pathogens calls for R&D on MERS and SARS coronaviruses, among other diseases
2015
-
-4 years
Pre-fusion structure of a human coronavirus spike protein published
2016
-
-19 days
SARS-CoV-2 Sequence publicly shared
2020
- 0 days
WHO declares PHEIC -
64 Days
First real-time PCR test granted WHO EUL -
65 days
WHO report 1 million confirmed COVID-19 cases -
84 days
Launch of mRNA vaccine Phase 1/2 trial, 4 candidates evaluated in parallel -
85 days
ACT-A launched -
126 days
Gavi Global Vaccine Summit, launched and raised $505 million for COVAX AMC to support LMICs -
138 days
RECOVERY trial reports dexamethasone reduces mortality for those with severe disease -
167 days
150 countries in COVAX (75 self-financing) -
179 days
Phase 2/3 trial starts with selected mRNA vaccine candidate -
216 days
WHO approves dexamethasone as a COVID-19 therapeutic -
236 days
First Rapid Diagnostic Test granted WHO EUL -
259 days
SOLIDARITY Trial published interim results on therapeutics -
284 days
Phase 3 data show efficacy, will file for EUA once safety milestones met -
307 days
First SRA approval of vaccine (MHRA of Pfizer/BioNTech) -
336 days
WHO issued its first emergency use validation for a COVID-19 vaccine - 336 days
1 established example for each of diagnostics, therapeutics and vaccines
2021
-
538 days
Partnership with Biovac to supply doses for Africa -
574 days
Partnership with Eurofarma to supply doses for Latin America -
601 days
FDA EUA for COVID-19 vaccine booster
100 Days Mission Timeline
Preparing for the 100 Days, Today
- Global epidemiological and genomic surveillance: continuous surveillance and established mechanisms for pathogen prioritisation and data sharing
- Fundamental research: ongoing research on pathogens and immunity
- Global regulatory harmonisation: streamline and harmonise regulatory processes, and establish emergency procedures
- Advance candidate vaccines and therapeutics for pathogens with epidemic or pandemic potential into early-stage clinical studies
- Establish the platforms and partnerships needed for R&D and clinical trials
- Build and sustain manufacturing capacity , kept warm through application of innovative technologies to endemic diseases
- Pathogens sequenced and shared globaly through integrated data and sample sharing mechanisms
The 100 Days Response
- 0 days
WHO declares PHEIC - Industry pivots existing programmes to address new target, leveraging past and new private and public investments
- Pandemic rules of the road take effect, facilitating sharing of samples and materials
- WHO and regulatory authorities issue guidance documents and target product profiles, including expectations for safety data
- Clinical trials of most promising candidates launched using clinical trial via regionally linked clinical trials networks
- Advance market commitments enable manufacturing at risk while clinical trials are ongoing
- Manufacturers rapidly expand production capacity, including through regional manufacturing hubs
- Safe and efficacious DTV achieve EUA
-
100 days
an armamentarium of DTV ready to be deployed, consisting of- Accurate and approved rapid diagnostic tests
- An initial regimen of therapeutics
- Vaccines ready to be produced at scale
Post – 100 Days Actions
- R&D improvements to thermostability and yields, enabling alternative routes of administration (e.g. nasal)
- Vaccines are evaluated for duration of protection, effectiveness against emerging variants, when used to boost immunity
Chapter 2
Progress Since Carbis Bay
The 100DM report made 25 actionable recommendations for governments, industry, philanthropic organisations, civil society and international organisations to take forward to achieve the Mission’s goals.[footnote 12] As set out in the June 2021 report, recommendations need to be underpinned by sustained financing ahead of a pandemic to enable implementation.
Key to improving global pandemic preparedness is effective surveillance coupled with pathogen analysis, so we can spot threats earlier and respond immediately. Once surveillance has spotted a disease risk, our best weapons are DTVs.
To prepare effectively we need to deliver against the following goals:
-
Invest in Research and Development (R&D) to fill the gaps in our arsenal: adopting a mission focused, public-private sector approach to prepare prototype DTVs to identify and treat pathogens of greatest pandemic potential, and to develop vaccines and therapeutic technologies that can be readily adapted and easily manufactured for an unknown diseases of pandemic potential, or ‘Disease X’.
-
Make the exceptional routine by embedding best practice between pandemics: including regionally and globally linked clinical trial networks, streamlined regulation and simplified transferable manufacturingprocesses (as the norm) ‘kept warm’ through global adult vaccination programmes.
-
Agree on the rules of the road for a pandemic: protocols formed as part of a wider suite of the WHO guidance, agreed in advance and demonstrating a step-change from business as usual when a PHEIC is declared.
Strengthening Global Surveillance
Achieving the 100DM and improving global pandemic preparedness requires, firstly, effective surveillance and pathogen analysis so we can spot pandemic threats earlier and respond immediately.
In early August, the WHO convened an Implementation Consultation Group (ICG) of 19 global experts to develop a conceptual approach for anInternational Pathogen Surveillance Network (IPSN). In line with Sir Jeremy Farrar’s Pathogen Surveillance Report,[footnote 13] the IPSN aims to bring genomic surveillance to speed and scale, so that it provides quality, timely and representative data within the broader surveillance architecture to better inform decision-making and public health action. Building on existing disease surveillance networks and based on expert inputs, the IPSN will rapidly undertake pilots to establish a ‘mesh network’ of pre-existing expert centres and active nodes. This will serve as the basis for the medium-to-long term strategic vision to span the molecular epidemiology needs for new and emerging threats, endemic diseases and other enduring public health challenges.
The WHO Hub for Pandemic and Epidemic Intelligence was established in Berlin to support the work of public health experts and policy-makers in all countries with the tools needed to forecast, detect and assess epidemic and pandemic risks so they can take rapid decisions to prevent and respond to future public health emergencies. The IPSN will build on the WHO Hub and other expert centres on pandemic and epidemic intelligence.
G7 partners have incorporated global surveillance considerations into national policies, building their domestic genomic capabilities:
-
The U.S. Centres for Disease Control and Prevention (CDC) has established the National SARS-CoV-2 Strain Surveillance (NS3) system to track new variants of concern, alongside the Center for Epidemic Forecasting and Outbreak Analytics.
-
The UK Health Security Agency and the UK’s Centre for Pandemic Preparedness were launched[footnote 14] to spearhead the UK’s contribution to developing a global early warning system to detect new infectious disease threats, alongside the New Variant Assessment Platform (NVAP) offering UK capacity and expertise to detect and assess new variants of SARS-CoV-2. An extensive genomic sequencing and analytics capacity is already in operation.
-
Canada, through Genome Canada, launched the COVID-19 Genomics Network (CanCOGeN) for large-scale SARS-CoV-2 and human host sequencing to track viral origin, spread and evolution. The Network, supported by its research arm ‘the Coronaviruses Variants RapidResponse Network’ (CoVaRR-Net), is scaling up sequencing of COVID-19 viruses and will further contribute to building national capacity to address future outbreaks and pandemics.
-
France has established a national genomic surveillance network, through the Santé Publique France and ANRS Emerging Infectious Disease, with the short term objective of characterising, describing and monitoring the circulation of SARS-CoV-2 variants and the long term objective of a sequencing network in support of surveillance activities on emerging infectious diseases. The Pasteur network also has SARS-CoV-2 surveillance activities overseas and is currently proactively monitoring the emergence of new viral pathogens.[footnote 15]
-
The Japanese Ministry of Health, Labour and Welfare (MHLW) and the National Institute of Infectious Diseases (NIID) has launched the National COVID-19 genomic surveillance system to track and monitor variants in collaboration with local public health institutes, academic institutions and commercial laboratories, alongside building the genome sequencing capacity at the local government level.
Investing in R&D to Fill the Gaps in Our Arsenal
Demonstrable progress has been made in the R&D of DTVs for COVID-19 and endemic diseases.
-
CEPI is developing a ranking methodology to assess the likelihood of a ‘Disease X’ emerging from a family of viruses, and are sharing information with the US’s National Institute of Allergy and Infectious Diseases (NIAID) who have developed their own approach to prioritisation. CEPI will work with the WHO (in its normative role) to agree the priority virus family list next year. This will determine the priorities against which prototype libraries of DTVs will be developed. CEPI will launch a call for proposals for pilot projects to develop vaccine libraries against two pilot virus families from the initial priority list, the Paramyxoviridae and Arenaviridae. CEPI is communicating closely with NIAID to ensure funding towards Disease X programmes are synergistic.
-
DeepMind has used its state-of-the-art AI system, AlphaFold, to generate protein structure predictions for 32 viruses curated by CEPI. These served as a starting point for discussing how AlphaFold might support the work of the 100DM in the discovery of targets for future DTVs. With the AlphaFold system now publicly available via open source code, DeepMind[footnote 16] is exploring with CEPI how it may play an advisory role to support structural virologists in making high-quality models for key targets. To increase AlphaFold’s relevance to vaccine development, further work will be needed to address certain limitations of the current system; these are areas of active research.
-
Industry and academia are adapting ‘programmable’ platformtechnologies,[footnote 17] (deployed during COVID-19), for use against wider endemic diseases. For example, Oxford University has started clinical trials of a plague vaccine using its viral vector technology[footnote 18] and Moderna is looking at using messenger ribonucleic acid (mRNA) technology to make vaccines against Nipah virus and HIV.[footnote 19] Pfizer has commenced a Phase 1 study of mRNA vaccine against influenza in healthy adults.[footnote 20] The WHO recently approved the world’s first vaccine against malaria which could prevent the deaths of 23,000 children each year in sub-Saharan Africa.[footnote 21]
-
Wellcome and CEPI are stimulating innovation in vaccine platform technology and manufacturing processes, including by co-funding the ‘Leap Programme’ for disruptive innovations in mRNA technologies.[footnote 22] The programme seeks to exponentially increase the number of biologic products that can be designed, developed, and produced every year, reducing their costs and increasing equitable access; and create a self-sustaining network of manufacturing facilities providing globally distributed, state-of-the-art surge capacity to meet future pandemic needs.
-
There have been several promising breakthroughs for therapeutics against COVID-19, including Merck & Co.’s oral antiviral molnupiravir, now approved by the UK’s Medicines and Healthcare products Regulatory Agency (MHRA), which was shown to reduce the risk of hospitalisation or death in stage 3 trials.[footnote 23] Pfizer’s novel protease inhibitor paxlovid, when taken with the oral antiviral ritonavir, was shown to reduce the risk of hospitalisation or death by 89%, in a randomised study of non-hospitalised adult patients with COVID-19 who are at high risk of progressing to severe illness.[footnote 24] Both Merck & Co. and Pfizer have signed voluntary licensing agreements with the Medicines Patent Pool (MPP) to help create broad access to oral antivirals.
-
The market for both antigen detecting rapid diagnostic tests (RDT) including lateral flow devices, and automated polymerase chain reaction (PCR) tests has expanded, with over 1,150 commercially available tests now on the market.[footnote 25] The average price of an RDT is falling, as industry has rapidly scaled up capacity and capabilities,[footnote 26] which is helping to create manufacturing capacity in, and provide increased access to LMICs. However, further work on regulatory approval is needed to expand access.
Work to strengthen the role of the international system in R&D capability and coordination for DTVs has also advanced:
-
CEPI’s Board has endorsed an approach to pursue activities related to therapeutics consistent with their current mandate, and will consider extending its mandate further to enable a broader scope of therapeutics’ efforts to be undertaken.
-
The Foundation for Innovative New Diagnostics (FIND) will strengthen international coordination and collaboration across the diagnostics ecosystem in pursuit of the 100DM, including supporting R&D to accelerate development of prototype diagnostics against priority virus families, and strengthen engagement with LMICs to improve access and promote uptake.
-
The International Readiness for Preventing Infectious Viral Disease Alliance (INTREPID) has recruited industry participants and formalised initial financial commitments to begin the rapid translation of promising academic discoveries onto an industrialised platform.
-
The Rapidly Emerging Antiviral Drug Development Initiative (READDI), in close collaboration with INTREPID and others, is developing small molecule antiviral drugs effective against priority virus families, as well as advancing clinical trials and manufacturing plans for rapid efficacy testing and production.
Embedding Best Practice Between Pandemics
There have been preliminary developments in the work to improve clinical trials and regulatory coordination, alignment and prioritisation:
-
The WHO recently convened a roundtable of globally diverse clinical trials experts[footnote 27] to agree collective actions to address the steps needed to make clinical trials more efficient, more effective and faster[footnote 28] so that DTVs are ready to be deployed within 100 days of a PHEIC. The WHO is scoping how an international network of regionally linked clinical trial mechanisms could be implemented, including optimisation of the infrastructure needed to support well designed and efficient trials and strengthening ethical and regulatory approvals processes.
-
The Good Clinical Trials Collaborative (GCTC) has developed streamlined guidance to support those involved in randomised controlled trials (RCTs) to adopt flexible, innovative and proportionate approaches to trial design and delivery based on a set of key underlying principles for good RCTs.[footnote 29]
-
The MHRA and the European Medicines Agency (EMA) have established a working group within the International Coalition of Medicines Regulatory Authorities (ICMRA) that will develop a pandemic preparedness protocol for regulators, reflecting best practice and lessons learned from COVID-19, to be published in 2023.
-
The MHRA and the U.S. Food and Drug Administration’s (FDA) Center for Devices and Radiological Health have committed to working with G7 counterparts to scope proposals to streamline diagnostics regulatory processes, prioritising the most impactful areas for mutual recognition agreements.
Global Manufacturing Capacity: Gavi, the WHO, CEPI and regional initiatives, supported by the G20,[footnote 30] are increasing vaccine distribution, administration and local manufacturing capacity in LMICs, including through technology transfer hubs in various regions. Development of this network will support enhanced access to DTVs, strengthening future preparedness and response. Individual companies are establishing extensive manufacturing capacity.
Rules of the Road
The procedures and protocols to come into effect in a future pandemic are being developed:
-
Data Access and Sharing: In response to the Science Academies of the Group of Seven’s (S7) statement on the “need for a better level of ‘data readiness’ for future health emergencies” the Global Pandemic Data Alliance (GPDA) has been established.[footnote 31] The GPDA has started work on a two-year implementation roadmap to meet the challenges set out by the S7 to ensure the availability and accessibility of data as a source for critical insights in public health emergencies.[footnote 32]
-
Biological Sample Collection and Sharing: The WHO BioHub System, if guided and ultimately approved by WHO Member States, could offer a mechanism for WHO Member States to voluntarily share biological materials with pandemic potential and must be designed to complement existing, functional systems such as the Global Influenza Surveillance and Response System (GISRS). A few countries[footnote 33] have signed up to participate in the Pilot Testing Phase and the WHO will report on progress to the World Health Assembly following consultation with WHO Member States.
-
Finance and Health Governance: In October, G20 Health and Finance ministers committed to establishing a Joint Finance-Health Task Force to enhance dialogue and global cooperation on issues relating to pandemic prevention, preparedness and response (pandemic PPR). The Task Force will promote the exchange of experiences and best practice, develop coordination arrangements between Finance and Health Ministries, promote collective action, assess and address health emergencies with cross-border impact, and encourage effective stewardship of resources for pandemic PPR, while adopting a One Health approach.[footnote 34] In early 2022, the Task Force will report on modalities to establish a financial facility for ensuring adequate and sustained financing for pandemic prevention, preparedness and response.
-
Pandemic Governance: The Working Group for Strengthening WHO Preparedness and Response (WGPR) reached consensus to recommend that Member States move forward with an intergovernmental process to draft and negotiate a convention, agreement, or other international instrument on pandemic preparedness and response. WHO Member States will discuss this at the special session of the World Health Assembly in November 2021.
The Challenges Ahead
Pandemic Financing and Equitable Access: This pandemic has seen areliance by developing countries on grant funding and a stark asymmetry in access to DTVs. Multilateral efforts have sought to expand pandemic response coordination. The Multilateral Leaders Task Force (composed of major multilateral international organisations) is a good starting point in shining a light on the challenges that need to be addressed to remove barriers to export and import of DTVs and increasing production in developingcountries. Despite this effort, a key priority for 2022 will be mobilising sustainable sources of finance to support equitable access.
Chapter 3
Looking Ahead: Mission Roadmaps for DTVs
In the following chapter we outline forward looking roadmaps for delivering safe, effective and affordable DTVs ready for global deployment within 100 days. The indicative and iterative roadmaps set out the key outcomes we expect to see in 2026 and the milestones against which we can track and report progress annually. They were developed collaboratively with G7Governments, the Mission’s implementing partners, the WHO, and experts from around the world.[footnote 35] They will be evaluated and adjusted annually through the G7 CSA review to reflect advances in Science and Technology (S&T) and changes in the geopolitical, societal, environmental, legal and economic environment.
Diagnostics: The aim is to create prototype diagnostics against the priority virus families (agreed by CEPI and the WHO), with a focus on the respiratory viruses, using a growing diversity of platforms which can be readily adapted to a new pathogen. Normalising the use of accurate diagnostics in point-of-care, non-clinical settings (including for coronaviruses and influenza viruses) and within pathogen surveillance systems will stimulate investment and innovation in the sector. FIND, working with governments and the diagnostics industry, aims to reduce the price of a rapid test to under $1.00.[footnote 36] Integrating diagnostics into routine healthcare and pathogen surveillance systems will allow industry to develop high-quality diagnostics, and scale up manufacturing rapidly, when a new pathogen is detected.[footnote 37]
Therapeutics: The aim is to have 25 high-quality therapeutic candidates that have completed Phase 1 studies in humans, covering the high priority respiratory virus families.[footnote 38] Once a new pathogen emerges, these will be ready to be rapidly transitioned into Phase 2/3 trials, and manufactured at pace and scale.[footnote 39] The aim is to make monoclonal antibodies (mAbs) more affordable to LMICs, through reducing manufacturing complexity (supporting technology transfer) and streamlining regulatory pathways,[footnote 40] whilst also advancing programmable therapeutics[footnote 41] using the latest computational chemistry.[footnote 42]
Vaccines: The aim is to develop vaccine libraries against ten high priorityvirus families,[footnote 43],[footnote 44] which can then be readily adapted to a new pathogen. Investing in vaccine development for endemic diseases will stimulate innovation in novel platform technology and sustain capability to rapidly pivot to create vaccines against Disease X.[footnote 45] This will include R&D to increase the thermostability of vaccines and accelerate development of needle-free formulations, which will help enable large scale production, overcome deployment challenges and promote equitable access.[footnote 46] Industry, with the WHO, will strengthen existing manufacturing capacity in LMICs and build additional capability where required.
A critical first step in the development of prototype DTVs to prepare for a future Disease X is to determine an agreed list of priority virus families. The WHO will work with CEPI (alongside FIND, NIAID and INTREPID) to develop and agree a list of virus families against which development of prototype DTV libraries could be prioritised in early 2022. This list could also inform R&D funding decisions (e.g. governments, philanthropic, private sector) and efforts in industry and academia in pursuit of the 100DM.
Once research is underway to develop DTVs against priority pathogens, prioritisation of product development is critical. Our experience developing DTVs during COVID-19 has demonstrated the importance of navigating the complex research landscape to inform expert scientific assessment of emerging products, conducted at an international level (e.g. by a panel of experts, under the authority of appropriate international organisations), so the most promising products are prioritised for testing and regulatory approval. Candidate treatments that meet the needs of the various patient populations, and of different health systems, should be prioritised and adapted to the designs and capacities of available platform trials. We recommend further work is conducted in early 2022 to progress this proposal, including identifying an appropriate joined up mechanism to ensure that the flow of products from different sources can be logged, monitored and prioritised.
Sustained R&D funding for development of DTVs is critical to achieve the Mission. Relying purely on current market incentives will be insufficient to act on the speed and scale needed to achieve the 100DM. Innovators need to be supported by incentive structures which align with the Mission, including strong and sustained public and private investment in R&D, fostering the level of certainty needed to stimulate rapid development of DTVs ready for global deployment within 100 days of a PHEIC. CEPI, through the replenishment event in March 2022, is seeking $3.5 billion investment from governments, global health organisations, and strategic partners to implement its five-year strategy to fund R&D to compress timelines and enable equitable access to lifesaving vaccines. Parallel investments to accelerate therapeutic and diagnostic development will be required to fill the gaps in our DTV arsenal and we recommend funding to stimulate R&D and innovation is consideredas part of any review of pandemic financing taken forward by the G20Finance-Health Task Force.
The ability to rapidly develop and deploy novel DTVs is dependent on a timely and systematic approach to data, information and biological sample sharing between industry, academia, international organisations, civil society and governments and strengthened public-private partnerships which build on those forged in the current pandemic.[footnote 47]
Over the next five years CEPI, alongside other key stakeholders including the WHO, will coordinate regular live fire exercises or ‘germ games’[footnote 48] to simulate the early warning phase and the first 100 days of a novel virus epidemic, where Day Zero marks the sequencing of the virus. The exercises will focus on the role of governments, in collaboration with key international organisations and international non-governmental organisations (INGOs), in developing safe, effective, available, and affordable DTVs that are ready to be produced at scale for global deployment. In the future these exercises could be extended to test the effectiveness of preparedness capabilities, including to assess collective resilience to potential future health threats.
Diagnostics Roadmap
Access to rapid, accurate diagnostic tests is critical to pathogen identification, interrupting transmission chains, and tracking and preventing the spread of a pathogen. In the COVID-19 pandemic, diagnostic developers were initially hindered in their efforts to create accurate tests due to slow access to samples, a lack of clear specifications, and insufficient regulatory harmonisation, which contributed to slow rollout in many jurisdictions.
The lack of investment in affordable, fast and accurate diagnostics before COVID-19, including point-of-care platforms capable of differentiating among pandemic threats like influenza and coronaviruses, resulted in limited research and production capacity which meant the market was underprepared.[footnote 49] Access to accurate tests in LMICs[footnote 50] and integration of diagnostics within surveillance networks[footnote 51] remain fundamental issues, alongside the need for thorough regulatory routes for diagnostics, including mutual recognition agreements between Stringent Regulatory Authorities (SRAs), that better define criteria and standards for effectiveness, quality and use cases.
Intense collaboration between academia and industry[footnote 52] did yield significant advances, with automated PCR tests available in 64 days from the declaration of the SARS-CoV-2 PHEIC and the first RDT, not requiring extensive laboratory infrastructure, receiving WHO approval in 236 days. Private sector innovation and increased demand from governments is helping drive down the price of RDTs across the world. The average cost of a rapid test has dropped from $5.00 to $2.50 in just over a year, as industry has rapidly scaled up capacity and capabilities,[footnote 53] which is helping increase access to LMICs.[footnote 54]
Aims
By 2026, the aim is to have ‘accurate and approved rapid point-of-care diagnostic tests’ available within 100 days (ideally sooner) of a PHEIC being declared. This includes developing prototype diagnostic libraries on a range of test platforms (against priority virus families) that can be easily and quickly adapted to an emerging pathogen. Governments and international organisations will increase demand for diagnostics by normalising in point-of-care and non-clinical settings (including integrating into pathogen surveillance systems), thereby continuing to stimulate the private sector to innovate and strengthen manufacturing capacity, which will drive down prices for both PCR and RDTs around the world.
Summary Plans
Global Coordination: FIND is working with the WHO, the diagnostics industry and the WHO to strengthen international coordination on diagnostics R&D,[footnote 55] including in pursuit of the 100DM, and to support equitable access to diagnostics. In January 2022, FIND will submit a joint report with CEPI on diagnostics innovation for pandemics for the 100DM at the World Economic Forum in Davos. Following this they will convene meetings with key stakeholders and partners to refine a diagnostics plan and budget (ahead of the Annual Meetings of the World Bank Group and the IMF in Spring 2022) and kick-start implementation in Q2 2022.
Prototype Diagnostics: The diagnostics industry, coordinated by FIND, is developing diagnostic libraries using a diversity of platforms – both those that can detect pathogens across an entire virus family (e.g. pan-coronavirus) and ‘pathogen-agnostic’ platforms with the potential to detect Disease X.[footnote 56] They have launched a programme to develop several new point-of-care PCR platforms for differentiating multiple pathogens, the first of which should be commercially available in 2023.[footnote 57] The CEPI vaccine library approach will generate reagents and standards that can be used by others to rapidly develop diagnostics for pathogens with Disease X potential.
Normalising Diagnostics: Governments have a significant role to play in creating push incentives for diagnostics R&D and innovation, as well as pull incentives, by normalising the use of diagnostics in point-of-care and non-clinical settings. The U.S. Government aims to have affordable and accessible diagnostics ready to meet national needs for daily, at home, testing within weeks after the recognition of an emerging pandemic threat.[footnote 58] Japan has expanded its use of RDTs in both clinical and non-clinical settings and Canada has prioritised regulatory submissions of point-of-care COVID-19 tests, as well as convening an Advisory Panel to provide advice to support the pivot to testing in point-of-care and non-clinical settings. France is currently mainstreaming the use of duplex PCR tests (influenza, SARS-CoV-2) for the “at risk” population, although this is almost exclusively carried out in a hospital context.
Access to LMICs: FIND, working with industry and wider partners, aims to reduce the price of a rapid test to under $1.00[footnote 59] which will help achieve the Access to COVID-19 Tools Accelerator (ACT-A) target of minimum coverage of ‘100 tests / 100k people / day’ in LMICs over the next 12 months.[footnote 60] FIND will support the expansion of the manufacturing base in LMICs, and will work toward creating modular manufacturing processes, which are transferable and scalable, allowing for rapid production of key diagnostic tests.[footnote 61] The supply of accurate diagnostics needs to be matched by public sector demand to enable sustainable global production capacity at affordable prices.
Surveillance: Building on the conceptual approach of the IPSN, partners including FIND and the diagnostics industry will continue to work with the WHO to enhance diagnostics capability and capacity for global genomic surveillance. They will leverage current efforts by the ACT-A for SARS-CoV-2 sequencing and building capacity for key endemic diseases, such as arboviral diseases and antimicrobial resistant bacteria. This is part of the WHO’s Global Genomic Strategy that seeks to strengthen and scale genomic surveillance for quality, timely and appropriate public health actions within local to global surveillance systems.
Diagram 3
Proposed 100 Days Mission Roadmap for Diagnostics
Successfully achieving the milestones for the following recommendations will help us attain accurate and approved rapid diagnostics tests within 100 days
| 2022 Milestones |
2023 Milestones |
2024 Milestones |
2025 Milestones |
2026 Outcomes |
|
|
Recommendation 2 Build prototype diagnostic libraries applicable to representative pathogens of pandemic potential (FIND supported by CEPI, WHO and industry) |
WHO works with CEPI (alongside FIND) to agree priority virus families for accelerated development of prototype diagnostic libraries. FIND, working with industry and academia, identifies two or more fit-for-purpose molecular (PCR) platforms for near-patient testing for multiple pathogens. |
FIND demonstrates progress in developing prototype libraries for detection of four priority list virus families (Coronavirusae, Orthomyxoviridae, Filovirusae and Flaviviridae). FIND advances at least two PCR platforms and establishes target supply chain for regional production of PCR and antigen rapid tests (RDTs). |
FIND matures prototype diagnostic libraries for four priority virus families. Adds additional virus families for development (decided in conjunction with CEPI and WHO based on surveillance data). FIND secures regional supply chains for 100DM response for PCR and antigen RDTs. FIND demonstrates effective prototype library stand-up in ‘germ games’. |
FIND matures diagnostic libraries across further four - six families of viruses including Paramyxoviridae, Bunyaviridae, Togaviridae and Flaviviridae, subject to funding. FIND demonstrates effective pathogen-agnostic platforms with the potential to detect Disease X. |
Prototype diagnostics developed providing broad coverage for 8-10* virus families. *final number subject to funding |
|
Recommendation 6 Strengthen the role of the international system in R&D capability and coordination for diagnostics (CEPI, FIND, WHO with academia and industry) |
FIND and CEPI to create a ‘contract mechanism’ for FIND to coordinate diagnostics work on the 100DM. FIND present strategy (alongside CEPI) to WEF, highlighting the fundamental role of academia and industry in diagnostic innovation for 100DM. FIND convene industry to work with WHO and SRAs to drive improvements in regulation, clinical trials and manufacturing for diagnostics. |
FIND, working with academia and industry, CEPI and the WHO begin implementing diagnostics strategy for 100DM. Partnerships formed across diagnostics sector and governments in both HICs and LMICs. FIND demonstrate improvement in global diagnostics coordination, including facilitating knowledge exchange and collaboration on 100DM. |
FIND, working with academia and industry, reports progress in developing partnerships across diagnostic sector and with governments to stimulate innovation and take up of diagnostics (see Rec 7). FIND works with partners (SRA, WHO, Industry) to drive improvements in standards, clinical trials, regulation and manufacturing / supply chain for diagnostics. |
FIND reports progress in developing partnerships across diagnostic sector and with governments to stimulate innovation and take up of diagnostics (see Rec 7). FIND works with partners (SRA, WHO, Industry) to drive improvements in standards, clinical trials, regulation and manufacturing / supply chain for diagnostics. |
Strengthened international coordination between governments, industry and international organisations on diagnostics R&D. |
|
Recommendation 7 Governments should normalise the use of accurate diagnostics for coronavirus and influenza in point-of-care and non-clinical settings (G7 Govts, FIND, WHO, academia and industry) |
G7 governments, working with FIND and WHO, produce plans to normalise the use of diagnostics domestically, including annual milestones. |
G7 governments, with FIND and WHO, develop implementation plan to normalise use of diagnostics and promote adoption via G20 and LMICs. | G7 governments, with FIND and WHO, implement plan to normalise use of diagnostics and promote adoption via G20 and LMICs. | G7 governments, with FIND and WHO, implement plan to normalise use of diagnostics and promote adoption via G20 and LMICs. | Diagnostics used routinely in point-of-care and non-clinical settings for priority pathogens. |
|
Recommendation 8 WHO should support an enhanced role for diagnostics in the surveillance of pandemic threats (WHO) |
WHO establish pilots for the International Pathogen Surveillance Network, which will serve as the basis of a rapid establishment of a ‘beta version’. WHO will report on the utilisation of the IPSN conceptual approach to strengthen genomic surveillance of pandemic threats before April 2022. |
A stepwise process is being used to develop and inform the conceptual approach, and to provide options for IPSN implementation. Future milestones will be determined in consultation with G7 CSAs and in line with the IPSN report due April 2022. |
A stepwise process is being used to develop and inform the conceptual approach, and to provide options for IPSN implementation. Future milestones will be determined in consultation with G7 CSAs and in line with the IPSN report due April 2022. |
A stepwise process is being used to develop and inform the conceptual approach, and to provide options for IPSN implementation. Future milestones will be determined in consultation with G7 CSAs and in line with the IPSN report due April 2022. |
Diagnostics integrated into the International Pathogen Surveillance Network (IPSN) to maximise its coverage and utility. |
Therapeutics Roadmap
Therapeutics are vital for treating infections, reducing morbidity and mortality, as well as mitigating the long-term damage to people’s health. Therapeutics can also be used as prophylaxis, to prevent symptoms and the spread of the disease in community settings, easing pressure on hospitals. Vaccines will not work against every pathogen, therefore having an initial regimen of high-quality therapeutics ready to go is vital in strengthening pandemic preparedness.
Despite warnings from scientists that a ‘stockpile’ of broad-spectrum ‘Phase 2-ready’ antivirals would be essential to respond to future outbreaks,[footnote 62] there were no approved antivirals for coronaviruses prior to the COVID-19 pandemic.[footnote 63] Notable successes early in the COVID-19 pandemic were as a result of repurposed drugs, such as dexamethasone, which have since saved countless lives.[footnote 64] There are now eleven approved repurposed antiviral drugs for treating COVID-19 globally and, while many antivirals specific to COVID-19 remain in preclinical development,[footnote 65] there are promising signs of significant breakthroughs, such as molnupiravir[footnote 66] and paxlovid.[footnote 67]
To date, at least six mAbs have been granted emergency use authorisation, with another five in late stage development.[footnote 68] The first generation of approved mAbs products[footnote 69] require high doses delivered by injection or infusion, which increases the cost of production and delivery and adds to the burden on health care systems which are already stretched. mAbs hold challenges for local manufacturing because they require highly specialised facilities, need to be adapted on an ongoing basis for new variants,[footnote 70] which results in limited supply and high prices.[footnote 71] Producers are currently insufficiently incentivised to invest in manufacturing innovations, and there is inadequate global capacity to produce mAbs at scale. These challenges must be addressed to increase access to mAbs to combat future health threats.
Aims
To rapidly prepare ‘an initial regimen of therapeutics’ against a future Disease X, we need to move from reactive response to proactive planning.[footnote 72] By 2026, the aim is to have 25 high-quality small molecule antiviral candidates which have completed Phase 1 studies in humans, developed against the high priorityvirus families.[footnote 73] These will be ready to be rapidly transitioned into phase 2/3 trials, and manufactured at pace and scale.[footnote 74] The aim is to make ‘programmable’ mAbs more affordable to LMICs by reducing complexity in administration, simplifying manufacturing processes, expanding manufacturing capacity and streamlining regulatory pathways.[footnote 75]
This will be underpinned through strengthened international coordination on therapeutics R&D, bringing together different initiatives, creating an enhanced end-to-end pipeline for small molecule antivirals and ‘programmable’ mAbs to truncate development timelines and promote equitable access. CEPI will work as a connector to ensure a coordinated approach, working closely with industry (including public-private product development partnerships already focused on therapeutics), through INTREPID, and READDI along with INGOs and wider stakeholders.
Summary Plans
Global Collaboration: A priority for 2022 is to create a forum to bring together complementary therapeutics initiatives to share information and reduce duplication. The specific remit of this forum is yet to be scoped, but CEPI – working with partners such as the Hever Group, Bill and Melinda Gates Foundation (BMGF), Wellcome, the International Federation ofPharmaceutical Manufacturers & Associations (IFPMA), and INTREPID – will play a key role as a convener, as well as contributing to evidence generation and tracking of novel and repurposed therapeutics. Such a therapeutics forum could provide an avenue for interested parties to share pre-competitive information (such as in silico predictions and animal models data analysis).
Developing Phase 1 Antivirals: Industry is working to develop 25high-quality small molecule antiviral candidates. INTREPID, an emerging industry-led consortium, seeks to accelerate this process through enhanced pre-competitive collaboration and jointly agreed targets (initially focused on an expanded panel of coronavirus targets). By the end of 2022, industry aims to develop ten high-quality antiviral candidates against priority virus families. READDI,[footnote 76] a public-private partnership, is developing five broad spectrumsmall molecule antiviral drugs, focusing initially on the Coronaviruses, Flaviviruses and Alphaviruses. Creating stockpiles of therapeutics against priority virus families should be considered. This would stimulate innovation, keep manufacturing warm and enable rapid containment of outbreaks of known pathogens.
Preparing Clinical Trial and Manufacturing Plans: READDI will form partnerships with manufacturers, and develop Phase 2/3 trial designs for antiviral candidates in advance of future viruses emerging.[footnote 77] This includes establishing “warm base” manufacturing plans to enable rapid scale up and distribution of small molecule antivirals around the world. READDI will also plan Phase 2/3 trials at pre-identified sites around the world for rapid efficacy testing in multiple patient populations. Other global partners, including the Global Research Collaboration for Infectious Disease Preparedness (GLoPID-R), will also play a key role in coordinating platform trials.
Reducing Cost of mAbs: Increasing access to mAbs will requireimprovements in deliverability and scale of production.[footnote 78] Working with the proposed therapeutics forum and existing public-private partnerships, industry will invest in manufacturing innovations for developing accessible mAbs, such as improving thermostability,[footnote 79] developing alternative routes of administration,[footnote 80] and reducing the size and/or number of doses: all of which would significantly reduce the costs for producers and the manufacturing capacity required for increased equitable access.[footnote 81] Strategies which target upstream productivity, such as continuous processing and alternative microbial hosts,[footnote 82] are likely to have the greatest impact on lowering costs.[footnote 83] Once established, integrating manufacturing innovations as new platform processes for new facilities, as well as retrofitting existing infrastructure, will ensure these innovations are widely adopted and lead to increased access to mAbs.
Alternative Therapeutic Modalities: mAbs may not be the most accessible therapeutic modality in some settings, therefore the development of other therapeutics modalities is essential. Several initiatives are exploring the possibilities of RNA based therapeutics[footnote 84] which could be a valuable tool in pandemic response, if issues around delivery to the tissue and cell type are addressed.[footnote 85] Targeting of gene expression through RNA interference (RNAi) is being developed as a new therapeutic strategy, has been proven for several conditions and there are licensed medicines available. Whilst it holds significant promise, there are a number of technical issues to be overcome for treating infections.
Diagram 4
Proposed 100 Days Mission Roadmap for Therapeutics
Successfully achieving the milestones for the following recommendations will help us attain an initial regime of therapeutics within 100 days
| 2022 Milestones |
2023 Milestones |
2024 Milestones |
2025 Milestones |
2026 Outcomes |
|
|
Recommendation 3 Develop prototype antiviral therapeutics, including antibody therapies, for respiratory pathogens of pandemic potential. (Research-based pharmaceutical industry initiatives such as READDI and INTREPID) |
WHO works with CEPI (alongside INTREPID) to develop priority virus families for accelerated development of prototype antivirals. Industry, working with INTREPID, agrees priority viral families for antiviral development and testing. Initiates development of ten high-quality antiviral candidates (small molecule antivirals). READDI launches broad spectrum antiviral discovery efforts for multiple priority virus families. |
Industry, working with INTREPID and wider partners, develops 20 antiviral candidates, against the agreed priorities. READDI advances lead antiviral compounds into preclinical testing. |
Industry, working with INTREPID and wider partners, develops 25 antiviral candidates against the agreed priorities. READDI, with INTREPID and industry and other partners, launch late stage preclinical and early clinical development and establishes clinical trial plans. |
Industry, working with INTREPID, and wider partners, develops 25 antiviral candidates, which have advanced to Phase 1 testing in humans, against the agreed priority pathogen list. READDI, with INTREPID and industry and other partners, identify small molecule antiviral candidates to advance through Phrase 1 testing, and develops Phase 2/3 clinical trial plans. |
25 high-quality therapeutic candidates developed against priority (respiratory) virus families. |
|
Recommendation 5 Invest in simplified cheaper routes for producing monoclonal antibodies and other new therapeutic modalities (G7 Govts, Industry (IFPMA)) |
Working with the proposed therapeutics forum, industry (with partners) targets investment to develop: i. mAbs – to enable smaller or less frequent doses, improve thermostability, routes of administration and pursue manufacturing innovation. ii. Novel programmable antivirals (including new approaches e.g. siRNA, stapled peptides or inferring inhibitors) against future Disease X. |
Industry, working with the proposed therapeutics forum, targets investment to address mAbs deployability challenges. Industry (with partners)>develops programmable antiviral platform technology (including new approaches) to address priority endemic diseases. | mAbs demonstrate improved thermostability and administration, with smaller or less frequent doses for the same treatment. Innovations contribute to reducing average cost of producing marketable mAbs to less than $75/gram. Programmable antiviral platform technologies developed against priority endemic diseases, proving efficacy. |
mAbs demonstrate improved thermostability and administration, with smaller or less frequent doses for the same treatment. Innovations contribute to reducing average cost of producing marketable mAbs to less than $50/gram. Programmable antiviral platform technologies developed against wider endemic diseases, proving efficacy. |
Cost of producing marketable mAbs reduced to less than $25/gram.a Programmable antivirals available, able to be rapidly re-purposed to Disease X. |
|
Recommendation 6 Strengthen the role of the international system in R&D capability and coordination for therapeutics by expanding CEPI’s remit to cover therapeutics, (CEPI with funding support from G7 Govts). |
INTREPID, READDI, the Hever Group, Wellcome, BMGF, CEPI and other key stakeholders agree a therapeutics forum to share information and reduce duplication of efforts. CEPI sets out its strategic objectives and investment plan for therapeutics. |
Future milestones will be agreed by the proposed therapeutics forum and reported by CEPI in 2022. INTREPID works with key industry partners to enable the rapid translation of promising academic discoveries onto industrialised platforms. |
Future milestones will be agreed by the proposed therapeutics forum and reported by CEPI in 2022. INTREPID works with key industry partners to enable the rapid translation of promising academic discoveries onto industrialised platforms. |
Future milestones will be agreed by the proposed therapeutics forum and reported by CEPI in 2022. INTREPID works with key industry partners to enable the rapid translation of promising academic discoveries onto industrialised platforms. |
A sustainable R&D ecosystem and improved international coordination and funding for therapeutics R&D. |
| a. The cost of producing monoclonal antibodies is currently estimated to be ~$100/gram, per Stamatis, C. and Farid, S.S. (2021). Process economics evaluation of call-free synthesis for the commercial manufacture of antibody drug conjugates. Biotechnology Journal, 16(4), p.2000238. Included within gram measurement are both the bulk drug substance and the final drug product, per Wellcome (2020). Expanding access to monoclonal antibodies. [online] Wellcome. Available at: https://wellcome.org/reports/expanding-access-monoclonal-antibodies. |
Vaccines Roadmap
Vaccines against COVID-19 have been developed and deployed in record time in this pandemic.[footnote 86] As of 24th November 2021, 7.78 billion doses have been administered globally, and 28.26 million are now administered each day.[footnote 87] This incredible success was the result of years of ongoing research on prototype vaccines against other coronaviruses, including those that cause Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), which began after the SARS outbreak in 2003.[footnote 88] This resulted in an unprecedented level of investment in early 2020[footnote 89] which allowed companies to run multiple trials in parallel and manufacture vaccines at risk,[footnote 90] and regulators moving at unprecedented speed.[footnote 91]
Recently developed ‘programmable’ vaccine platforms,[footnote 92] such as viral vector and mRNA,[footnote 93] have transformed the vaccine landscape because of their versatility, high potency, capacity for rapid development and potential for low-cost manufacture and safe administration.[footnote 94] These technologies also have the potential to be manufactured rapidly at scale[footnote 95] and remove the need to build new manufacturing facilities for vaccines developed against different diseases.[footnote 96] A facility dedicated to mRNA production can rapidly manufacture vaccines against multiple targets, with minimal adaptation to processes and formulation.[footnote 97]
The WHO estimates that up to 50% of all vaccines (although considerably less for COVID-19 vaccines due to demand) may be wasted globally every year because of lower than anticipated demand, temperature control, logistics and shipment-related issues.[footnote 98] Improving the stability of new ‘programmable’ platforms, and reducing the need for needle and syringe, offers a chance to reduce the pressure on healthcare systems and increase speed and scale of global distribution and access.[footnote 99]
We may learn more about vaccines this year alone than in previous decades combined, because of the detailed information collected on clinical volunteer demographics and humoral and cellular responses.[footnote 100] Rapid advances in structural biology[footnote 101] and artificial intelligence[footnote 102] (including protein structure predictions) have the potential to significantly accelerate timelines for identifying targets for diagnostic, vaccine and drug discovery, towards our goal of having vaccines ready to be produced at scale for global deployment within 100 days of the WHO declaring a PHEIC. In January 2022, CEPI and McKinsey will publish a report outlining lessons learned on vaccines from COVID-19, focusing on how we can truncate approval timelines for vaccines without compromising on safety. The report will also outline the current global landscape of vaccine platform technology, highlighting key opportunities for future investments.
Aims
-By 2026, the aim is to have developed libraries of prototype vaccines against priority virus families[footnote 103] and advanced platform technology, such that it can rapidly pivot to a future Disease X. This investment will also be targeted towards increasing the stability of vaccine platforms to enable large scale production of thermostable vaccines, as well as R&D to accelerate development of alternative routes of administration which will help overcome the logistical challenges and promote equitable access.[footnote 104],[footnote 105] Vaccine manufacturing capacity will be expanded to match the demand to COVID-19 vaccines, and kept “warm” to maintain capabilities able to pivot in response to a future pandemic.
Summary Plans
Prototype Vaccines: CEPI is developing libraries of prototype vaccines against ten high priority virus families, based on novel computational antigen designs and using rapid response platforms that can be quickly adapted if related viruses emerge.[footnote 106] CEPI will conduct extensive preclinical and clinical (Phase 1) testing of a subset of vaccines within each virus family to develop the regulatory support and ability to rapidly deploy a vaccine when a new pathogen emerges. In 2022, they will begin two pilot projects to create vaccine libraries against two high priority virus families, the Paramyxoviruses and Arenaviruses. Development of candidate vaccine libraries will provide industry with the starting materials for vaccine development against a new pathogen, as well as the critical assay reagents and manufacturability data generated through this work.
Platform Technology: Investments into prototype vaccine libraries will accelerate wider industry research into vaccines against endemic diseases.[footnote 107] By 2026, CEPI aims to have supported the development of licenced vaccines against two endemic diseases (current programmes are focused on Nipah, Lassa fever and chikungunya viruses). In addition to preventing serious illness and death from endemic diseases, this research will advance existing platform technology, which can be used to rapidly develop vaccines against a new pathogen. Industry and academia continue to advance platform technology through creating vaccines against COVID-19 and known endemic diseases, proving efficacy in humans (where possible). Vaccine seed stocks and batch versions of primary or variant Virus-like particles[footnote 108] should be produced to contain outbreaks of known pathogens. This would also create demand to keep manufacturing facilities “warm” beyond the current pandemic.
Reduce Complexity: Vaccine manufacturers are investing in reducing the complexity of the manufacturing process of these new platforms to enable large scale production. Pfizer and BioNTech have initiated a Phase 3 study evaluating a lyophilised (freeze-dried) formulation of their COVID-19 vaccine designed to be refrigerator-stable.[footnote 109] Similarly, Moderna and CureVac (with GlaxoSmithKline) continue to explore mechanisms that could produce more thermostable mRNA vaccines, removing the need for ultra cold chain storage for the mRNA platform.[footnote 110] A recent discovery of ionizable adamantane-based Lipid Nanoparticles (LNPs), a new type of nanoparticles that are capable of transporting nucleic acids of varying lengths into cells, could be transformational.[footnote 111] These efforts are complemented by academic and biotech companies’ research into how to make the RNA structure itself more stable.
Alternative Routes of Administration: Breakthroughs witnessed in injectable vaccines have not yet translated to licenced needle free vaccines. Proving the vaccine efficacy of needle-free formulations (oral, intranasal, thinfilm) should be the priority for R&D moving forward, including to understand correlates of protection and measure immune responses, but will require sustained investments from governments and industry. Using novel technologies to deliver influenza vaccines in clinical efficacy studies may provide the fastest route to determining vaccine efficacy in humans, and multiple technologies could be compared in one large trial. Currently, there are 13 (four oral and nine intranasal) needle free vaccines in clinical trials, with many more in pre-clinical trials. Should these trials prove successful, it could transform the global distribution of vaccines in a pandemic.[footnote 112]
Manufacturing: Gavi, the WHO, CEPI and regional initiatives, supported by the G20,[footnote 113] are increasing vaccine distribution, administration and local manufacturing capacity in LMICs, including through technology transfer hubs in various regions. The Partnership for African Vaccine Manufacturing (PAVM) is leading the establishment of a new mRNA hub in South Africa and the Pan-American Health Organization (PAHO) is coordinating the development of hubs in Argentina and Brazil. The WHO is due to launch an Expression of Interest for hubs specialising in viral vector vaccines in 2022. BioNTech announced in August that it was looking to establish malaria and tuberculosis vaccine production sites using mRNA technology in Rwanda and Senegal. [footnote 114] Similarly, in October, Moderna announced plans to invest up to $500 million to build a factory in Africa to make up to 500 million doses of mRNA vaccines annually.[footnote 115] The challenge ahead will be to ensure this manufacturing capacity stays “warm” post the current pandemic, and is embedded as business as usual practice through expanding vaccination programmes around the world.
Diagram 5
Proposed 100 Days Mission Roadmap for Vaccines
Successfully achieving the milestones for the following recommendations will mean vaccines are ready to be produced at scale for global deployment within 100 days
| 2022 Milestones |
2023 Milestones |
2024 Milestones |
2025 Milestones |
2026 Outcomes |
|
|
Recommendation 2 Build prototype vaccine libraries applicable to representative pathogens of pandemic potential. (CEPI) |
WHO works with CEPI (alongside NIAID) to develop and agree priority virus families for accelerated development of prototype vaccine libraries. CEPI conducts annual ‘germ games’ to test preparedness to future Disease X. CEPI begins two pilot (discovery) projects to create vaccine libraries against two high priority virus families (Arenaviridae and Paramyxoviruses). |
CEPI conducts annual ‘germ games’ to inform international preparedness plans for future Disease X. CEPI stimulates (academic and industry) R&D to develop candidate vaccine libraries for two virus families (Arenaviridae and Paramyxoviruses) to preclinical phase and commences discovery of vaccines against further virus families. |
CEPI conducts annual ‘germ games’ to inform international preparedness plans for future Disease X. CEPI supports preclinical testing of subset of initial vaccine library and progresses development of further candidates to preclinical phase. |
CEPI conducts annual ‘germ games’ to inform international preparedness plans for future Disease X. CEPI supports clinical testing of subset of initial vaccines library and conducts preclinical testing of further vaccines to preclinical phase. |
Prototype vaccines libraries developed for ten high priority virus families. |
|
Recommendation 4 Invest in modernising vaccine technology by targeting vaccine preventable diseases. (Industry (IFPMA), academia, CEPI, Gavi) |
CEPI (with McKinsey) catalogues advances in platform technology and (with Wellcome) invests in disruptive innovations to improve vaccine platform technology and manufacturing processes. Industry and academia develops vaccine platform technology and applies to priority endemic diseases (e.g. Nipah, Ebola, Influenza, Plague, Lassa fever), proving tailored efficacy in humans. |
CEPI, working with funding and delivery partners (academia, industry) progresses and reports on disruptive innovations to improve vaccine platform technology and manufacturing processes. Industry and academia develops vaccine platform technology and applies to wider set of endemic diseases, proving tailored efficacy in humans. |
CEPI, with partners, progresses disruptive innovations to innovate in vaccine platform technology and manufacturing processes. Industry and academia develops vaccine platform technology and applies to wider set of endemic diseases, proving tailored efficacy in humans. Manufacturing capacity is sustained through demand for adult vaccinations (including in LMIC). |
CEPI works with industry and government to innovate and rapidly transition novel vaccine technology and manufacturing innovations into business as usual. Industry and academia develops vaccine platform technology and applies to wider set of endemic diseases, proving tailored efficacy in humans. Manufacturing capacity is sustained through demand for adult vaccinations (including in LMIC). |
Readily programmable vaccine platform technology available, able to be rapidly re-purposed to an emerging Disease X threat. |
|
Recommendation 12 Stimulate a move towards innovative technologies to reduce the complexity of vaccine manufacturing processes and make technology transfer and scalable manufacturing easier in a pandemic by investing in R&D. (Industry (IFPMA), academia, CEPI, Gavi) |
CEPI (with Wellcome) invests in disruptive innovations to improve technology platforms and manufacturing processes. Industry and academia collaborate to understand the causes of instability and to drive improvements in Lipid Nanoparticles (LNPs). Industry and academia, working with governments invests in R&D to accelerate development of needle-free vaccine formulations, including to prove vaccine efficacy. |
Programmable vaccine platform technologies demonstrate measurable improvements in thermostability. Industry and academia, working with governments invests in R&D to accelerate development of needle-free vaccine formulations, with initial proof of vaccine efficacy (in humans).. |
Innovations, such as 2nd generation LNPs, are established addressing key thermostability issues. Multiple vaccine candidates are in preclinical trials to be administered needle-free. Efficacy of needle-free formulations proved for use in humans using multiple platforms. |
Innovations, such as 2nd generation LNPs, are established and able to be transferred to facilities globally. Multiple vaccine candidates are in late stage clinical trials to be administered needle-free. Efficacy of needle-free formulations proved for use in humans using multiple platforms. |
Vaccine platforms optimised for large scale production and simplified routes of administration and storage. |
|
Recommendation 16 Governments and industry to share risk to maintain vaccine manufacturing capacity. (Gavi, WHO, CEPI, regional initiatives, industry, Developing Countries Vaccine Manufacturing Networks) |
Gavi, WHO, CEPI and regional initiatives agree a medium to long term plan to expand capabilities of existing manufacturers in LMICs, and establish sustainable capacity in regions with no significant capacity. Industry and Governments invest in sustainable regionalised manufacturing capability. |
Gavi, WHO, CEPI and regional institutions mobilise public and private finance behind the implementation plan and begins supporting scale up of manufacturing capacity. Industry and Governments invest in sustainable regionalised manufacturing capability. |
Gavi, WHO, CEPI, regional institutions and industry partners work with development finance institutions and private investors to mobilise finance and implement medium term plan. Regional facilities producing SRA approved or WHO emergency use listed or pre-qualified products for regional and international use. Industry and Governments invest in sustainable regionalised manufacturing capability. |
Gavi, WHO, CEPI, regional institutions and industry partners work with development finance institutions and private investors to mobilise finance and implement medium term plan. Regional facilities producing SRA approved or WHO emergency use listed or pre-qualified products for regional and international use. Industry and Governments invest in sustainable regionalised manufacturing capability. |
Expanded capabilities of existing manufacturers in LMICs, enhancing capacity in all low capacity regions. |
Chapter 4
Cross Cutting Mission ‘Enablers’
To achieve the 100DM goals of (i) accurate and approved rapid diagnostictests; (ii) an initial regimen of therapeutics; and (iii) vaccines ready to be produced at scale for global deployment, and improve future pandemic preparedness, we need to make the exceptional routine by embedding best practice in business-as-usual activity. We also need to agree ‘rules of the road’ that come into effect in a pandemic, agreeing these ahead of time so no time is wasted negotiating the basics.[footnote 116]
The 100DM provides a set of actionable recommendations[footnote 117] to embed best practice and improve standards and protocols necessary to accelerate the DTV development pipeline and improve preparedness. These address a number of fundamental cross-cutting enablers and fall across the four categories outlined below:
Diagram 6
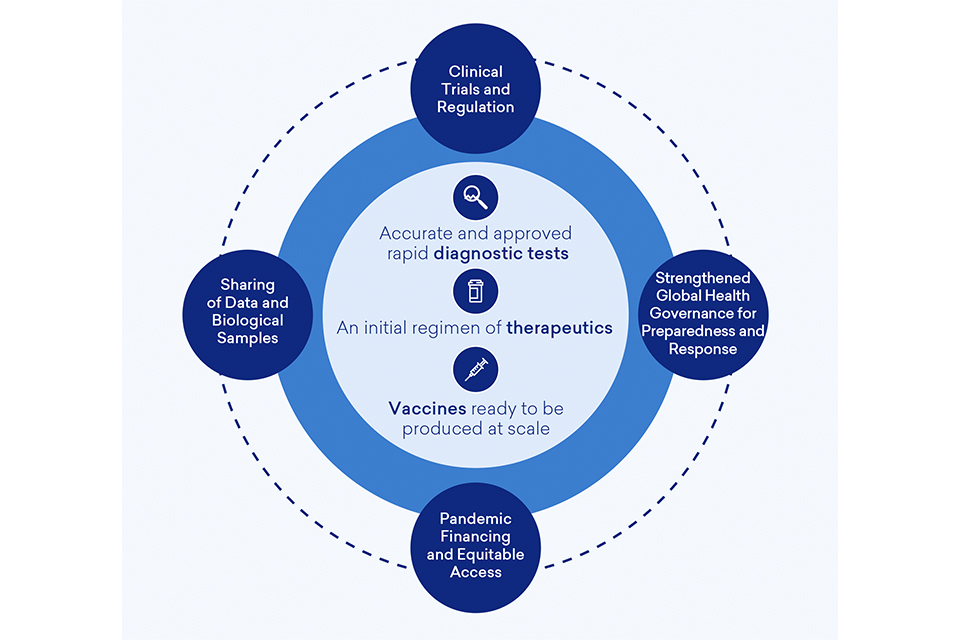
Diagram 6
Improvements to Clinical Trials Capability and Regulation Processes
The 100DM recommends scoping work is undertaken (led by the WHO) to determine how a network of clinical trial platforms could be implemented to enable a coordinated and efficient approach to the testing of DTVs[footnote 118] and the creation of regional mechanisms to coordinate and prioritise clinical trials of DTVs.[footnote 119]
Success of the 100DM clinical trials recommendations would ensure a significant increase in the proportion of trials designed to yield actionable information on relevant clinical questions. Evidence suggests that only 5% of COVID-19 therapeutic clinical trials were randomised and adequately powered, and 74% of participants took part in clinical trials that were either not randomised, not adequately powered, or neither.[footnote 120] Transparency is critical to yielding actionable information. COVID-19 demonstrated the need for industry and academia to follow WHO rules on transparency and utilise the International Clinical Trials Registry Platform (ICTRP).[footnote 121] To efficiently combat a future pandemic, the infrastructure, capability and regulatory system supporting trials must be prepared to substantially improve these metrics.
The WHO is scoping how to embed best practice from COVID-19 to make clinical trials more efficient and effective. This will ensure that improvements in clinical trials infrastructure, prioritisation and coordination are deployed both in public health emergencies and for regional and global issues of public health importance. This complements the implementation of the G7 Therapeutic and Vaccine Clinical Trials Charter[footnote 122] agreed by health ministers in June 2021, which will help deliver high-quality, reliable, and comparable evidence from international clinical trials to speed up access to approved DTVs.
The WHO (supported by the UK Government) recently hosted the first in a series of roundtable discussions and scoping work to apply lessons from COVID-19 and learning from experts who led multi-country or large national clinical trials such as RECOVERY.[footnote 123] The work has included a rapid research assessment of clinical trials infrastructure, gaps and opportunities across the world to understand what enhancements to infrastructure, capacity and coordination need to be made to enable a faster response to testing of DTVs (noting trials for diagnostics were particularly weak during COVID-19) in the future. More detailed scoping will be conducted by February 2022 to understand how best to strengthen and coordinate clinical trials infrastructure to enable a faster response to future epidemic threats and to respond more effectively to ongoing issues of global health importance, including by supporting regionally linked clinical trials infrastructure. Based on the outcome of this scoping, the WHO (with UK support) will develop an implementation plan to fund, develop and strengthen infrastructure for clinical trials in a sustainable way. This implementation plan will involve engaging with clinical trial funders to explore how to improve coordination, including prioritising which DTVs should be evaluated in clinical trials. Head-to-head clinical trials may be needed to enable DTVs to be tested at speed and industry should be engaged during the implementation plan to explore how to make industry-led DTVs available for trials during a pandemic.
The 100DM also recommends that Stringent Regulatory Authorities work to transform the approach to clinical trial regulation, shortening the time to authorise trials and streamlining the requirements and guidelines relating to trials conduct.[footnote 124] GCTC is developing guidance setting out the underlying principles for effective RCTs. Public consultation on the guidance has concluded and it will be published in December 2021. Regulators, funders and industry should engage with the guidance and, as appropriate, reflect the good principles for RCTs in their own requirements and guidance, both during a pandemic and outside of one.
The MHRA and EMA are co-leading a Clinical Trials Working Group within the ICMRA. In 2022, the ICMRA Executive Committee will prepare a position paper endorsing an off-the-shelf pandemic preparedness protocol for regulators, reflecting best practice and lessons learned from Covid-19. The ICMRA will publish the protocol in 2023 to guide regulators on appropriate clinical trials practice.
Safe Data Access and Use for Health Emergencies
The GPDA was formed in September 2021 to drive forward implementation of the S7 recommendations to improve safe data access and use for health emergencies.[footnote 125],[footnote 126] Members currently include data.org, Digital Health & AI Research Collaborative (I-DAIR), the Royal Society, the Open Data Institute (ODI), and The Trinity Challenge. The GPDA is working closely with the WHO, Wellcome, and other stakeholders, in particular current and incoming presidencies from the G7 and the G20.
The GPDA has started work on a two-year implementation roadmap to meet the challenges set out by the S7 to ensure the availability and accessibility of data as a source for critical insights in public health emergencies. Through this roadmap, the GPDA aims to lay out a plan to convene a cross-sector, transdisciplinary community, develop a research agenda, build the right technology infrastructure, and upskill public health practitioners in support of better use of data for health emergencies. The roadmap will outline plans to unlock data to support progress in three priority areas: developing a global disease surveillance and risk monitoring system, building and strengthening public health capacity and accelerating the development and delivery of DTVs.
By the end of 2022, data.org will have successfully concluded the Privacy Preserving Technologies Challenge[footnote 127] and developed and published at least two prototypes for analysing commercially sensitive or privately held data in a privacy-preserving way, via academic publications and open, tech-facing documentation on GitHub. Additionally, they will have started development of the Epiverse set of open-source tools for epidemiological data analysis.[footnote 128] The Trinity Challenge will have helped to scale and connect eight data solutions[footnote 129] and is planning to raise funds to run at least one additional innovation challenge for using data and analytics for health emergencies.
By 2026, the GPDA aims to make significant steps towards an open, trusted and trustworthy data ecosystem for pandemic response which is resilient, well-governed, preserves privacy and where skills and insights are shared and developed internationally and with global equity in mind. The GPDA is conducting a mapping exercise to capture existing regional and multilateral data initiatives, data governance approaches and fundamental data governance considerations and principles for how to design, develop and deploy a global data architecture. This will include a curated suite of open-source tools that can help access, process and analyse data for health emergencies in a cost effective, scalable, interoperable and reproducible way, including from non-traditional data sources. These tools will be integrated into the epidemiological community and key pandemic data analysis centres working closely with the WHO, Wellcome, and other relevant stakeholders.[footnote 130]
Sharing of Biological Samples
The 100DM recommended that scoping work (led by the WHO) be undertaken for a system that enables biological samples to be collected and shared immediately and unhindered in a pandemic. Although complicated, this has the potential to aid the development of DTVs, particularly diagnostics, and track pathogen evolution.
The WHO BioHub Facility[footnote 131] aims to promote rapid and timely sharing of Biological Materials with Epidemic or Pandemic Potential (BMEPP); facilitate rapid access to such pathogens and their information by relevant and qualified entities for the development of effective and safe public health products including DTVs; and ensure fair and equitable access to such products by all countries, based on public health needs.[footnote 132] The implementation of the BioHub will be determined by Member States and guidance from the 2022 World Health Assembly. By the end of 2022, the WHO aims to expand the scope and user base of the BioHub system to other pathogens beyond SARS-CoV-2 in the pilot phase, as well as to connect the system with existing repositories or laboratory networks.
Pandemic Financing and Equitable Access
The current crisis has highlighted the economic and financial impact that a health crisis can have on the global economy and the disproportionate burden borne by women and girls and certain ethnic groups. The 100DM report highlighted that there is a strong economic, financial and health case to have funding mechanisms in place before a pandemic to allow LMICs and Upper-Middle-Income Countries (UMICs) to purchase DTVs at speed, scale, and at risk when a threat materialises.[footnote 133] The international development finance community aims to have in place financial mechanisms that enable immediate access to funding for pandemic response, including advanced purchase agreements for DTVs, such that equitable access is at the heart of the next response. Beyond the complexities of financial structuring, the success of any new mechanism will depend on strong and broad-based political ownership, and the ability to coordinate and deliver effectively on the ground in times of crisis.
Ensuring sustainable sources of finance for pandemic preparedness is an essential enabler for achieving many of the 100DM recommendations. The International Development Association (IDA) is developing a toolkit for crisis response and preparedness as part of the ongoing IDA20 replenishment process, which will help strengthen preparedness and financing of response activities in the event of a future pandemic. The replenishment will continue to support capacity building and implementation, particularly in countries affected by fragility, conflict and violence, boosting crisis preparedness to address food insecurity and pandemic threats.[footnote 134]
The World Bank and the IMF should consider their existing mechanisms working closely with the G20 Joint Finance-Health Task Force who will conduct a review of existing pandemic financing mechanisms. The review, due to be completed in early 2022, will outline how to address potential financing gaps, including by mobilising an appropriate mix of existing multilateral financing mechanisms, and it will explore setting up new financing mechanisms.[footnote 135]
There will remain a need for grant financing from High-Income Countries (HICs) to both incentivise pandemic preparedness and also to deliver funding to actors who cannot access the Multilateral Development Bank (MDB) funding mechanisms. In March 2021, CEPI launched a new 5-year $3.5 billion (£2.48 billion) strategy to reduce the time for development and approval of new vaccines against future pandemic threats to 100 days, and to ensure large-scale manufacturing capacity is quickly in place for rapid global supply. The UK is due to host the CEPI replenishment conference in March 2022, which will be an important moment to mobilise the resources (of sovereign governments, philanthropic foundations, and the private sector) needed to deliver CEPI’s goals which are also central to delivering the 100DM for DTVs, particularly R&D to fill the gaps in our current DTV arsenal.
Since June, action has been taken to address the need for collective action for financing of pandemic PPR to become more adequate, more sustainable and better coordinated. The World Bank is looking at lessons from COVAX and African Vaccine Acquisition Trust (AVAT), which will help inform the design of robust, pooled procurement mechanisms for the future.[footnote 136] Such mechanisms will be critical to help secure and guarantee timely, affordable, and equitable access to vaccines and other medical countermeasures (oxygen therapeutics, diagnostic kits, personal protective equipment, etc.) for developing countries. These mechanisms will require at-risk financing from the World Bank, wider MDBs, and other donors.
The lack of international coordination has been a significant challenge and has hindered global access to DTVs. In response the Multilateral Leaders Task Force on COVID-19 DTVs was established in June 2021 jointly by the IMF, World Bank, the WHO and WTO to help developing countries access and deliver COVID-19 tools. A key role of the Multilateral Taskforce and others could be to bring focus to issues such as monitoring the calendar of deliveries for vaccines in line with the WHO targets, supporting an increased role of the private sector; ensuring effective vaccination on the ground; sharing information on the most effective financial responses; and coordinating with ACT-A, AVAT, and other DTV financing initiatives. The ACT-Accelerator and Multilateral Leaders Task Force (MLT) recently launched the Global COVID-19 Access Tracker which measures progress towards the global targets for access to COVID-19 vaccines, treatments, tests, PPEs, other tools, and delivery of donor pledges.[footnote 137]
A Sustained Global Response
The COVID-19 pandemic has shown the shortcomings of our international system. There has been insufficient global coordination across the health, finance and political architecture before and during a crisis and there is broad agreement that strengthened coordination is essential to improve dialogue, global cooperation and oversight of pandemic readiness. Alongside the 100DM, the Independent Panel for Pandemic Preparedness and Response (IPPPR), the G20 High Level Independent Panel on Financing the Global Commons for Pandemic Preparedness and Response (HLIP) and the Pan-European Commission on Health and Sustainable Development (Pan-European Commission) have all made recommendations to strengthen oversight for pandemic preparedness.
In response, the G20 have agreed to establish a Joint Finance-Health Task Force to enhance dialogue and global cooperation on issues relating to pandemic PPR, promoting the exchange of experiences and best practices, developing coordination arrangements between Finance and HealthMinistries, promoting collective action, assessing and addressing health emergencies with cross-border impact, and encouraging effectivestewardship of resources for pandemic PPR, while adopting a One Health approach.[footnote 138]
The Task Force will initially be jointly chaired by the 2021 and 2022 G20 Presidencies. It will meet before the end of 2021 and report to Health and Finance Ministers in early 2022. The Task Force will be assisted by a secretariat housed at the WHO, with the support of the World Bank[footnote 139] and will play an important role that was missing in this pandemic, linking health and finance ministries and reviewing finance mechanisms.
In late November 2021 a special session of the World Health Assembly will discuss the benefits of developing a WHO convention, agreement, or other international instrument on pandemic preparedness and response, taking into consideration the recommendations of the WGPR to establish an intergovernmental negotiating process. This is a unique window of opportunity to advance much-needed reforms and to strengthen global health governance for preparedness and response.
Potential targeted amendments to the existing International HealthRegulations (IHRs) are also under consideration by the WGPR, as a means of strengthening the global response to future pandemics.[footnote 140]
Chapter 5
A Mission Approach to Implementation
G7 CSAs recommend that the 100DM is managed with the seriousness of purpose and commitment akin to an Apollo mission, focusing innovation to rapidly fill the gaps in our DTV arsenal and strengthening our collective defences. The work to implement this, as set out in the roadmaps, will be achieved through a wide array of international organisations. In order to secure cumulative progress and coordination of these various workstreams, we propose establishing:
-
Annual independent G7 CSA Implementation Reviews, aligned with G7 leaders summits and supported by a secretariat, providing sustained support to the incumbent G7 presidency CSA (or equivalent) chair until2026.
-
A Science and Technology Expert Group (STEG), including world class scientific experts, to broaden international scientific input, leadership and oversight of the 100DM S&T recommendations.
As well as galvanising international engagement and progress, the G7 CSAs and STEG will bolster global support and investment in the S&T capabilities needed to achieve the 100DM.
The Annual G7 CSA Implementation Review
The 100DM report recommended that G7 CSAs or equivalent should meet at the end of 2021 to review implementation and regularly thereafter until satisfied. We therefore recommend that an annual independent G7 CSA Implementation Review should take place until the Mission is delivered in 2026 to advise the G7 and implementing partners.
A G7 100DM Secretariat could be formed to provide support to the incumbent G7 CSA in delivering the annual Implementation Review with fellow CSAs (until the Mission completes in 2026). Responsibilities could include:
-
Supporting implementation throughout the year, brokering partnershipsand assisting the recommendations implementation partners where necessary;
-
Convening and delivering the annual G7 CSA Implementation Review, chaired by the incumbent Presidency’s CSA;
-
Compiling the CSA Implementation Report to share with leaders,including commissioning updates from those organisations leading implementation of the recommendations, revising the implementation roadmaps, and determining the priorities over the upcoming year;
-
Linking the 100DM implementation to the work led by the G20 Joint Finance-Health Task Force, including working with the G20 CSA to commission updates on G20-led work;
-
Garnering the input of experts on critical issues and consider establishing working groups that focus on specific challenges or issues, commission or conduct assessments (e.g. tabletop exercises) and ensuring that the capabilities are accessible to all; and
-
Aligning 100DM plans and delivery with the proposed STEG. The proposal is that the STEG would be responsible for guiding the implementation of the 100DM S&T recommendations, with the 100DM Secretariatresponsible for reporting progress across all recommendations to G7 leaders annually.
The Science and Technology Expert Group
Achieving the 100DM will require the successful creation of a variety of ambitious S&T capabilities in a very short timeframe. For the S&T recommendations, an independent, dedicated group of cross-sectoral technical experts should have the delegated responsibility to help drive progress towards the 100DM, accessing other experts as and when necessary. As part of its major project management and evaluation role, it could:
-
Support and challenge the pace of 100DM technical implementation by reviewing progress towards the S&T goals of the 100DM;
-
Identify key technological and scientific opportunities, risks and challenges for relevant recommendations; and
-
Identify complementary initiatives and investments from the public, private and philanthropic sectors that could be aligned with, or integrated into, the 100DM.
The group would not have authority to make or change funding or political decisions; rather the global scientific and technological standing to make strong recommendations that will be influential with 100DM implementing organisations and funders.
Diagram 7
100 Days Mission Reporting Timeline: The Year Ahead
2021
November
- First G7 CSA 100DM Implementation Review (Convened by 100DM Secretariat)
- Special session of World Health Assembly (Other contribution)
December
- 7-8: G20 Sherpa meeting, Jakarta
- 11-12: G7 Foreign and Development Ministers meeting
2022
January
- UK handover G7 Presidency to Germany
March
- CEPI 2.0 Replenishment conference (Other contribution)
Spring
- Inaugural STEG meeting, and subsequent monthly meetings (Convened by 100DM Secretariat)
- World Bank and IMF meeting where multilateral development banks will report on loans for DTV during a pandemic (Other contribution)
- WHO Implementation Group reports to G7 on its implementation of Pathogen Surveillance Report (Other contribution)
- G20 meeting
April
- G7 Science Academies report on data capture, standards, sharing and analysis (Other contribution)
- SRA to report on new, common regulatory framework on diagnostics, an international knowledge-sharing alliance and regulatory evaluation best practices (Other contribution)
TBC
- Recommended second G7 CSA 100DM Implementation Review (Convened by 100DM Secretariat)
- G7 Leaders Summit (Germany)
- G7 Finance and Health Ministerial meetings
- G20 Health and Finance Ministers stands up a permanent Task Force
December
- Completion of CEPI 2.0 Replenishment (Other contribution)
Annex A
Progress and Plans, 100 Days Mission Recommendations
These recommendations are predicated on an effective global surveillance system and public health response capability. For each recommendation to work effectively we recommend a true partnership between the public and private sectors. We have also focused on existing institutions, with simplified accountability wherever possible. There are a large number of existing, and excellent, international health institutions and our approach has been to recommend better use of what we have, rather than creating additional international organisations. These recommendations should undergo further development by the proposed leads, including to ensure the right organisations are included and to assess costs and financing sources.
| 100DM Recommendations | 100DM Recommendation Lead & Milestones | Summary Progress Update | Proposed Future Reporting and Key Milestones |
|---|---|---|---|
| Overall Better Pandemic Preparedness | |||
| 1. A regular review of the implementation of the recommendations in this report, beginning with an initial stocktake before the end of 2021. | G7 Chief Scientific Advisers, or equivalents, to meet at the 2021 Implementation Review, ahead of the 2022 G7 summit, and regularly thereafter until the Mission is complete (2026). | • The 2021 Implementation Review, the first of its kind, was chaired by Sir Patrick Vallance and took place on 22nd November 2021 to review 100DM progress since the June 2021 Carbis Bay G7 summit. • CSAs and equivalents recommended conducting annual 100DM Implementation Reviews (2022 to 2026), reporting to Leaders ahead of the annual G7 summit. • The incumbent G7 presidency CSA or equivalent would chair the review. This Implementation Report provides an independent review of progress across the 100DM recommendations in 2021 and sets out suggested priorities and plans for the years ahead. |
2022 • The 2022 Implementation Review should review progress and agree updated milestones for the 100DM, including clear ownership of actions and delivery timelines against milestones. • CSAs were supportive in principle of the proposal to establish a dedicated 100DM Secretariat in early 2022 to support incumbent G7 CSAs in hosting future annual implementation reviews and drafting the implementation report. The Secretariat will liaise with the proposed Science and Technology Expert Group, implementing organisations, the WHO, wider G7 and G20 tracks, and the G20 Joint Finance-Health Task Force to ensure coherent reporting across 100DM implementation. • CSAs recommend a new Science and Technology Expert Group (STEG) is established in Q1 of 2022 to provide technical assurance of delivery across the 100DM S&T recommendations. It will meet with 100DM implementing partners and international science leaders and experts regularly to strengthen assurance and review and report progress, including to G7 CSAs. Future Years • CSAs recommend reporting to to G7 Leaders annually by publishing an Implementation Report on the 100DM and providing a verbal update at the annual G7 Leaders Summit. |
| R&D to fill the gaps in our arsenal | |||
| 2. Build prototype vaccines and diagnostic libraries applicable to representative pathogens of pandemic potential. | CEPI, as per the Pathogen Surveillance Report, works with industry, biotechnology companies, research institutions, academia, and others supported by G7 governments. CEPI will report on this annually from October 2021. | • CEPI has reported to the 100DM Secretariat on progress working with industry and academia to mature plans for CEPI 2.0, including delivery of the Disease X programme. • DeepMind has used its state-of-the-art AI system. AlphaFold, to generate protein structure predictions for 32 viruses curated by CEPI. These served as a starting point for discussing how AlphaFold might support the work of the 100DM. |
2022 • CEPI (with partners) will provide a written annual report setting out progress in developing prototype vaccine libraries ahead of the annual Implementation Review. • CEPI will create pilot vaccine libraries for two priority virus families as well as invest in R&D to advance platform technologies. FIND, with CEPI, the WHO and industry and academic partners will develop a diagnostics innovation plan for the 100DM and commence development of prototype diagnostics libraries. • Development of prototype libraries will be informed by regular live fire exercises or ‘germ games’ to simulate the early warning phase (and the first 100 days) of a novel respiratory virus epidemic, where Day Zero marks the sequencing of the virus. • CEPI will secure funding through the replenishment event in the UK in March 2022. CEPI and FIND will develop and agree on a funding plan for development of prototype diagnostics. Future Years • By 2026 we should aim to have prototype vaccine libraries developed for the ten virus families, building on CEPI’s two pilot vaccines libraries and conducting preclinical and clinical trials of selected subset of its vaccines library. • On diagnostics, FIND will work with the WHO, industry and academia to support development of prototype diagnostics libraries providing broad coverage for ten virus families and develop pathogen-agnostic platforms with the potential to detect ‘Disease X’. |
| 3. Develop prototype antiviral therapeutics, including antibody therapies, for respiratory pathogens of pandemic potential. | Research-based pharmaceuticals, supported by initiatives like INTREPID, to work with academia, biotechnology companies, CEPI, The Global Fund, philanthropic funders and G7 governments. INTREPID to report annually from October 2021 until 2026. | • INTREPID reported progress in supporting pharmaceutical industry initiatives to develop prototype antiviral therapeutics to the 100DM Secretariat ahead of the 2021 Implementation Review. • INTREPID has recruited industry participants and formalised initial financial commitments to begin the rapid translation of promising academic discoveries onto an industrialised platform. • READDI has received initial financial commitments and commenced broad spectrum antiviral discovery and development efforts. |
2022 • INTREPID (supporting industry and academic partners) will report annually, setting out progress in developing prototype antiviral therapeutics prior to the annual Implementation Review. • Industry, supported by INTREPID (in collaboration with CEPI, FIND and the WHO), should agree priorities for antiviral development and testing, and develop ten high-quality antiviral candidates, informed by the agreed list of priority virus families (the WHO with CEPI). • READDI will annually report progress on small molecule antiviral development as well as advances in manufacturing and clinical trials planning. Future Years • By 2026, industry, supported by INTREPID, will develop 25 high-quality therapeutic candidates against priority (respiratory) pathogens that have completed Phase 1 studies in humans. READDI will develop five broad spectrum antivirals and agree Phase 2/3 trial designs for antiviral candidates in advance of future viruses emerging. |
| 4, Invest in modernising vaccine technology by targeting vaccine preventable diseases. | Industry [supported by IFPMA] to work with biotechnology companies, research institutions, CEPI and supported by G7 governments. CEPI and IFPMA to report separately and annually from October 2021. | • IFPMA reported to the 100DM Secretariat on its progress ahead of the 2021 Implementation Review. They highlighted rapid developments in ‘plug and play’ vaccine platform technology which academia and industry are aiming to apply to endemic diseases. • IFPMA also reported that the biopharmaceutical industry supports the ambition of the 100DM and is committed to investing in R&D to build a portfolio of promising candidate vaccines, treatments, and technologies against pathogens of pandemic or epidemic importance. • CEPI, working with McKinsey, is conducting a review of the mRNA vaccine developer landscape, with a focus on key technological advances and critical gaps. This analysis will inform future investments to demonstrate the effectiveness and safety of platform technology for selected pathogens to licensure for application to Disease X threats. Developments will be designed to be accessible for use by all populations in all regions. The McKinsey report will be published in January 2022. • CEPI is investing with philanthropic partners to accelerate disruptive innovations in vaccine platform technologies and manufacturing processes through their LEAP programme. |
2022 • IFPMA, working with industry partners, will provide a written annual report on industry’s progress to modernise vaccine platform technology including by targeting endemic diseases (proving tailored efficacy) prior to the annual Implementation Review. • In 2022 CEPI, working with partners including IFPMA and Wellcome, will invest in disruptive innovations (LEAP Programme) to improve mRNA technology platforms and manufacturing processes, and facilitate the establishment of global research networks. Future Years • By 2026, the goal is to have readily ‘programmable’ vaccine technology platforms available, able to be rapidly re-purposed and deployed globally at scale to an emerging Disease X. |
| 5. Invest in simplified cheaper routes for producing monoclonal antibodies and other new therapeutic modalities. | Industry [supported by IFPMA], to work with biotechnology companies, research institutions, academia and other relevant institutions. IFPMA to report annually from October 2021. | • IFPMA reported to the 100DM Secretariat on current investments and work is underway to develop simpler and cheaper routes for producing monoclonal antibodies ahead of the 2021 Implementation Review. They highlighted the importance of innovation to simplify routes of administration and improve manufacturing efficiency, to realise the full benefits of mAbs. • CEPI reported to its Investors Council in September 2021 on the emerging therapeutics landscape catalysed by COVID-19 and CEPI’s role to address gaps within it. The landscape analysis revealed extensive interest in upstream therapeutics R&D but limited research and attention focused on mid or down- stream R&D. CEPI is well positioned to make selected investments in monoclonal antibodies given its overlap with their existing work on mRNA vaccines. Conversations are ongoing within CEPI to shape their strategy for therapeutics. |
2022 • IFPMA (with CEPI, industry and academic partners) will provide a written annual report on industry’s progress to develop cheaper routes for producing monoclonal antibodies and other new therapeutic modalities (including ‘programmable’ therapeutics) prior to the annual Implementation Review. • Industry, working with partners, will progress developments towards smaller or less frequent doses, improve thermostability, pursue alternative routes of administration and advances in manufacturing innovation (e.g. platform capability). Future Years • By 2026 this should reduce the cost of producing marketable mAbs to less than $25 / gram. This outcome will greatly improve access for LMICs. |
| 6. Strengthen the role of the international system in R&D capability and coordination for therapeutics and diagnostics by expanding CEPI’s remit to cover therapeutics and diagnostics, in addition to vaccines. | CEPI with funding support from G7/G20 governments. [The replenishment process is expected to be completed by 2022] | • CEPI and FIND reported to the 100DM Secretariat on their progress in strengthening the role of the international system in R&D capability and coordination for DTVs ahead of the 2021 Implementation Review. • The recommendation to expand CEPI’s role to cover therapeutics aligns with and builds upon CEPI’s 2022-2026 Strategy (“CEPI 2.0”). CEPI’s Board has endorsed an approach to pursue activities related to therapeutics consistent with their current mandate, and will consider extending it to enable a broader scope of therapeutics’ efforts to be pursued. • FIND is working in partnership with CEPI and the WHO to strengthen international coordination on diagnostics in pursuit of the 100DM (CEPI has no plans to expand into a diagnostics coordination role). |
2022 • Diagnostics: In January 2022, CEPI and FIND will submit a joint report on diagnostics innovation for pandemics for the 100DM at the World Economic Forum in Davos, Switzerland. FIND, working with CEPI and the WHO, will provide a written report annually on strengthening diagnostics R&D coordination (global) and capability development ahead of the annual Implementation Review. • Therapeutics: a forum will be established to bring together complementary therapeutics initiatives. The specific remit of this forum is yet to be scoped but CEPI will play a key role as a convener, as well as contributing to evidence generation and tracking of novel and repurposed therapeutics. The forum provides a potential avenue for interested parties to share pre-competitive information. • CEPI, working with the proposed therapeutics forum, will report annually on strengthening therapeutics R&D coordination (global) and capability development ahead of the annual Implementation Review. • In March 2022 the UK government will host the CEPI replenishment conference to secure funding to deliver ‘CEPI 2.0’, including efforts in support of the 100DM. Future Years • By 2026 strengthened international R&D capability and coordination across therapeutics and diagnostics (as for vaccines) will have led to an effective end-to-end R&D pipeline for DTVs, with sustained funding to support delivery of 100DM. • FIND will report progress in developing partnerships across the diagnostic sector and governments and improvements in regulation, clinical trials, manufacturing and supply chains for diagnostics. |
| 7. Governments should normalise the use of accurate diagnostics for coronavirus and influenza in point-of-care and non-clinical settings. | G7 governments to work with FIND and other relevant organisations. G7 CSAs will report on their country’s progress at the 2021 Implementation Review and agree next steps. | • G7 CSAs reported to the 100DM Secretariat on their government’s progress implementing this recommendation ahead of the 2021 Implementation Review. Many G7 countries have stimulated their domestic diagnostic markets through procurement and deployment of rapid diagnostic tests for COVID-19. • In the US, the programme “Say Yes! COVID-19 Test’’ has been rolled out across selected local jurisdictions where residents can order and have rapid at-home COVID-19 tests or pick them up at designated sites. • Japan has introduced ‘Guidelines for COVID-19 Antibody Testing’ which outlines a number of testing methods for relevant laboratories. The use of rapid tests in Japan is being enhanced. All COVID-19 tests are publicly funded in Japan. • France is currently mainstreaming the use of duplex PCR diagnostic tests (influenza, SARS-CoV-2) for the “at risk” population, although this is almost exclusively carried out in a hospital context. • The Canadian government has worked closely with the private sector to drive technology development and scale workplace testing programmes and support access to free tests in the community. Canada is aiming to move away from the PCR lab-based testing as the sole diagnostic test and, as such, are prioritising regulatory submissions of point-of-care COVID-19 tests and have convened a COVID-19 Testing and Screening Advisory Panel to provide evidence-informed advice to support point-of-care COVID-19 test implementation across the country. • The UK is changing the model of diagnostics services to be more proactive, taking advantage of new technologies and changed public behaviour, to focus on prevention and prediction. Enhanced diagnostics capacity and capability will improve preparedness for future pandemics as this can be pivoted to developing tests against a new pathogen. |
2022 • G7 governments, working with FIND and partners including WHO, will produce plans to normalise the use of diagnostics domestically including annual indicative milestones. • In 2022, FIND will focus on promoting affordability of RTD/PCRs for endemic diseases. Future Years • By 2026 diagnostics will be used routinely in point-of-care and non-clinical settings. FIND, working with academia and industry (and with the WHO), aims to develop diagnostics that are affordable to LMICs and support uptake in those countries. |
| 8. WHO should support an enhanced role for diagnostics in the surveillance of pandemic threats. | The WHO Implementation Group to report on progress in early 2022 to the G7 Presidency as part of its implementation of the Pathogen Surveillance Report. | • The WHO convened an Implementation Consultation Group (ICG) to develop a conceptual approach for an International Pandemic Surveillance Network (IPSN). A stepwise process is being used to develop the conceptual approach, and to provide options for IPSN implementation. The steps include elucidating the features of the IPSN, identifying its use cases, mapping existing capacities including in countries and networks/initiatives to assess barriers and identify opportunities, and developing the work packages and pilots needed for implementation. • The IPSN pilots are being scoped to serve as the ‘beta version’ of the IPSN by the end of 2021. • The IPSN and the ACT-A Genomic Surveillance working groups, are working towards the strategic goal of ensuring that genomic surveillance is strengthened and scaled for quality, timely, and appropriate public health actions within local and global surveillance systems. The WHO is conducting a global consultation with stakeholders in 2021, and the strategy outcomes are strongly interconnected with the WHO Berlin Hub to strengthen pandemic and epidemic intelligence. |
2022 • Pilots for the network will be established, which will serve as a ‘beta version’ of the IPSN. The pilots will run in parallel to the IPSN ICG finalising the conceptual approach to inform a global strategic vision. • The WHO will report on the utilisation of the IPSN conceptual approach to strengthen genomic surveillance of pandemic threat before April 2022. • The WHO will finalise and publish the WHO Global Genomics Strategy in March 2022. Future Years • By 2026, the WHO, working with partners, aims to integrate diagnostics into the IPSN to maximise its coverage and utility. • By 2026, the IPSN will be harmonised with complementary work streams including the Global Pandemic Data Alliance’s work and the Food and Agriculture Organization (FAO), Organization for Animal Health (OIE), and UN Environment Programme (UNEP). |
| Make the exceptional routine by embedding best practice and preparation in business as usual activity | |||
| 9. Scope out how an international network of clinical trial platforms could be implemented to enable a coordinated and efficient approach to testing of DTVs. | The WHO to work with G20 governments, industry, philanthropic organisations and academia. The WHO will propose a collaboration model at the 2021 Implementation Review. | • The WHO Special Programme for Research and Training in Tropical Diseases is conducting an initial rapid research assessment of regionally linked clinical trials infrastructure, gaps and opportunities across the world. This showed the existence of clinical trials networks in all regions. • More detailed scoping will be conducted by February 2022 to understand how best to strengthen and coordinate regionally linked clinical trials infrastructure to enable a faster response to future epidemic threats and to respond more effectively to ongoing issues of global health importance. • Based on the outcome of this scoping, the WHO (with UK support) will develop an implementation plan to fund, develop and strengthen appropriate infrastructure for clinical trials in a sustainable way. |
2022 • The WHO will focus on identifying and optimising the type of infrastructure needed to support efficient trials and confirm the action plan to deliver this, including securing funding and partners for implementation. • The WHO will provide an update on their clinical trials action plan and the potential implementation of an international network of regionally linked clinical trials mechanisms to G7 CSAs at the 2022 annual Implementation Review. Future Years • Milestones for delivery will be included in the WHO action plan and updated at the 2022 G7 CSA review. |
| 10. Develop a common regulatory framework that better defines criteria and standards for effectiveness, quality and use cases for diagnostics. | Stringent Regulatory Authorities to work through the International Medical Device Regulators Forum. SRA will report on progress by April 2022. | • The International Medical Device Regulators Forum (IMDRF) formally rejected this recommendation as it was considered as outside the remit of the organisation. • The MHRA (UK) and the FDA’s Center for Devices and Radiological Health (US) have committed to working with G7 counterparts to define the scope of this recommendation, given limited global resources, and prioritise the most impactful areas for mutual recognition. A bilateral paper will be produced by the end of 2021 that sets out the scope and sequencing of this recommendation. |
2022 • Following the release of the bilateral collaboration and prioritisation paper in late 2021, the MHRA and FDA will collaborate with their G7 counterparts to implement proposals to streamline regulatory processes over 2022-26. • This could include a streamlined process for new product applications, and will explore the possibility of reciprocal agreements on regulatory approval to negate the need for the same product to be approved in multiple jurisdictions. Future Years • By 2026 SRAs, working with the WHO and FIND, will have implemented recommendations on developing mutual recognition agreements to enable the rapid approval of diagnostic tools once a PHEIC is declared. |
| 11. Transform the approach to clinical trial regulation, shortening the time to authorise trials and streamlining the requirements and guidelines relating to trial conduct. | Stringent Regulatory Authorities to work with the WHO and the Good Clinical Trials Collaborative. SRA will report on progress at the 2021 Implementation Review. | • The Good Clinical Trials Collaborative (GCTC) reported to the 100DM Secretariat on its progress ahead of the 2021 Implementation Review. • The GCTC has reviewed existing rules, such as the Good Clinical Practice (GCP) clinical trials guidance, and developed new guidance with a cross-sectional, global stakeholder group. Public consultation has taken place and the new guidance will be published in December 2021. The GCTC guidance will focus on foundational scientific and ethical principles that enable innovative, efficient and robust randomised trials of promising interventions. |
2022 • In 2022 the GCTC will conduct a comprehensive engagement and communications plan to embed their guidance (published in December 2021) within the clinical regulation community and conduct a capacity strengthening assessment of existing guidance. • GCTC will collect feedback from stakeholders and promote implementation of the good RDT principles by regulators, funders and governments. Future Years • By 2023 major regulatory authorities’ updated guidance should be substantively influenced by these principles and they should be applied in industry clinical trials. |
| 12. Stimulate a move towards innovative technologies to reduce the complexity of vaccine manufacturing processes and make technology transfer and scalable manufacturing easier in a pandemic by investing in R&D. | Pharmaceutical industry [supported by IFPMA] to work in partnership with biotechnology companies, CEPI, Gavi, research institutions, academia, the public sector and relevant organisations. IFPMA will report annually from October 2021. | • IFPMA reported to the 100DM Secretariat summarising industry progress in moving towards innovative technologies (platform technologies) as a route to simplify future manufacturing processes. Industry and the public sector are investing in improving the scalability of viral vector manufacturing technology platforms. Approaches vary according to the vector and include high-yield cell lines, suspension cell cultures, process intensification and automation, and modular manufacturing processes. • The report noted that some companies are developing lyophilized vaccines, enabling their use outside of the cold chain. Pfizer and BioNTech have initiated a Phase 3 study evaluating a lyophilized (freeze-dried) formulation of their COVID-19 vaccine designed to be refrigerator-stable. Similarly, Moderna and CureVac continue exploring mechanisms that could produce more thermostable mRNA vaccines. 2nd generation Lipid Nanoparticles (LNPs) capabilities may simplify encapsulation and aid tech transfer. • Novel (needle-free) routes of administering vaccines are also under development (including nasal sprays). Proving the efficacy of needle-free routes will be key. |
2022 • IFPMA (and partners) will provide a written report annually on progress towards innovative technologies to reduce the complexity of manufacturing prior to the annual Implementation Review. • CEPI (with partners) will invest in disruptive innovations to improve platform technology and manufacturing processes. • Industry and academia will collaborate to understand the causes of instability of mRNA formulations and to drive development of routes to stabilise future products (e.g. improvements in LNPs). Industry, working with governments, develops needle-free vaccine formulations, proving efficacy. Future Years • In 2026, vaccine platforms will be optimised for large-scale production and simplified routes of administration and storage. |
| 13. The IMF to explore expanding their Article IV consultation with member countries to include a pandemic preparedness assessment, and draw on the analysis and expertise of others. Concurrently, multilateral development banks continue to support investment to strengthen and prepare health systems as part of their core day-to-day business. | The IMF to include a pandemic preparedness assessment in its Q3-4 2021 Article IV consultation with member countries. Multilateral development banks to report by the 2022 Annual Meetings. |
• The IMF reported to the 100DM Secretariat on this recommendation ahead of the Implementation Review and provided a helpful assessment of the recommendation. This recommendation will not be taken forward because Article IV reports focus on the most macro-critical issues facing each country. This can make standardised requirements problematic as priority issues vary substantially across countries and time. • G7 CSAs recognise that readiness assessments for each country are critical to achieving full PPR. The financing review being undertaken by the G20 Joint Finance-Health Taskforce will assess current pandemic financing mechanisms and review if they should be strengthened or if new mechanisms are required. |
2022 • The G20 Joint Finance-Health Taskforce will meet before the end of 2021 and report to G20 Health and Finance Ministers by early 2022 on modalities to establish a financial facility, to be designed inclusively, to ensure adequate and sustained financing for pandemic PPR. The Task Force will initially be jointly chaired by the 2021 and 2022 G20 Presidencies, and will be assisted by a secretariat housed at the WHO, with the support of the World Bank. Future Years • The financing review led by the G20 Task Force will recommend next steps to the G20 Health and Finance ministers. • The Task Force could work with the IMF and other stakeholders to coordinate and mobilise critical financing such as grants and concessional lending with the aim of improving LMIC access to DTVs. |
| 14. Establishing a Global Health Board reporting to the G20 to provide oversight of pandemic readiness on an annual basis. | G20 governments to consider this at the October 2021 meeting of G20 Health and Finance Ministers for adoption in 2022. | • The G20 Health and Finance Ministers met in October 2021 and agreed to establish a G20 Joint Finance-Health Task Force aimed to enhance dialogue and global cooperation on issues relating to pandemic PPR; promoting the exchange of experiences and best practices; developing coordination arrangements between Finance and Health Ministries; promoting collective action; assessing and addressing health emergencies with cross-border impact; and encouraging effective stewardship of resources for pandemic PPR, while adopting a One Health approach. |
2022 • The G20 Joint Finance-Health Taskforce will meet before the end of 2021 and will report to G20 Health and Finance Ministers by early 2022 on modalities to establish a financial facility, to be designed inclusively to ensure adequate and sustained financing for pandemic PPR. The Task Force will initially be jointly chaired by the 2021 and 2022 G20 Presidencies, and will be assisted by a secretariat housed at the WHO, with the support of the World Bank. Future Years • Following the 2022 Spring assessment, the G20 will consider permanent governance arrangements to provide oversight on pandemic PPR. |
| 15. Governments should build in conditions into DTV funding contracts for LMIC access to access DTVs at not for profit and scale, which is to be enacted if a Public Health Emergency of International Concern is declared. | G7 governments, through its CSAs or equivalents, to provide an update at the 2021 Implementation Review. | • G7 CSAs reported to the 100DM Secretariat on their progress implementing this recommendation ahead of the 2021 Implementation Review. G7 governments are considering the domestic implementation of this recommendation. • The US, as per procurement laws and the Federal Acquisition Regulation, already negotiates with companies to get favourable prices for rapid DTVs and has plans to donate many of them to LMICs. • Canada supports the recommendation in principle and is looking at solutions (e.g. COVAX Facility) to purchase DTVs at lower costs while complying with existing intellectual property policies. • The UK is developing common principles for the management of research outputs to standardise the approach in research funding (grants and contracts) to encourage equitable access for less developed countries. |
2021/2022 • Further work is required by all G7 governments to align their equitable access commitments with DTV funding contracts. In late 2021/early 2022 the G7 CSAs should identify a domestic single point of contact to clarify country domestic positions on this recommendation. • WHO Member States should consider whether equitable access in DTV funding contracts should also be captured in forthcoming discussions on a potential WHO Convention, agreement, or other instrument on pandemic preparedness and response, if appropriate. Future Years • By 2026 G7 governments should consider including clauses in DTV funding contracts and grants that support equitable access for LMICs. This could also be considered in the discussion on a WHO convention, agreement, or other international instrument (if appropriate). |
| 16. Governments and industry should share risk to maintain vaccine manufacturing capacity. | COVAX Manufacturing Task Force,to work with industry and G7 governments. The Task Force will publish a plan by October 2021. | • The COVAX Vaccine Manufacturing Task Force, supported by partner organisations and the G20,141 is increasing vaccine distribution, administration and local manufacturing capacity in LMICs, including through technology transfer hubs in various regions. • The Partnership for African Vaccine Manufacturing (PAVM) is leading the establishment of a new mRNA hub in South Africa and the Pan-American Health Organisation (PAHO) is coordinating the development of hubs in Argentina and Brazil. |
2022 • In 2022 the COVAX Vaccine Manufacturing Task Force will demonstrate a viable model for vaccine manufacturing hubs to build regional production capacity, with stronger industry participation. • New industry partnerships will be established with manufacturers in Africa, Latin America and Asia that expand capacity across priority technology platforms. • A platform will be established to support collaboration across international and regional organisations on vaccine manufacturing investment and market shaping. Future Years • By 2026 there will be expanded capabilities of existing manufacturers in LMICs, and established sustainable capacity in regions where there is currently no significant capacity. |
| Agree different rules of the road to come into effect in a pandemic | |||
| 17. As part of the proposed WHO Treaty on Pandemic Preparedness setting guidance for pandemics, the WHO should define ‘rules of the road’ and set out guidance on good practice for all relevant stakeholders in a pandemic, pre-negotiated with governments, industry and international organisations. | The WHO to work with industry, CEPI, Gavi, FIND, The Global Fund, and other relevant organisations. WHO to report at the special session of the World Health Assembly in November 2021. | • In late November 2021 a special session of the World Health Assembly will discuss the benefits of developing a WHO convention, agreement, or other international instrument, taking into consideration the recommendations of the WGPR to establish an intergovernmental negotiating process. This is a unique window of opportunity to advance much-needed reforms and to strengthen global health governance for preparedness and response. • Potential targeted amendments to the existing International Health Regulations (IHRs) are also under consideration by the WGPR, as a means of strengthening the global response to future pandemics.142 |
2022 • In late November 2021 a special session of the World Health Assembly will discuss the benefits of developing a WHO convention, agreement, or other international instrument intended to provide the long-term, overarching framework for better global health security and accountability. Future Years • By 2026 the WHO should set good practice guidance for a pandemic, to be considered by Member States during the discussions on a potential WHO convention, agreement, or other international instrument in late November 2021. |
| 18. Explore the creation of regional mechanisms to coordinate and prioritise clinical trials of DTVs. | The WHO to work with G20 governments, industry, philanthropic organisations and academia. The WHO will report on progress by October 2021 and present a proposal at the 2022 Implementation Review. | • In October 2021, the WHO hosted the first of a series of roundtable discussions. A work plan to deliver this recommendation has been developed, including to improve the coordination and prioritisation of clinical trials both in pandemics and for ongoing issues of global health importance. • The work plan includes consideration of mechanisms to improve global coordination of research questions and testing of DTVs, developing recommended shared protocols to be triggered in a public health emergency, strengthening and accreditation of national ethical review boards, and facilitating the rapid, effective and equitable uptake of the results of trials into policy and practice. |
2022 • The WHO will conclude the exploratory roundtable discussions and work with relevant stakeholders to implement the action plan. • At the 2022 Implementation Review the G7 CSAs will assess the outcome of WHO’s exploratory work and update the roadmap for this recommendation accordingly. Future Years • The WHO should lead the implementation action plan, working with global partners, and aim to have delivered its plan by 2026. |
| 19. Stringent Regulatory Authorities and the WHO should form an international alliance in a pandemic to support timely exchange of knowledge and information relating to standards and guidelines for DTVs. 20. Stringent Regulatory Authorities and the WHO exchange experience and best-practice on regulatory evaluation of other types of studies (e.g. human challenge trials, immunogenicity studies) during pandemics to support the development of appropriate protocols and guidelines. |
Stringent Regulatory Authorities to work in partnership with the WHO. SRA will report on progress by April 2022. | • The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) reported to the 100DM Secretariat on its progress ahead of the 2021 Implementation Review. The update for recommendations 19 and 20 have been combined to reflect the integrated delivery mechanism. • The MHRA and the European Medicines Agency (EMA) have established and are co-leading a Clinical Trials Working Group within the International Coalition of Medicines Regulatory Authorities (ICMRA). One of the key outputs recommended by the ICMRA executive committee will be the development of a reflection paper to endorse an off-the-shelf pandemic preparedness protocol for regulators which will reflect best practice and lessons learned from COVID-19. Ireland, Italy, USA, Japan and Canada have expressed an interest to participate in the working group, while the MHRA and EMA are seeking representation from more regulators. |
2022 • The MHRA will provide a written report on progress of the pandemic protocol, on behalf of the ICRMA Clinical Trials Working Group, ahead of the annual Implementation Review. • The ICMRA Executive Committee will endorse a reflection paper and drafting of the protocols will begin by appropriate experts. These will be endorsed by the ICMRA Executive Committee. Future Years • The aim is for the protocol to be endorsed by the ICMRA in 2023 resulting in an off-the shelf pandemic preparedness protocol for regulators, reflecting best practice and lessons learned from COVID-19. |
| 21. Explore the scope for a system that enables biological samples to be collected and shared immediately and unhindered in a pandemic. | The WHO to identify what is possible with respect to the Nagoya protocol. The WHO will report on progress by October 2021 ahead of the 2021 Implementation Review. | • The WHO is developing a BioHub System with global reach to expand knowledge and advance technical work on high-threat pathogens. The System will allow Member States to share biological materials with epidemic or pandemic potential (BMEPP) on a voluntary basis with one of the trusted WHO BioHub Facilities under predetermined conditions. At an initial stage, these qualified entities will receive BMEPP for non-commercial purposes. The WHO will be conducting technical consultations, including on defining the conditions for sharing with commercial partners. • In parallel, the WHO Secretariat will work on a proposal to broaden the WHO BioHub System for the use of BMEPP by qualified entities, such as manufacturers, for commercial purposes as well as their fair and equitable allocation to countries in need. • Domestically, Japan is establishing a national biobank, the Repository of Data and Specimen of Infectious Disease (REBIND), to assess human samples with genomic sequence data in order to address R&D challenges. |
2022 • In May 2022 the WHO will report to the World Health Assembly on the progress of the WHO BioHub System. G7 CSAs should note progress in their annual Implementation Review. Future Years • Implementation (including timing) of the BioHub will be determined by Member States and guidance from the 2022 World Health Assembly. • The BioHub could encourage the sharing of biological materials soon after the detection of a pathogen with epidemic or pandemic potential in order to support rapid characterisation, provide informed review of the risk under Annex 1 of the International Health Regulations (2005)143 and foster greater international cooperation for health emergency preparedness and response. |
| 22. Support the recommendations of the Science Academies of the G7 and endorse the development of a roadmap towards a more systematic approach to data capture, standards, sharing and analysis for health emergencies. | Science Academies of G7 countries (S7) to work with the WHO and partners and report on progress by April 2022. | • The Global Pandemic Data Alliance (GPDA) is now leading implementation of this recommendation, working with the WHO. • The GPDA has developed a two-year implementation pandemic data roadmap (2021-2023) to meet the challenges set out by the Science Academies of the Group of Seven (S7) to ensure the availability and accessibility of data for critical insights during public health emergencies. • The GPDA’s health data roadmap will unlock data to support progress in three priority areas: developing a global disease surveillance and risk monitoring system, building and strengthening public health capacity and accelerating the development and delivery of DTVs. Funding has been secured for the first round of major programmes planned by GPDA partner organisations and work has commenced. |
2022 • In 2022 the Global Pandemic Data Alliance will produce a written report on progress on the pandemic data roadmap ahead of the annual Implementation Review. • The GPDA will secure further funding to deliver the roadmap, including to build a suite of open-source tools that can help access, process and analyse data for health emergencies. • The GPDA will conduct a mapping exercise of the existing regional and multilateral initiatives to focus efforts on gaps, avoid duplication and maximise impact. • The GPDA will publish a mapping of current data governance approaches, and frameworks for identifying and evaluating considerations around data governance and data architecture. Future Years • By 2023 the GPDA will have delivered its pandemic data roadmap to ensure safe access to data and data analysis for health emergencies. GPDA’s tools will be integrated into the epidemiological community and key pandemic data analysis centres. |
| 23. A PHEIC should trigger the activation of an automatic mechanism to procure and distribute DTVs. Further work is needed to determine how such a facility could operate and we recommend considering basing this on advance commitments that are pre-negotiated well before a pandemic. | World Bank to work with Gavi, The Global Fund, CEPI and the WHO. The World Bank will facilitate agreement on financing mechanisms by the 2022 Spring Meetings and report to the 2022 G20 Presidency. | • The World Bank and the IMF reported to the 100DM Secretariat on their progress ahead of the 2021 Implementation Review. The Multilateral Leaders Task Force is coordinating with the World Bank, the IMF, WHO and the World Trade Organization (WTO) to align funding plans, offerings and resource opportunities. • The World Bank is reviewing lessons from COVAX and AVAT to inform the design of robust, pooled procurement mechanisms for the future. These mechanisms will be critical to help secure and guarantee timely, affordable and equitable access to vaccines and other medical countermeasures (oxygen therapeutics, diagnostic kits, personal protective equipment, and more) for developing countries. These mechanisms will require at-risk financing from the World Bank and donors. • The G20 Health and Finance Ministers are establishing a G20 Joint Finance-Health Task Force that could support a range of possible functions, including enhancing dialogue and global cooperation on issues relating to pandemic PPR, promoting the exchange of experiences and best practices, developing coordination arrangements between Finance and Health Ministries, promoting collective action, assessing and addressing health emergencies with cross-border impact, and encouraging effective stewardship of resources for pandemic PPR, while adopting a One Health approach. |
2022 • The G20 Joint Finance-Health Task Force will be established in late 2021. The Task Force could present in early 2022 an assessment on modalities to establish a financial facility, to ensure adequate and sustained financing for pandemic prevention, preparedness and response. Future Years • By 2026, the aim is to have strengthened (pre-negotiated) financing mechanisms in place for DTVs prior to a PHEIC being declared. The G20 Joint Finance-Health Task Force or its replacement arrangements could lead the work, working closely with the World Bank and other partners. |
| 24. As part of countries’ bilateral DTV procurement, any advance purchase agreements with manufacturers should include a requirement for products provided to LMICs to be provided at not for profit. This must also be done within a similar timeframe to when HICs are supplied. | G7 governments, through its CSAs or equivalents, to provide an update at the 2021 Implementation Review. | • G7 CSAs reported to the 100DM Secretariat on their government’s progress implementing this recommendation ahead of the 2021 Implementation Review. • In late November 2021 a special session of the World Health Assembly will discuss a potential WHO convention, agreement, or other international instrument intended to provide the long-term, overarching framework for better global health security and accountability that the world needs. |
2021/22: • Further work is required by all G7 governments to align their equitable access commitments with DTV procurement protocols. • In early 2022 the G7 CSAs should identify a domestic point of contact to discuss and determine a way forward on the recommendation. • Advance Purchase Agreements (APAs) could be considered as part of discussions oin a potential WHO convention, agreement, or other international instrument being discussed as part of the 2021 World Health Assembly special session. This would support aims towards equitable access to DTVs. Future Years • By 2026 G7 governments should consider including clauses in DTV funding contracts and grants which support equitable access to DTVs for LMICs. |
| 25. Multilateral development bank loans should be made available so LMICs can purchase DTVs above the 30% provided through the DTV financing facility in line with recommendation 23. Normal access limits or policies applied by multilateral development banks should not prevent countries receiving urgent finance during a pandemic. | Multilateral development banks to report to the G20 Presidency by the Spring Meetings of the World Bank and the IMF. | • The World Bank and the IMF will conduct further work to fill any pandemic financing gaps, and the conclusion of the International Development Association (IDA) replenishment will determine the measures required. • The World Bank is exploring strengthening its existing core funding mechanisms – IDA and the International Bank for Reconstruction and Development (IBRD) – in support of pandemic preparedness and response. In the ongoing IDA20 replenishment process the World Bank is exploring a package of measures to enhance IDA’s toolkit of crisis response and preparedness to strengthen preparedness and financing of response activities in the event of a future pandemic. • The G20 Joint Finance-Health Task Force as part of their assessment could include a review of current finance mechanisms and assess if strengthening is required. |
2022 • The G20 Joint Finance-Health Task Force could report to G20 Health and Finance Ministers by early 2022 on modalities to establish a financial facility, to be designed inclusively, to ensure adequate and sustained financing for pandemic PPR. Future milestones will be developed following the conclusion of the G20 Joint Finance-Health Task Force in early 2022. Future Years • 2026 milestones to be informed by the work of the G20 Joint Finance-Health Task Force. |
| 141: G20 Presidency (2021). G20 Rome Leaders’ Declaration. [online] G20.org. Available at: https://www.g20.org/wp-content/uploads/2021/10/G20-rome-leaders-declaration. |
| 142: Independent Panel for Pandemic Preparedness and Response (2021). COVID-19: Make it the Last Pandemic. [online] Available at: https://theindependentpanel.org/wp-content/uploads/2021/05/COVID-19-Make-it-the-Last-Pandemic_final.pdf. |
| 143: World Health Organization (2016). International Health Regulations (2005) Third Edition. [online] WHO. Available at: https://www.who.int/publications/i/item/9789241580496. |
Annex B
G7 Chief Scientific Advisers’ Biographies
|
United Kingdom: Sir Patrick Vallance, Government Chief Scientific Adviser Sir Patrick Vallance is the Government Chief Scientific Adviser and Head of the Government Science and Engineering profession. Patrick worked at GlaxoSmithKline (GSK) from 2006 until 2017, including as President of R&D from 2012. Prior to joining GSK, he was a clinical academic, Professor of Medicine and led the Division of Medicine at UCL. His personal research was in the area of diseases of blood vessels and endothelial biology. |
|
|
United States: Dr Eric Lander, Science Advisor to the President of the United States, and Director of the White House Office of Science and Technology Policy Dr. Eric Lander is President Biden’s science advisor and the Director of the White House Office of Science and Technology Policy. He was formerly President and Founding Director of the Broad Institute, a research institute focused on genomic medicine. He was one of the principal leaders of the Human Genome Project and he has served as a scientific advisor to the federal government across multiple administrations and in multiple departments. |
|
|
Germany: Professor Lothar Wieler, President of the Robert Koch Institute Professor Lothar Wieler is president of the Robert Koch Institute in Berlin, the national Public Health Institute in Germany. He is co-founder of the German national research platform on zoonoses, and deputy spokesperson for the research consortium InfectControl 2020, which pursues intersectoral approaches to preventing and treating infections from a One Health perspective. Professor Wieler is also a member of the Strategic and Technical Advisory Group for Infectious Hazards of the World Health Organisation, the scientific advisory board of the Global Research Collaboration for Infectious Disease Preparedness and the WHO Europe Advisory Committee on Health Research. |
|
|
Japan: Dr Yasumasa Fukushima, Councillor, Office of Healthcare Policy, Cabinet Secretariat; and Chief Medical Officer, Ministry of Health, Labour and Welfare Dr. Yasumasa Fukushima was appointed as the Chief Medical Officer of the Ministry of Health, Labour and Welfare (MHLW) Japan in August 2020, after serving as the president of the National Institute of Public Health from 2018. Dr. Fukushima started his career with the MHLW in 1987 and also served as a public health administrator at national, prefectural and municipal levels. |
|
|
Canada: Dr Mona Nemer, Chief Science Advisor of Canada Dr. Mona Nemer is Canada’s Chief Science Advisor. Her role is to provide science advice to the Prime Minister and his Cabinet. An internationally renowned cardiovascular scientist and a distinguished academic leader, Dr. Nemer has made seminal contributions to several fields ranging from gene regulation to molecular cardiology. |
|
|
Italy: Professor Stefano Vella, Professor of Global Health, Catholic University of Rome Professor Stefano Vella is Adjunct Professor of Global Health at the Catholic University of Rome, and member of the Program Committee of Horizon Europe – Health Cluster, the Research Program of the European Commission (2021-2027). He was President of the Italian Medicines Agency from 2017 to 2018. Professor Vella has conducted specific research on major pandemics, specifically on HIV/AIDS and tuberculosis. |
|
|
France: Professor Stewart Cole, President of Institut Pasteur, France Professor Stewart Cole, Professor of Microbial Pathogenesis, is President of the Institut Pasteur, Paris, and vice-president of the Pasteur network (33 members in 25 countries). He is an expert in tuberculosis. |
|
|
France: Professor Yazdan Yazdanpanah, Head of Infectious Disease department at Bichat Claude Bernard Hospital and Professor of Medicine at Paris Diderot University, France Professor Yazdan Yazdanpanah is Director of the ANRS Emerging Infectious Disease French Funding Agency, French Aviesan Institute of Immunology, Inflammation, Infectiology, and Microbiology. He is also the Co-Chair of the Global Research Collaboration for Infectious Disease Preparedness International Network (GLoPID-R) and the Chair of The European & Developing Countries Clinical Trials Partnership board. |
|
|
France: Professor Jean-Francois Delfraissy, Chair, COVID-19 Scientific Council Professor Jean-Francois Delfraissy has been president of the National Consultative Ethics Committee since January 2017. In 2019, he was appointed co-chair of the Collaborative Group on Social Participation. He received the INSERM Honorary Award in 2019. Since March 2020, he is the President of the Covid-19 Scientific Council. Professor Delfraissy is a specialist in HIV and emerging viruses and has published more than 500 articles in the field of immunology, internal medicine and HIV infection. |
|
|
Representative of the European Union: Sir Peter Piot, Special Advisor to the President of the European Commission on COVID-19 Sir Peter Piot MD PhD is Special Advisor to the President of the European Commission and is based in his native Belgium. He is the Handa Professor of Global Health at the London School of Hygiene & Tropical Medicine of which he was the Director. He was the founding Executive Director of the Joint United Nations Programme on HIV/AIDS and Under Secretary General of the United Nations, Belgium. |
Annex C
Glossary of Terms
-
ACT-A – Access to COVID-19 Tools Accelerator, launched by WHO and partners in April 2020
-
Advance commitment – a promise or agreement to take some future action. It here refers to the buying or selling of an asset (DTVs) at some future time, with pre-agreed terms
-
Antibody therapy – treatment that uses antibodies, in this report referring to their use in infectious diseases, for instance by binding to viral surface proteins to block entry into human cells
-
Antiviral therapeutics – therapeutics to treat or prevent viral infections
-
AP3 – American Pandemic Preparedness Plan, published in September 2021
-
APA – Advance Purchase Agreement, a commitment to purchase (DTVs) for a certain volume, at a price, ahead of the product being on the market, helping to cover industry risks needed to e.g. upscale manufacturing
-
AVAT – African Vaccine Acquisition Trust
-
Biological samples – such as blood samples, cell cultures or organisms. Such biological products are vital in the preparation of DTVs
-
BMEPP – Biological Materials with Epidemic or Pandemic Potential
-
BMGF – Bill and Melinda Gates Foundation
-
Business as usual – in this report, referring to the time when the world is not combatting a pandemic
-
CanCOGeN – Canadian COVID-19 Genomics Network
-
CDC – Centres for Disease Control and Prevention (US)
-
CEPI – Coalition for Epidemic Preparedness Innovations, an organisation that provides R&D funding for vaccines to stop future epidemics
-
Clinical trial – a type of research that studies new tests and treatments and evaluates their effects on human health outcomes
-
Clinical trial platforms – bring together different stakeholders to facilitate clinical research
-
COVAX – The vaccine pillar of ACT-A, co-led by Gavi and CEPI. Also the COVID-19 vaccine procurement pool led by Gavi and CEPI
-
COVAX AMC – a financing instrument created by Gavi that will support the participation of 92 LMICs in the COVAX Facility
-
COVAX Manufacturing Task Force – a group set up from 14 May 2021 as part of ACT-A’s vaccine pillar, COVAX, to identify and resolve issues impeding equitable access to vaccines through COVAX
-
CoVaRR-Net – Coronaviruses Variants Rapid Response Network
-
COVID-19 – the disease caused by the virus SARS-COV-2
-
CSA – Chief Scientific Adviser (or Chief Scientific Adviser-equivalent) of a government
-
Diagnostics – products which diagnose diseases, commonly known as tests
-
Disease – a deviation from normal healthy functioning, in this report typically refers to infectious diseases that affect humans
-
Disease X – represents the knowledge that a serious international epidemic could be caused by a pathogen currently unknown to cause human disease (WHO definition)
-
DTVs – diagnostics, therapeutics and vaccines
-
EMA – European Medicines Agency
-
Endemic – a disease that is regularly found in a population or area
-
Epidemic – a disease that affects a large number of people within a region, population or community
-
Equitable access – when those with equal needs have equal access. In this report usually referring to DTVs such that DTVs are distributed globally based on clinical need
-
FAO – Food and Agriculture Organization of the United Nations, an agency leading on nutrition and food security
-
FDA – Food and Drug Administration (US), in this report referred to in its capacity as a health regulator
-
FIND – Foundation for Innovative New Diagnostics, an organisation aiming to ensure equitable access to reliable diagnostics around the world
-
G7 – the Group of 7 nations, an intergovernmental organisation consisting of Canada, France, Germany, Italy, the United Kingdom, the United States and the representatives of the European Union
-
G20 – the Group of 20, a forum for international economic cooperation between 19 countries and the European Union
-
Gavi – the Vaccine Alliance, an organisation aiming to increase access to immunisation in developing countries
-
GCP – Good Clinical Practice, the international ethical, scientific and practical standard to which all clinical research is conducted
-
GCTC – Good Clinical Trials Collaborative, a partnership launched in June 2020 to develop guidance to enable and promote informative, ethical and efficient RCTs
-
GISRS – Global Influenza Surveillance and Response System
-
Global Fund – an international financing and partnership organisation fighting AIDS, Tuberculosis and Malaria epidemics
-
GLoPID-R – Global Research Collaboration for Infectious Disease Preparedness
-
GPDA – Global Pandemic Data Alliance
-
HERA – Health Emergency Preparedness and Response Authority (EU), launched in 2020 and aimed at improving Europe’s capacity and readiness to respond to health emergencies
-
HICs – High-Income Countries
-
HIV – Human Immunodeficiency Virus
-
Human challenge trial – clinical trials in which participants are intentionally challenged with an infectious disease organism
-
HLIP – High Level Independent Panel on Financing the Global Commons for Pandemic Preparedness and Response
-
IBDR – International Bank for Reconstruction and Development
-
ICMRA – International Coalition of Medicines Regulatory Authorities, a voluntary coordinating and advocacy group of regulatory authorities
-
ICG – Implementation Consultation Group
-
ICTRP – International Clinical Trials Registry Platform
-
IDA – International Development Association
-
I-DAIR – Digital Health & AI Research Collaborative
-
IFPMA – International Federation of Pharmaceutical Manufacturers & Associations, an international industry association representing research- based pharmaceutical companies and associations
-
IHRs – International Health Regulations
-
IMDRF – International Medical Device Regulators Forum, a voluntary forum for medical device regulators to accelerate international medical device regulatory harmonisation and convergence
-
IMF – International Monetary Fund
-
Immunogenicity study – measures any adverse immune response generated by a therapeutic or vaccine such as reduced efficacy or autoimmune, allergic and anaphylactic reactions in the body
-
Infectious diseases – diseases, caused by pathogens, that can be spread between organisms
-
INGOs – international organisations and international non-governmental organisations
-
INTREPID – International Readiness for Preventing Infectious Viral Disease Alliance, the research-based pharmaceutical industry initiative to discover and develop antivirals for future pandemics
-
IPPPR – the WHO Independent Panel for Pandemic Preparedness and Response
-
IPSN – International Pathogen Surveillance Network
-
LMICs – Low- and Lower-Middle-Income Countries
-
LNPs – Lipid Nanoparticles
-
mABs – monoclonal antibodies
-
MDB – Multilateral Development Bank, an international financial institution chartered by two or more countries, with a purpose to encourage economic development in developing countries
-
MERS – Middle East Respiratory Syndrome
-
MHRA – Medicines and Healthcare products Regulatory Agency (UK)
-
MHLW – Ministry of Health, Labour and Welfare (Japan)
-
MLT – ACT-Accelerator and Multilateral Leaders Task Force
-
Modular manufacturing processes – using an assembly line type process for manufacturing, in this report referring particularly to vaccine manufacturing
-
mRNA – Messenger ribonucleic acid: in vaccines it stimulates/teaches cells to make a specific protein which generates an immune response
-
Nagoya Protocol – ‘The Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization to the Convention on Biological Diversity’ is an international agreement aiming at sharing the benefits arising from the utilisation of genetic resources in a fair and equitable way
-
NIAID – National Institute of Allergy and Infectious Diseases (US)
-
NIID – National Institute of Infectious Diseases (Japan)
-
NS3 – National SARS-CoV-2 Strain Surveillance
-
NVAP – New Variant AssessmentPlatform (UK)
-
OIE – World Organisation for Animal Health, an intergovernmental organisation focused on animal health
-
One Health Approach – an approach to health policy that recognises the interconnection between people, animals, plants and their shared environment
-
PAHO – Africa and the Pan-American Health Organisation
-
Pandemic – an epidemic occurring worldwide, or over a very wide area, crossing international boundaries and usually affecting a large number of people
-
Pandemic PPR – pandemic prevention, preparedness and response
-
Pan-European Commission – Pan-European Commission on Health and Sustainable Development
-
Pathogen – an organism causing disease to its host
-
Pathogen Surveillance Report – shorthand for the report by Sir Jeremy Farrar, ‘A proposal to develop an equitable global pathogen surveillance network in 2021 that can prevent and respond to emerging and endemic infectious diseases at speed and at scale’, published in May 2021
-
PAVM – Partnership for African Vaccine Manufacturing
-
PCR – Polymerase chain reaction, in this report referenced mainly for its use in diagnostic tests
-
Peptide inhibitors – a type of therapeutic treatment which block a virus from binding to human cells and therefore prevent disease
-
Phase 2 – the second phase of clinical trials, following Phase 1 safety studies. Phase 2 trials look at safety as well as how well the product works. It precedes (and is sometimes combined with) large-scale Phase 3 trials which test effectiveness
-
PHEIC – Public Health Emergency of International Concern, declared by WHO
-
Priority pathogens – a global priority pathogens list of antibiotic- resistant bacteria, developed by WHO to help in prioritising the R&D of new and effective antibiotic treatments
-
“Programmable” technologies – denotes the transformative impact of new technology platforms and approaches, like mRNA, which allow scientists to rapidly amend medical tools to respond to a specific pathogen
-
Prototype diagnostic/therapeutic/vaccine – the preparation of DTVs for prototype pathogens, such that they are broad-spectrum or generic in response to a class of pathogen e.g. a coronavirus vaccine, that could be rapidly adapted to respond to a specific type of pathogen e.g. COVID-19
-
Prototype pathogen – pathogen groups or families with similar characteristics, against which it is possible to produce prototype DTVs
-
Pull incentives – create incentives for private sector engagement by creating viable market demand, paying for “results”
-
Push incentives – incentivising industry via reducing industry’s costs usually during the R&D stages
-
R&D – Research and Development
-
RCTs – randomised controlled trials
-
RDT – Rapid Diagnostic Test
-
READDI – Rapidly Emerging Antiviral Drug Development Initiative
-
REBIND – Repository of Data and Specimen of Infectious Disease (Japan)
-
Rules of the road – in this report we refer to rules of the road to denote the need to agree different rules of the road in a pandemic so that no time is wasted negotiating the basics. These protocols should form part of a wider suite of guidance WHO sets out (for instance, covering travel and PPE) which must be agreed in advance and demonstrate a step-change from business as usual when a PHEIC is declared.
-
RNA – ribonucleic acid
-
RNAi – ribonucleic acid interference
-
S7 – Science Academies of theGroup of Seven
-
SARS – Severe Acute Respiratory Syndrome
-
S&T – Science and Technology
-
Small molecule antivirals – chemical compounds typically comprising only 20-100 atoms. These drugs can enter cells easily due to low molecular weight
-
Spring Meetings – comprised of the joint World Bank-IMF Development Committee and the IMF’s International Monetary and Financial Committee events. The World Bank Group and the IMF also organise and host a number of related meetings of groups of country delegations, such as the G20, G-24, Commonwealth, the Civil Society Policy Forum, Brazil, Russia, India, China, and South Africa (BRICS), and other events.
-
SRA – Stringent Regulatory Authorities, national drug regulatory authorities which are members or observers or associates of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, as defined by WHO
-
STEG – Science and Technology Expert Group
-
Structural biology – a branch of biology focused on the molecular structure of biological substances. In this report referring to its use to define likely common surface protein targets or enzyme targets, so as to aid in the development of prototype vaccines and therapeutics
-
Technology transfer – in this report, referring to the complex process of transferring the knowledge, physical objects, skills and technology management required to manufacture DTVs with a particular emphasis on the challenges and complexity of vaccine manufacturing technology transfer
-
Therapeutics – the branch of medicine concerned with the treatment of disease and the action of remedial agents. Commonly referred to as medicines or treatments
-
UNEP – UN Environment Programme
-
UMICs – Upper-Middle-Income Countries
-
UNEP – UN Environment Programme
-
Vaccine – A product that stimulates a person’s immune system to produce immunity to a specific disease, protecting the person from that disease
-
Viral vector vaccine – these vaccines use the body’s own cells to produce antigen. They do this by using a modified virus (the vector) to deliver genetic code for antigen into human cells. By infecting cells and instructing them to make large amounts of antigen, which then trigger an immune response, the vaccine mimics what happens during natural infection with certain pathogens – especially viruses
-
Virus – a submicroscopic infectious agent that replicates only inside the living cells of an organism
-
Virus families – the taxonomy of different viruses, classified according to characteristics
-
WGPR – Working Group for Strengthening WHO Preparedness and Response
-
World Health Assembly – the decision-making body of the World Health Organization
-
WHO – UN World Health Organization, dealing with major health issues around the world. Sets standards for disease control, healthcare, and medicines; conducts education and research programs; and, publishes scientific papers and reports
-
World Economic Forum – international organisation that brings together its membership of political and business leaders on a yearly basis to discuss major issues concerning the world political economy
-
WTO – World Trade Organization
-
ZEPAI – Centre for Pandemic Vaccines and Therapeutics (Germany)
-
World Health Organization (2021). WHO COVID-19 dashboard. [online] covid19.who.int. Available at: https://covid19.who.int/. ↩
-
Gopinath, G. (2020). Reopening from the Great Lockdown: Uneven and Uncertain Recovery. [online] IMF. Available at: https://blogs.imf.org/2020/06/24/reopening-from-the-great-lockdown-uneven-and-uncertain-recovery/. ↩
-
The Economist (2021). What is the economic cost of covid-19? [online] The Economist. Available at: https://www.economist.com/finance-and-economics/2021/01/09/what-is-the-economic-cost-of-covid-19. ↩
-
The White House (2021). American Pandemic Preparedness: Transforming Our Capabilities. [online] Available at: https://www.whitehouse.gov/wp-content/uploads/2021/09/American-Pandemic-Preparedness-Transforming-Our-Capabilities-Final-For-Web.pdf. ↩
-
Mcmichael, A., Campbell-Lendrum, D., Corvalán, C., Ebi, K., Githeko, A., Scheraga, J. and Woodward, A. (2003). Climate change and human health – Risks and Responses. [online] Available at: https://www.who.int/globalchange/publications/climchange.pdf. ↩
-
Coalition for Epidemic Preparedness Innovations (2021). CEPI launches plan to tackle risk of future pandemics and epidemics. [online] CEPI. Available at: https://cepi.net/news_cepi/cepi-launches-plan-to-tackle-risk-of-future-pandemics-and-epidemics/. ↩
-
G7 Pandemic Preparedness Partnership (2021). 100 Days Mission to respond to future pandemic threats. [online] gov.uk. Available at: https://www.gov.uk/government/publications/100-days-mission-to-respond-to-future-pandemic-threats. ↩
-
Department of Health and Social Care (2021). Government and life science industry join forces on 100 Days Mission for future pandemics. [online] Gov.uk. Available at: https://www.gov.uk/government/news/government-and-life-science-industry-join-forces-on-100-days-mission-for-future-pandemics. ↩
-
Defined by when the World Health Organization (WHO) declaring a public health emergency of international concern (PHEIC). ↩
-
European Commission (2021). European Health Emergency preparedness and Response Authority (HERA): Getting ready for future health emergencies. [online] European Commission. Available at: https://ec.europa.eu/commission/presscorner/detail/en/IP_21_4672. ↩
-
The International Federation of Pharmaceutical Manufacturers & Associations (2021), 100DM Implementations Report submission to Cabinet Office ↩
-
G7 Pandemic Preparedness Partnership (2021). 100 Days Mission to respond to future pandemic threats. [online] gov.uk. Available at: https://www.gov.uk/government/news/government-and-life-science-industry-join-forces-on-100-days-mission-for-future-pandemics. ↩
-
Farrar, J. (2021). A proposal to develop an equitable global pathogen surveillance network in 2021 that can prevent and respond to emerging and endemic infectious diseases at speed and at scale. [online] Gov.uk. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/990314/Pathogen_Surveillance_report_to_the_UK_G7_Presidency_2021.pdf. ↩
-
Department of Health and Social Care (2021). UK and US agree new partnership to fight future pandemics and tackle health inequalities. [online] Gov.uk. Available at: https://www.gov.uk/government/news/uk-and-us-agree-new-partnership-to-fight-future-pandemics-and-tackle-health-inequalities. ↩
-
Pasteur International (2021). Pasteur International Network. [online] pasteur-network.org. Available at: https://pasteur-network.org/en/about/who-we-are/. ↩
-
Hassabis, D. (2021). Putting the power of AlphaFold into the world’s hands. [online] DeepMind. Available at: https://deepmind.com/blog/article/putting-the-power-of-alphafold-into-the-worlds-hands. ↩
-
Including Viral Vector and mRNA. ↩
-
Oxford Vaccine Group (2021). Investigating a Vaccine Against Plague (PlaVac) - Trials. [online] trials.ovg.ox.ac.uk. Available at: https://trials.ovg.ox.ac.uk/trials/plague. ↩
-
Dance, A. (2020). Coronavirus vaccines get a biotech boost. Nature, 583(7817), pp.647–649. ↩
-
Pfizer (2021). Pfizer Starts Study of mRNA-Based Next Generation Flu Vaccine Program - Pfizer. [online] Pfizer.com. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-starts-study-mrna-based-next-generation-flu-vaccine. ↩
-
Hogan, A.B., Winskill, P. and Ghani, A.C. (2020). Estimated impact of RTS,S/AS01 malaria vaccine allocation strategies in sub-Saharan Africa: A modelling study. PLOS Medicine, 17(11), p.e1003377. ↩
-
Wellcome (2021). R3 - Wellcome Leap: Unconventional Projects. Funded at Scale. [online] wellcomeleap.com. Available at: https://wellcomeleap.org/r3/. ↩
-
Merck (2021). Merck and Ridgeback Biotherapeutics Provide Update on Results from MOVe-OUT Study of Molnupiravir, an Investigational Oral Antiviral Medicine, With Mild-to-Moderate COVID-19. [online] Merck.com. Available at: https://www.merck.com/news/merck-and-ridgeback-biotherapeutics-provide-update-on-results-an-investigational-oral-antiviral-medicine-in-at-risk-adults-with-mild-to-moderate-covid-19/. ↩
-
Pfizer (2021). Pfizer’s Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk of Hospitalization or Death by 89% in Interim Analysis of Phase 2/3 EPIC-HR Study - Pfizer. [online] Pfizer.com. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate. ↩
-
Foundation for Innovative New Diagnostics (2021). SARS-CoV-2 diagnostic pipeline. [online] FIND. Available at: https://www.finddx.org/covid19/pipeline/?avance=Commercialized&type=all&test_target=all&status=all§ion=show-all&action=default. ↩
-
World Health Organization (2021). Global partnership to make available 120 million affordable, quality COVID-19 rapid tests for low- and middle-income countries. [online] www.who.int. Available at: https://www.who.int/news/item/28-09-2020-global-partnership-to-make-available-120-million-affordable-quality-covid-19-rapid-tests-for-low–and-middle-income-countries. ↩
-
WHO Roundtable discussion on ‘Improving clinical trials for the post-COVID-19 world’, 19 October 2021. Chaired by Professor Quarraisha Abdool with experts from South Africa, Kenya, Philippines, UK, USA, Nigeria, India, Belgium, Colombia, Brazil, Singapore (not exhaustive). ↩
-
So that DTVs are ready to be deployed within 100 days of a new pandemic threat being identified. ↩
-
The Good Clinical Trials Collaborative (2021). The Good Clinical Trials Collaborative. [online] goodtrials.org. Available at: https://www.goodtrials.org/. ↩
-
G20 Presidency (2021). G20 Rome Leaders’ Declaration. [online] G20.org. Available at: https://www.g20.org/wp-content/uploads/2021/10/G20-rome-leaders-declaration. ↩
-
Royal Society (2021). Global Pandemic Data Alliance – UK Invites the Global Pandemic Data Alliance to Deliver on S7 Recommendations on Health Emergencies. [online] Available at: https://royalsociety.org/-/media/news/2021/Global-Pandemic-Data-Alliance-Press-Release.pdf. ↩
-
Royal Society (2021). Global Pandemic Data Alliance – UK Invites the Global Pandemic Data Alliance to Deliver on S7 Recommendations on Health Emergencies. [online] Available at: https://royalsociety.org/-/media/news/2021/Global-Pandemic-Data-Alliance-Press-Release.pdf. ↩
-
Italy, Japan, Peru, Thailand, Egypt, Luxembourg, Switzerland as of December 2021. ↩
-
G20 Presidency (2021). G20 Rome Leaders’ Declaration. [online] G20.org. Available at: https://www.g20.org/wp-content/uploads/2021/10/G20-rome-leaders-declaration. ↩
-
The UK Government Chief Scientific Adviser held a roundtable discussion with industry and academic leaders, who contributed to the development of these roadmaps. CEPI, FIND, INTREPID, READDI, selected companies and academic experts have also provided expertise, in addition to G7 Government officials. ↩
-
Foundation for Innovative New Diagnostics (2021). COVID-19 & pandemic preparedness. [online] FIND. Available at: https://www.finddx.org/pandemics/. ↩
-
Abbott (2021). How Do We Help Prevent the Next Pandemic? Collaboration. [online] Abbott. Available at: https://www.abbott.com/corpnewsroom/diagnostics-testing/how-do-we-help-prevent-the-next-pandemic-collaboration.html. ↩
-
The International Readiness for Preventing Infectious Viral Disease Alliance (2021). International Readiness For Preventing Infectious Viral Disease. [online] INTREPID Alliance. Available at: https://www.intrepidalliance.org/#approach. ↩
-
Rapidly Emerging Antiviral Drug Development Initiative (2021). The best time to fight a global pandemic is now. And always. [online] READDI. Available at: https://www.readdi.org/. ↩
-
Wellcome (2020). Expanding access to monoclonal antibodies. [online] Wellcome. Available at: https://wellcome.org/reports/expanding-access-monoclonal-antibodies. ↩
-
Moderna (2021). Moderna’s Pipeline. [online] Moderna. Available at: https://www.modernatx.com/pipeline. ↩
-
Evotec (2021). Evotec: Action Plan 2025. [online] Evotec. Available at: https://actionplan.evotec.com/. ↩
-
Coalition for Epidemic Preparedness Innovations (2021). CEPI – Investment Case. [online] CEPI. Available at: https://endpandemics.cepi.net/. ↩
-
Graham, B.S. and Corbett, K.S. (2020). Prototype pathogen approach for pandemic preparedness: world on fire. Journal of Clinical Investigation, 130(7). ↩
-
Coalition for Epidemic Preparedness Innovations (2021). CEPI launches plan to tackle risk of future pandemics and epidemics. [online] CEPI. Available at: https://cepi.net/news_cepi/cepi-launches-plan-to-tackle-risk-of-future-pandemics-and-epidemics/. ↩
-
Chakamba, R. (2021). The cold chain storage challenge. [online] Devex. Available at: https://www.devex.com/news/the-cold-chain-storage-challenge-99869. ↩
-
Deloitte (2015). 2019 Global life sciences sector outlook. [online] Deloitte. Available at: https://www2.deloitte.com/global/en/pages/life-sciences-and-healthcare/articles/global-life-sciences-sector-outlook.html. ↩
-
Sample, I. (2021). Bill Gates call for huge global effort to prepare for future pandemics. [online] The Guardian. Available at: https://www.theguardian.com/us-news/2021/nov/04/bill-gates-call-for-huge-global-effort-to-prepare-for-future-pandemics. ↩
-
Venkatesan, P. (2020). COVID-19 diagnostics—not at the expense of other diseases. The Lancet Microbe, [online] 1(2), p.e64. Available at: https://www.thelancet.com/journals/lanmic/article/PIIS2666-5247(20)30041-0/fulltext. ↩
-
Ondoa, P., Oskam, L., Loembe, M.M. and Okeke, I.N. (2021). Transforming access to diagnostics: how to turn good intentions into action? The Lancet, [online]. Available at: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02182-6/fulltext. ↩
-
Vandenberg, O., Martiny, D., Rochas, O., van Belkum, A. and Kozlakidis, Z. (2020). Considerations for diagnostic COVID-19 tests. Nature Reviews Microbiology, 19(171–183). ↩
-
Vandenberg, O., Martiny, D., Rochas, O., van Belkum, A. and Kozlakidis, Z. (2020). Considerations for diagnostic COVID-19 tests. Nature Reviews Microbiology, 19(171–183). ↩
-
World Health Organization (2021). Global partnership to make available 120 million affordable, quality COVID-19 rapid tests for low- and middle-income countries. [online] www.who.int. Available at: https://www.who.int/news/item/28-09-2020-global-partnership-to-make-available-120-million-affordable-quality-covid-19-rapid-tests-for-low–and-middle-income-countries. ↩
-
World Health Organization (2021). ACT now, ACT together 2020-2021 Impact Report. [online] who.int. Available at: https://www.who.int/publications/m/item/act-now-act-together-2020-2021-impact-report. ↩
-
Recommendation 6 of 100 Days Mission. ↩
-
The Foundation for Innovative New Diagnostics (2021), 100DM Implementations Report submission to Cabinet Office ↩
-
The initial focus includes Coronaviridae, Orthomyxoviridae, Filoviridae and Flaviviridae. ↩
-
The White House (2021). American Pandemic Preparedness: Transforming Our Capabilities. [online] Available at: https://www.whitehouse.gov/wp-content/uploads/2021/09/American-Pandemic-Preparedness-Transforming-Our-Capabilities-Final-For-Web.pdf. ↩
-
Foundation for Innovative New Diagnostics (2021). COVID-19 & pandemic preparedness. [online] FIND. Available at: https://www.finddx.org/pandemics/. ↩
-
World Health Organization (2021). ACT-Accelerator Strategic Review. [online] www.who.int. Available at: https://www.who.int/publications/m/item/act-accelerator-strategic-review. ↩
-
Foundation for Innovative New Diagnostics (2021). Accelerating access to diagnostics. [online] FIND. Available at: https://www.finddx.org/wp-content/uploads/2019/03/FIND_Access-overview.pdf. ↩
-
Dolgin, E. (2021). The race for antiviral drugs to beat COVID — and the next pandemic. Nature, 592(7854), pp.340–343. ↩
-
Dolgin, E. (2021). The race for antiviral drugs to beat COVID — and the next pandemic. Nature, 592(7854), pp.340–343. ↩
-
Ledford, H. (2020). Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature, [online] 582(7813), pp.469–469. Available at: https://www.nature.com/articles/d41586-020-01824-5. ↩
-
Venkadapathi, J., Govindarajan, V.K., Sekaran, S. and Venkatapathy, S. (2021). A Minireview of the Promising Drugs and Vaccines in Pipeline for the Treatment of COVID-19 and Current Update on Clinical Trials. Frontiers in Molecular Biosciences, 8(637378). ↩
-
Medicines and Healthcare products Regulatory Agency (2021). First oral antiviral for COVID-19, Lagevrio (molnupiravir), approved by MHRA. [online] gov.uk. Available at: https://www.gov.uk/government/news/first-oral-antiviral-for-covid-19-lagevrio-molnupiravir-approved-by-mhra. ↩
-
Mahase, E. (2021). Covid-19: Pfizer’s paxlovid is 89% effective in patients at risk of serious illness, company reports. BMJ, 375(2713), p.n2713. ↩
-
Corti, D., Purcell, L.A., Snell, G. and Veesler, D. (2021). Tackling COVID-19 with neutralizing monoclonal antibodies. Cell, 184(12), pp.3086–3108. ↩
-
World Health Organization (2021). Therapeutics and COVID-19: living guideline. [online] www.who.int. Available at: https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-therapeutics-2021.3. ↩
-
Chen, R.E., Zhang, X., Case, J.B., Winkler, E.S., Liu, Y., VanBlargan, L.A., Liu, J., Errico, J.M., Xie, X., Suryadevara, N., Gilchuk, P., Zost, S.J., Tahan, S., Droit, L., Turner, J.S., Kim, W., Schmitz, A.J., Thapa, M., Wang, D. and Boon, A.C.M. (2021). Resistance of SARS-CoV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nature Medicine, [online] 27(717–726), pp.1–10. Available at: https://www.nature.com/articles/s41591-021-01294-w. ↩
-
World Health Organization (2021). ACT-Accelerator Strategic Review. [online] www.who.int. Available at: https://www.who.int/publications/m/item/act-accelerator-strategic-review. ↩
-
The International Readiness for Preventing Infectious Viral Disease Alliance (2021). International Readiness For Preventing Infectious Viral Disease. [online] INTREPID Alliance. Available at: https://www.intrepidalliance.org/#approach. ↩
-
The International Readiness for Preventing Infectious Viral Disease Alliance (2021). International Readiness For Preventing Infectious Viral Disease. [online] INTREPID Alliance. Available at: https://www.intrepidalliance.org/#approach. ↩
-
Rapidly Emerging Antiviral Drug Development Initiative (2021). The best time to fight a global pandemic is now. And always. [online] READDI. Available at: https://www.readdi.org/. ↩
-
Wellcome (2020). Expanding access to monoclonal antibodies. [online] Wellcome. Available at: https://wellcome.org/reports/expanding-access-monoclonal-antibodies. ↩
-
Rapidly Emerging Antiviral Drug Development Initiative (2021). The best time to fight a global pandemic is now. And always. [online] READDI. Available at: https://www.readdi.org/. ↩
-
Meganck, R.M. and Baric, R.S. (2021). Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nature Medicine, [online] 27(3), pp.401–410. Available at: https://www.nature.com/articles/s41591-021-01282-0.pdf. ↩
-
The International Federation of Pharmaceutical Manufacturers & Associations (2021), 100DM Implementations Report submission to Cabinet Office ↩
-
He, F., Hogan, S., Latypov, R.F., Narhi, L.O. and Razinkov, V.I. (2010). High throughput thermostability screening of monoclonal antibody formulations. Journal of Pharmaceutical Sciences, 99(4), pp.1707–1720. ↩
-
Meganck, R.M. and Baric, R.S. (2021). Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nature Medicine, [online] 27(3), pp.401–410. Available at: https://www.nature.com/articles/s41591-021-01282-0.pdf. ↩
-
Wellcome (2020). Expanding access to monoclonal antibodies. [online] Wellcome. Available at: https://wellcome.org/reports/expanding-access-monoclonal-antibodies. ↩
-
Brady, J.R. and Love, J.C. (2021). Alternative hosts as the missing link for equitable therapeutic protein production. Nature Biotechnology, [online] 39(4), pp.404–407. Available at: https://www.nature.com/articles/s41587-021-00884-w. ↩
-
Mahal, H., Branton, H. and Farid, S.S. (2021). End-to-end continuous bioprocessing: Impact on facility design, cost of goods, and cost of development for monoclonal antibodies. Biotechnology and Bioengineering, 118(9). ↩
-
Moderna (2021). Moderna’s Pipeline. [online] Moderna. Available at: https://www.modernatx.com/pipeline. ↩
-
Meganck, R.M. and Baric, R.S. (2021). Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nature Medicine, [online] 27(3), pp.401–410. Available at: https://www.nature.com/articles/s41591-021-01282-0.pdf. ↩
-
Li, Y.-D., Chi, W.-Y., Su, J.-H., Ferrall, L., Hung, C.-F. and Wu, T.-C. . (2020). Coronavirus vaccine development: from SARS and MERS to COVID-19. Journal of Biomedical Science, [online] 27(1). Available at: https://jbiomedsci.biomedcentral.com/articles/10.1186/s12929-020-00695-2. ↩
-
Our World in Data (2021). Coronavirus (COVID-19) Vaccinations – Statistics and Research. [online] Our World in Data. Available at: https://ourworldindata.org/covid-vaccinations. ↩
-
Airfinity (2021). September 2021 snap shot COVID-19 data. [online] IFPMA. Available at: https://www.ifpma.org/wp-content/uploads/2021/09/Airfinity_September_2021_Snapshot_COVID-19_Data.pdf. ↩
-
G20 Presidency (2021). A Global Deal for our Pandemic Age – Report of the G20 High Level Independent Panel on Financing the Global Commons for Pandemic Preparedness and Response. [online] Available at: https://www.g20.org/wp-content/uploads/2021/07/G20-HLIP-Report.pdf. ↩
-
The International Federation of Pharmaceutical Manufacturers & Associations (2021). Momentum of COVID-19 vaccine manufacturing scale up sufficient for step change in distribution. [online] IFPMA. Available at: https://www.ifpma.org/resource-centre/momentum-of-covid-19-vaccine-manufacturing-production-scale-up-is-now-sufficient-for-step-change-in-distribution-and-opens-way-for-urgent-political-leadership-and-country-preparedness/. ↩
-
McKinsey (2021). Fast-forward: Will the speed of COVID-19 vaccine development reset industry norms? - McKinsey. [online] McKinsey. Available at: https://www.mckinsey.com/industries/life-sciences/our-insights/fast-forward-will-the-speed-of-covid-19-vaccine-development-reset-industry-norms. ↩
-
Adalja, A.A., Watson, M., Cicero, A. and Inglesby, T. (2020). Vaccine Platform Technologies: A Potent Tool for Emerging Infectious Disease Vaccine Development. Health Security, 18(1), pp.59–60. ↩
-
Ball, P. (2020). The lightning-fast quest for COVID vaccines — and what it means for other diseases. Nature, [online] 589(7840), pp.16–18. Available at: https://www.nature.com/articles/d41586-020-03626-1. ↩
-
Pardi, N., Hogan, M.J., Porter, F.W. and Weissman, D. (2018). mRNA vaccines — a new era in vaccinology. Nature Reviews Drug Discovery, [online] 17(4), pp.261–279. Available at: https://www.nature.com/articles/nrd.2017.243. ↩
-
Rosa, S.S., Prazeres, D.M.F., Azevedo, A.M. and Marques, M.P.C. (2021). mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine, 39(16). ↩
-
The International Federation of Pharmaceutical Manufacturers & Associations (2021), 100DM Implementations Report submission to Cabinet Office. ↩
-
Seqirus (2021). Seqirus Announces Investment in Next-Generation Influenza Vaccine Technology, Self-Amplifying Messenger RNA (sa-mRNA). [online] Seqirus. Available at: https://www.seqirus.com/news/seqirus-invests-in-self-amplifying-messenger-rna-technology. ↩
-
UN Environment Programme (2020). Why optimized cold-chains could save a billion COVID vaccines. [online] unep.org. Available at: https://www.unep.org/news-and-stories/story/why-optimized-cold-chains-could-save-billion-covid-vaccines. ↩
-
Ravi, A., Sadhna, D., Nagpaal, D. and Chawla, L. (2015). Needle free injection technology: A complete insight. International Journal of Pharmaceutical Investigation, [online] 5(4), p.192. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4675000/. ↩
-
Dolgin, E. (2021). How COVID unlocked the power of RNA vaccines. Nature, [online] 589(7841), pp.189–191. Available at: https://www.nature.com/articles/d41586-021-00019-w. ↩
-
Bárcena, M., Barnes, C.O., Beck, M., Bjorkman, P.J., Canard, B., Gao, G.F., Gao, Y., Hilgenfeld, R., Hummer, G., Patwardhan, A., Santoni, G., Saphire, E.O., Schaffitzel, C., Schendel, S.L., Smith, J.L., Thorn, A., Veesler, D., Zhang, P. and Zhou, Q. (2021). Structural biology in the fight against COVID-19. Nature Structural & Molecular Biology, 28(1), pp.2–7. ↩
-
Jumper, J., Evans, R., Pritzel, A., Green, T., Figurnov, M., Ronneberger, O., Tunyasuvunakool, K., Bates, R., Žídek, A., Potapenko, A., Bridgland, A., Meyer, C., Kohl, S.A.A., Ballard, A.J., Cowie, A., Romera-Paredes, B., Nikolov, S., Jain, R., Adler, J. and Back, T. (2021). Highly accurate protein structure prediction with AlphaFold. Nature, [online] 596(590–596), pp.1–11. Available at: https://www.nature.com/articles/s41586-021-03819-2. ↩
-
Graham, B.S. and Sullivan, N.J. (2017). Emerging viral diseases from a vaccinology perspective: preparing for the next pandemic. Nature Immunology, 19(1), pp.20–28. ↩
-
Chakamba, R. (2021). The cold chain storage challenge. [online] Devex. Available at: https://www.devex.com/news/the-cold-chain-storage-challenge-99869. ↩
-
Chakamba, R. (2021). The cold chain storage challenge. [online] Devex. Available at: https://www.devex.com/news/the-cold-chain-storage-challenge-99869. ↩
-
Contingent on the outcome of the March 2022 CEPI Replenishment process. ↩
-
Monrad, J.T., Sandbrink, J.B. and Cherian, N.G. (2021). Promoting versatile vaccine development for emerging pandemics. npj Vaccines, 6(1). ↩
-
Krammer, F. (2020). SARS-CoV-2 vaccines in development. Nature, [online] 586(516-527). Available at: https://www.nature.com/articles/s41586-020-2798-3. ↩
-
Gallagher, J. (2021). Prof Sarah Gilbert, Covid vaccine creator: Now let’s take on 12 more diseases. BBC News. [online] 16 Oct. Available at: https://www.bbc.co.uk/news/health-58898085. ↩
-
Moderna (2021). Moderna’s Pipeline. [online] Moderna. Available at: https://www.modernatx.com/pipeline. ↩
-
Hejdankova, Z., Vanek, V., Sedlak, F., Prochazka, J., Diederichs, A., Kereïche, S., Novotna, B., Budesinsky, M., Birkus, G., Grantz Saskova, K. and Cigler, P. (2021). Lipid Nanoparticles for Broad Spectrum Nucleic Acid Delivery. Advanced Functional Materials, p.2101391. ↩
-
Team Consulting (2020). Preparing for mass vaccination. [online] www.team-consulting.com. Available at: https://www.team-consulting.com/insights/preparing-for-mass-vaccination/. ↩
-
G20 Presidency (2021). G20 Rome Leaders’ Declaration. [online] G20.org. Available at: https://www.g20.org/wp-content/uploads/2021/10/G20-rome-leaders-declaration. ↩
-
Burger, L. (2021). BioNTech eyes Rwanda, Senegal for malaria, tuberculosis vaccine production. Reuters. [online] 27 Aug. Available at: https://www.reuters.com/world/africa/biontech-eyes-rwanda-senegal-malaria-tuberculosis-vaccine-production-2021-08-27/. ↩
-
Mason, J. and Fick, M. (2021). Moderna plans African vaccine plant as drugmakers urged to help poorest. Reuters. [online] 7 Oct. Available at: https://www.reuters.com/world/africa/moderna-plans-mrna-vaccine-factory-africa-2021-10-07/. ↩
-
G7 Pandemic Preparedness Partnership (2021). 100 Days Mission to respond to future pandemic threats. [online] gov.uk. Available at: https://www.gov.uk/government/publications/100-days-mission-to-respond-to-future-pandemic-threats. ↩
-
Recommendations 9 to 25 of the 100 Days Mission. Specific recommendations are set out in further detail at Annex A. ↩
-
Recommendation 9 of the 100 Days Mission. ↩
-
Recommendation 18 of the 100 Days Mission. ↩
-
G7 Pandemic Preparedness Partnership (2021). 100 Days Mission to respond to future pandemic threats. [online] gov.uk. Available at: https://www.gov.uk/government/news/government-and-life-science-industry-join-forces-on-100-days-mission-for-future-pandemics ↩
-
Concept by research.life (2020). COVID-19 Global Research Information & Resources. [online] covid19.researcher.life. Available at: https://covid19.researcher.life/article/analysis-of-the-who-ictrp-for-novel-coronavirus-clinical-trial-registrations/be81de42-faef-46a3-959d-7f71c2942fa0. ↩
-
Department of Health and Social Care (2021). G7 Therapeutics and Vaccines Clinical Trials Charter. [online] Gov.uk. Available at: https://www.gov.uk/government/publications/g7-health-ministers-meeting-june-2021-communique/g7-therapeutics-and-vaccines-clinical-trials-charter. ↩
-
Oxford Recovery Trial (2021). Recovery Trial. [online] www.recoverytrial.net. Available at: https://www.recoverytrial.net/. ↩
-
Recommendation 11 of the 100 Days Mission. ↩
-
Royal Society (2021). UK Invites the Global Pandemic Data Alliance to Deliver on S7 Recommendations on Health Emergencies. [online] Available at: https://royalsociety.org/-/media/news/2021/Global-Pandemic-Data-Alliance-Press-Release.pdf. ↩
-
Royal Society (2021). Data for international health emergencies: governance, operations and skills. [online] Available at: https://royalsociety.org/-/media/about-us/international/g-science-statements/G7-data-for-international-health-emergencies-31-03-2021.pdf. ↩
-
data.org (2021). Epiverse: Distributed Pandemic Tools Program. [online] data.org. Available at: https://data.org/initiatives/epiverse/. ↩
-
data.org (2021). data.org Awards £3M to the London School of Hygiene & Tropical Medicine to Transform Global Pandemic Response. [online] data.org. Available at: https://data.org/news/data-org-awards-3m-to-the-london-school-of-hygiene-tropical-medicine-to-transform-global-pandemic-response/. ↩
-
Trinity Challenge (2021). The Trinity Challenge Winners. [online] The Trinity Challenge. Available at: https://thetrinitychallenge.org/awards/winners/. ↩
-
Royal Society (2021). UK Invites the Global Pandemic Data Alliance to Deliver on S7 Recommendations on Health Emergencies. [online] Available at: https://royalsociety.org/-/media/news/2021/Global-Pandemic-Data-Alliance-Press-Release.pdf. ↩
-
World Health Organization (2021i). WHO BioHub. [online] www.who.int. Available at: https://www.who.int/initiatives/who-biohub. ↩
-
World Health Organization (2021i). WHO BioHub. [online] www.who.int. Available at: https://www.who.int/initiatives/who-biohub. ↩
-
G7 Pandemic Preparedness Partnership (2021). 100 Days Mission to respond to future pandemic threats. [online] gov.uk. Available at: https://www.gov.uk/government/publications/100-days-mission-to-respond-to-future-pandemic-threat. ↩
-
World Bank (2021). World Bank/IMF Annual Meetings 2021: Development Committee Communiqué. [online] World Bank. Available at: https://www.worldbank.org/en/news/press-release/2021/10/15/world-bank-imf-annual-meetings-2021-development-committee-communiqu. ↩
-
G20 Presidency (2021). G20 Rome Leaders’ Declaration. [online] G20.org. Available at: https://www.g20.org/wp-content/uploads/2021/10/G20-rome-leaders-declaration. ↩
-
World Bank (2021). World Bank/IMF Annual Meetings 2021: Development Committee Communiqué. [online] World Bank. Available at: https://www.worldbank.org/en/news/press-release/2021/10/15/world-bank-imf-annual-meetings-2021-development-committee-communiqu. ↩
-
Global Covid-19 Access Tracker (2021). COVID-19 Global Access Tracker v2. [online] Covid19globaltracker.org. Available at: https://covid19globaltracker.org/. ↩
-
G20 Presidency (2021). G20 Rome Leaders’ Declaration. [online] G20.org. Available at: https://www.g20.org/wp-content/uploads/2021/10/G20-rome-leaders-declaration. ↩
-
G20 Presidency (2021). The G20 established a joint Finance-Health Task Force to strengthen pandemic prevention, preparedness and response - G20. [online] g20.org. Available at: https://www.g20.org/the-g20-established-a-joint-finance-health-task-force-to-strengthen-pandemic-prevention-preparedness-and-response.html. ↩
-
Independent Panel for Pandemic Preparedness and Response (2021). COVID-19: Make it the Last Pandemic. [online] Available at: https://theindependentpanel.org/wp-content/uploads/2021/05/COVID-19-Make-it-the-Last-Pandemic_final.pdf. ↩
