ACMD advice on 2-benzyl benzimidazole and piperidine benzimidazolone opioids (accessible version)
Updated 10 December 2025
1. Introduction
1.1. New psychoactive substances (NPS) have presented a substantial public health challenge over the last 13 years, with new synthetic opioids (NSO) making an increasing recent contribution [United Nations Office on Drugs and Crime (UNODC), 2021]. These NSO give users similar effects to those of morphine and heroin, but some are much more potent. This means that substantially lower doses are needed to achieve the effects desired by users, but there is also a high risk of accidental overdose, and this may cause life- threatening toxicity including loss of consciousness, cardiorespiratory arrest and death.
1.2. The NSO most frequently encountered have been the fentanyl analogues, which have caused a large number of drug-related deaths internationally and which were the subject of an earlier ACMD report (Misuse of fentanyl and fentanyl analogues, January 2020). Fentanyl and its analogues have commonly been synthesised in China, but further legal controls affecting these compounds were enacted there between 2016 and 2019 [Bao et al., 2019], encouraging synthetic chemists in that country to explore the synthesis of other NSO that remain uncontrolled.
1.3. This report considers two similar but distinct types of NSO, the 2-benzyl benzimidazole (‘nitazene’) and the piperidine benzimidazolone (‘brorphine- like’) opioids.
1.4. 2-Benzyl benzimidazole (‘nitazene’) opioids were originally developed in the 1950s as analgesics. Several were shown to have potent opioid (heroin-like) effects [Blanckaert et al., 2020; EMCDDA, 2020; WHO, 2020], but none were subsequently marketed anywhere as human or veterinary medicines [Gross and Turrian 1957; Hunger et al., 1957; EMCDDA, 2020; WHO, 2020].
1.5. 2-Benzyl benzimidazole opioids have previously been encountered internationally as substances of misuse. For example, etonitazene was implicated in the deaths of 10 drug users in Moscow in 1998 and clandestine laboratories manufacturing this compound have been occasionally identified since then [Sorokin et al., 1999]. In 2003, etonitazene was manufactured by an American chemist in Utah and placed in nasal spray bottles, apparently for his own use [Desert News, 2003].
1.6. More recently, there have been many international reports of severe toxicity involving 2-benzyl benzimidazole opioids, especially isotonitazene. As of November 2021, 9 of these compounds had been reported to the UNODC Early Warning Advisory, specifically isotonitazene, 5-aminoisotonitazene, N- pyrrolidino-etonitazene, butonitazene, metonitazene, protonitazene, etodesnitazene (etazene), flunitazene and metodesnitazene (metazene) [UNODC, 2021].
1.7. 2-Benzyl benzimidazole opioids have been sold as powders or nasal sprays and administered by the intravenous, sublingual or nasal routes or by vaping. They may also be used to fortify heroin, or as constituents of counterfeit medicines [WHO, 2020; EMCDDA, 2020]. Users may be unaware of their inclusion and, like fentanyls, the high potency of some of these compounds provides a substantial risk of severe and potentially fatal overdose.
1.8. There is also recent evidence of fatal and non-fatal toxicity associated with the piperidine benzimidazolone opioid brorphine. This compound has been detected in seized powders, and use via the oral route or by inhalation (smoking, vaping) may be more common than use by injection [WHO, 2021]. Many other chemicals with this general structure have also been shown to have opioid actions, although to date there are no reports of their misuse.
1.9. This report examines the available evidence of harms caused by 2-benzyl benzimidazole and piperidine benzimidazolone opioids, and their prevalence and public health impact in the UK. It provides recommendations, including control under the Misuse of Drugs Act 1971 and scheduling via the Misuse of Drugs Regulations 2001.
2. Legal control
2.1. Internationally, etonitazene and clonitazene are controlled under the United Nations Single Convention on Narcotic Drugs of 1961 [UNODC, 1961; INCB, 2021). Isotonitazene was added to schedule I of the convention, as amended by the 1972 Protocol, in June 2021. Metonitazene and brorphine were subsequently also added to the same schedule in March 2022 [UNODC, 2022]. Further details of international controls are provided in Annex A.
2.2. In the UK, etonitazene and clonitazene are listed as Class A drugs in the Misuse of Drugs Act 1971 and in schedule 2 of the Misuse of Drugs Regulations 2001. All other 2-benzyl benzimidazole opioids and all piperidine benzimidazolone opioids (including brorphine) are not currently controlled under the Misuse of Drugs Act 1971, although, as psychoactive substances, import, supply, possession with the intent to supply and possession in a custodial institution are all offences under the Psychoactive Substances Act 2016.
3. Chemistry
3.1. The general structure of 2-benzyl benzimidazole opioids is shown in Annex C. They contain a benzimidazole ring with an ethylamine at the 1-position and a benzyl group at the 2-position [DEA, 2021a].
3.2. 2-Benzyl benzimidazole opioids are structurally unrelated to other opioid drug groups, including morphine, fentanyls and U-series analogues (‘utopioids’). The benzimidazole ring structure is, however, also found in a wide range of human and animal medicines, including anthelminthics (such as mebendazole, albendazole), proton pump inhibitors (such as omeprazole), antivirals (such as enviradine), antihypertensives (such as candesartan), antihistamines (such as astemizole) and fungicides (such as carbendazim) [Brishty et al., 2021].
3.3. The 2-Benzyl benzimidazoles are synthetic opioids and can be prepared in only a few steps from readily available, uncontrolled precursors [Pardeshi et al., 2021]. While there are preferred syntheses, a number of viable alternative methods and variations are available. These synthetic approaches are amenable to incorporation of structural changes to all parts of the nitazene molecule, including changes to the electron-withdrawing-nitro substituent, to the N,N-diethyl moiety at the end of the ethylamino sidechain and to the benzyl group at C2 (including varying substituents on the aryl ring and changing the methylene linker either by adding substituents or by swapping to other elements) [Ujváry et al., 2021]. The modular nature of the synthesis means that each part of the molecule can be varied independently, allowing access to a large number of analogues.
3.4. The general structure of piperidyl benzimidazolone opioids is shown in Annex D. They contain a benzimidazole-2-one ring system, substituted with a 4- piperidinyl group on one of the ring nitrogens, with a benzylic group attached to the piperidinyl nitrogen. They are chemically distinct from other opioid drug groups, although there are some structural similarities to fentanyl. However, the 4-piperidinyl benzimidazolone core is found in other medicines, including the antipsychotics benperidol and pimozide, and the dopamine antagonist domperidone, which is used to promote lactation and treat nausea and vomiting, and gastroparesis.
3.5. The piperidyl benzimidazolone opioid brorphine has been encountered recently in many countries in forensic casework and most evidence in this report on this group of materials concerns this compound. There are, however, other examples with opioid activity and other members of this group that are of interest, for instance the selective nociceptin opioid peptide (NOP) receptor antagonist J-113397 that is much used in research and was the lead for a new generation of NOP antagonists [Jong et al., 2004].
3.6. Brorphine and its analogues can be made in only a few steps from readily available precursors [Kennedy et al., 2018]. The synthetic method allows for many close analogues to be made and structural changes can easily be made to all parts of the molecule (e.g., R1 to R8 on Annex D). These modifications can include having substituents on the phenyl ring of the benzimidazolone group, varying the piperidine ring and varying the substituent attached to the piperidine nitrogen. The synthesis is modular, allowing access to a large number of analogues.
4. Pharmacology
4.1. The pharmacology and toxicology of opioid drugs have recently been described in detail (ACMD report - Misuse of fentanyl and fentanyl analogues, January 2020). Briefly, these drugs act by interacting with a series of receptors within the brain and nervous system termed mu (MOR), delta (DOR) and kappa (KOR) opioid receptors. Of these, the MOR is particularly important as the principal mediator of the analgesia, respiratory depression, euphoria and dependency associated with heroin, morphine, fentanyl and other opioids [Gill et al., 2019]
4.2. Early studies demonstrated that several 2-benzyl benzimidazole compounds, including isotonitazene and etonitazene, have potent centrally mediated analgesic effects [Hunger et al., 1960b]. A recent review of early studies administering these drugs to mice via the subcutaneous route indicated potencies compared to morphine ranging from 1 (equal potency) for flunitazene to 500 times more potent for isotonitazene and 1000 times more potent for etonitazene [Ujváry et al., 2021]. In a mouse tail-flick analgesia model, isotonitazene was 500 times more potent than morphine [Hunger et al., 1960b]. If these studies reflect the pharmacology in humans, a dose of isotonitazene would be expected to have the same analgesic effect as a morphine dose 500 times larger.
4.3. Across several in vitro assays of MOR activation isotonitazene was observed to be more potent (up to 10 times) than fentanyl in activating the receptor but with similar agonist efficacy. The rank order of potency of a series of nitazenes was reported to be etonitazene >= isotonitazene > protonitazene >= metonitazene > butonitazene >= etodesnitazene » 5-aminoisotonitazene = flunitazene > metodesnitazene [Blanckaert et al., 2020; Vandeputte et al., 2021].
4.4. The N,N-dialkyl component of nitazenes can be modified in a number of ways. Replacing the ethyl groups with other small alkyl groups retains significant activity and the N-desalkylforms of nitazenes, where 1 of the 2 alkyl groups on the amino group has been lost, are reported to be at least equipotent with their parent compounds. Fusion of the two alkyl groups of the N,N-diethyl component into a pyrrolidine ring gives N-pyrrolidino etonitazene (etonitazepyne). Preliminary unpublished data suggested that N-pyrrolidino etonitazene is among the most potent of the 2-benzyl benzimidazole opioids, being 20 times more potent than fentanyl and with MOR activation similar to that of etonitazene [Vandeputte and Stove unpublished, cited by Blankaert et al., 2021] while the N-piperidinyl form (etonitazepipne) is reported to have even greater potency.
4.5. Modifications at the 4- position of the benzyl structure have been extensively explored. Replacement of ethoxy by other small ether groups such as methoxy, propoxy and isopropoxy, retains very significant activity, with the isopropoxy form of etonitazene (isotonitazene) being the most potent variant, with around 500 times the potency of morphine. Replacement of the alkoxy group by halogens results in materials such as clonitazene, with much reduced potencies, similar to or slightly greater than that of morphine. Small alkyl groups at this position produce materials with greater potency, with propyl nitazene reported to have a potency around 50 times that of morphine. Replacement of the oxygen atom of these ethers by a sulphur atom to produce thioethers results in materials less potent than their ether equivalents but still significantly greater than that of morphine.
4.6. Removal of the nitro group results in a reduction in potency, with etodesnitazene (etazene, etonitazene without the nitro group) having a potency around 70 times that of morphine. Replacement of the nitro group with other electron-withdrawing groups, such as cyano or acetyl, results in materials with reduced but still significant potencies.
4.7. Some other variations of the etonitazene structure have also been reported to produce materials with significant MOR potency, though none of these variants have so far been observed in drug markets internationally.
4.8. Three of the nitazenes have had some preliminary clinical evaluation in a variety of settings; after single-dose oral administration, etonitazene was 80 to 120 times more potent than morphine as a euphoriant, while clonitazene (a low-potency nitazene of comparable potency to morphine in in vitro bioassays) was of slightly lower potency than morphine. Subcutaneous metonitazene (a moderately potent nitazene in vitro) was around 10 times more potent than morphine as an analgesic [Bromig, 1958; Ujváry et al., 2021]. From these data it appears that the in vitro bioassay data are reasonably predictive of clinical efficacy. None of these were taken forward as medicines, with respiratory depression/failure being noted.
4.9. Other factors are also important in determining pharmacological effects and the risk of toxicity. The ability to reach sites of action in the brain is essential and increased for lipid soluble substances. 2-benzyl benzimidazole opioids, including isotonitazene, are highly lipid soluble and, therefore, likely to cross rapidly into the brain [WHO, 2020].
4.10. 4-piperidinyl benzimidazolones were disclosed by Janssen in the 1960s [Janssen Pharmaceutica, 1965; Janssen and Van der Eycken, 1968] as potent morphine-like analgesics. Recent studies have shown members of this series (SR14968 and SR17018) have morphine-like analgesic effects in rhesus monkeys, though their limited solubility prevented a thorough investigation of their effects on respiration [Cornelissen et al., 2021]. SR- 14968 has also been shown to produce fentanyl-like discriminative stimulus effects in male and female rats [Schwienteck et al., 2019].
4.11. Replacing the central piperidine group with larger (7-membered azacycloheptane) or smaller (5-membered pyrolidine) rings, or moving the attachment point of the benzimidazolone group from the 4-position to the 3- position of the piperidine all resulted in reduced activity [Kennedy et al., 2018]. Groups other than a hydrogen or a methyl group on the benzylic carbon also results in substantial loss of MOR activity [Kennedy et al., 2018].
4.12. In assays of agonist activity (G protein activation) at the MOR, brorphine has been reported to be a potent, high-efficacy agonist [Kennedy et al., 2018; Vandeputte et al., 2020; Verougstraete et al., 2020; Grafinger et al., 2021] approximately 13 fold more potent than morphine [Kennedy et al., 2018] and with potency estimates compared to fentanyl ranging from slightly less potent (3 fold) [Vandeputte et al., 2020] to more potent (10 fold) [Grafinger et al., 2021]. A number of close analogues reported by Kennedy et al. (2018) show potency similar to or slightly greater than that of morphine.
4.13. Brorphine shows selectivity for the MOR over the KOR, being some 500 times more potent as an agonist at the MOR [Grafinger et al., 2021]. A number of synthetic opioids structurally related to brorphine have been reported in the scientific literature to be MOR agonists. Some have similar potency to brorphine [Kennedy et al., 2018; Schmid et al., 2017]
4.14. In mice, two brorphine analogues, SR-14968 and SR-17018, produced reward-associated behaviour and induced physical dependence which may indicate that they could be misused and produce physical dependence [Kudla et al., 2022].
4.15. In humans, brorphine is extensively metabolised – at least 11 metabolites have been identified [Krotulski et al., 2020; Grafinger et al., 2021]. The activities of the metabolites at the MOR have not been reported.
5. Toxicology
5.1. The acute toxicity of several 2-benzyl benzimidazole opioids has been studied in mice and rabbits [Gross and Turrian, 1957; Ujváry et al., 2021; EMCDDA, 2020]. There is a wide range in toxicity for the various compounds studied, with etonitazene associated with the lowest doses required to produce toxic effects. Limited information on toxicity is available for isotonitazene. It should be noted, however, that studies in rodents should be interpreted with caution as they do not accurately reflect opioid toxicity in humans.
5.2. Specific antidotes antagonise opioid effects on the MOR and reverse the toxic effects of heroin. Naloxone is widely used for this indication and has saved many lives after overdose. Research involving isolated tissues and animals has demonstrated that opioid antagonists, including naloxone or naltrexone, inhibit the opioid receptor binding and clinical effects of etonitazene [Hughes et al., 1975; Pert and Snyder, 1973; Barnett et al., 1975; Achat-Mendes et al., 2009; Blanckaert et al., 2020]. Information on the efficacy of naloxone specifically for treating intoxication from 2-benzyl benzimidazole opioids in humans is lacking, but in a human volunteer, life-threatening respiratory depression and coma caused by subcutaneous metonitazene (1mg) was reversed by 5mg of the opioid antagonist nalorphine administered intravenously [Bromig, 1958].
5.3. Some 2-benzyl benzimidazole metabolites (such as N-desethylisotonitazene, N-desethyletonitazene) also have high efficacy and potency. There is very little information available on their effects in humans and they may not be tested for as part of post-mortem toxicology, but their effects may add to those of the parent compounds. Toxicity in some animal species may differ from that observed in humans due to differences in rates or routes of metabolism.
5.4. The varying potency of different 2-benzyl benzimidazole opioids for analgesic and toxic effects means that very different doses would be needed to achieve desired effects in users. There is a risk that this would not be adequately taken into account by dealers or users switching from one compound to another.
6. Legitimate uses
6.1. 2-Benzyl benzimidazole and piperidinyl benzimidazolone opioids have legitimate uses as analytical reference standards and potentially for research, but no other legitimate uses have been identified [EMCDDA, 2020].
6.2. The Medicines and Healthcare products Regulatory Agency (MHRA) was consulted about legitimate medical uses of the following compounds: butonitazene, clonitazene, etazene, etonitazene, flunitazene, isotonitazene, metodesnitazene, metonitazene, nitazene and protonitazene. The MHRA confirmed that none of these compounds have ever been authorised in the UK as medicines. There are no licences either currently or previously for any of these substances and, as a consequence, none will have been marketed in the UK as a medicine. None of these compounds have been the subject of any request for scientific advice meetings with the MHRA by any company, there have been no imports for legitimate purposes (named patient prescriptions) and none are being used in a clinical trial where the sponsor has applied to the MHRA for their clinical trial.
6.3. In relation to the schedule 2 compounds of 26,000 import or export licences issued over the last year, only 5 involved etonitazene and 2 involved clonitazene. The companies and quantities involved were consistent with use as reference standards.
6.4. The MHRA have also stated that there are currently no marketing authorisation applications for medicinal use of brorphine in their applications system and, they were unable to find any evidence of clinical trials using brorphine in the UK or internationally.
6.5. The benzimidazole ring structure and the 4-piperidinyl benzimidazolone core are both found in a range of licensed non-opioid medicines (see ‘Section 3: Chemistry’) and it is important that these are not captured by any legislation based on generic chemical structure.
7. Misuse
7.1. Established opioids such as heroin, morphine, oxycodone and fentanyl have well-recognised dependency potential and addiction to these drugs accounts for a high proportion of problematic drug users in the UK and other countries.
7.2. Early evidence of the addictive potential in man of earlier 2-benzyl benzimidazole examples was recently reviewed by the EMCDDA [2020]. Orally administered single doses of clonitazene and etonitazene were compared with morphine in non-tolerant former morphine addicts. Clonitazene appeared to be about one-third to one-fifth as potent as morphine sulphate and roughly equivalent to codeine, while etonitazene was more than 80 to 120 times as effective as morphine as a euphoriant. Both drugs supressed symptoms of opioid withdrawal in opioid-dependent patients who had previously been stabilised on morphine, while abrupt discontinuation of etonitazene promoted abstinence syndrome [Anon, 1959; EMCDDA, 2020].
For etonitazene, the discriminative stimulus [Zhang et al., 2000], self- administration [Achat-Mendes et al., 2009; Ginsberg and Lamb 2018], and tolerance and dependence [Tang, 1982; Walker and Young, 2001] were similar or greater than those of morphine or fentanyl [WHO, 2000].
7.3. Limited specific evidence is available for the dependency potential of more recently encountered 2-benzyl benzimidazole opioids. There are, however, unverified user reports of withdrawal symptoms after use of isotonitazene, including fever, dizziness, flu-like feelings, blackouts, anxiety and panic attacks [WHO, 2020].
7.4. In studies using rats, brorphine fully substituted for the effects of morphine. No human studies are available relating to physical dependence or withdrawal symptoms in human users of brorphine, although users have reported development of tolerance and withdrawal on the social media site Reddit [WHO, 2021].
7.5. Verougstraete et al. [2020] described a 24-year-old male who presented with features of opioid withdrawal including generalised pain, tachycardia and sweating, He had used brorphine orally with etizolam for 1 to 2 months after a period of more than a year of abstention from opioid use. The patient reported effects similar to oxycodone or fentanyl, with a long-lasting ‘high’, the development of tolerance and intense cravings after waking.
7.6. There is limited information on the manufacture, trafficking, distribution and use of 2-benzyl benzimidazole or piperidinyl benzimidazolone opioids. It is reported that some of the isotonitazene sold in Europe was manufactured by chemical companies based in China [EMCDDA, 2021]. Brorphine and several 2-benzyl benzimidazoles have been available for sale via the dark net [Lamy et al., 2021]
7.7. Interest in NPS may be identified by posts made on social media sites before these substances are detected in samples from human users. Posts on the social media site Reddit mentioning isotonitazene were first made in January 2019 and brorphine in February 2019 [Barenholtz et al., 2021]. From automated scanning of online resources discussing NPS (January to August 2020), threads concerning etazene, brorphine and fluonitazene were among the most popular [Catalani et al., 2021].
7.8. Isotonitazene was first identified in samples from a March 2019 toxicology case in Alberta, Canada [WHO, 2020] and in illicit drug markets in Europe in April 2019, following a drug seizure in Estonia [EMCDDA, 2020]. It was first reported in the US in November 2019 from samples collected in August 2019. The most recent, Centre for Forensic Science Research and Education (CFSRE) trend report for January to March 2022 shows a peak in US isotonitazene detections in October to September 2019, with subsequent reductions, although positive detections continue at a lower rate.
7.9. Isotonitazene has been sold online as a powder, nasal spray or in counterfeit pills with reported routes of administration including vaping, intravenous, sublingual or intranasal (spray or insufflation). Reported doses ranged from 1mg to 10mg (intravenous, sublingual or via vaping) and 100 µg to 200 µg via nasal spray [WHO, 2021]. Isotonitazene-containing drug seizures reported to the EMCDDA have been of powders (brown, yellow, or white) or liquids, and may include the free base and/or the hydrochloride salt.
7.10. The 2-benzyl benzimidazole opioid etodesnitazene was detected in a police seizure made in Poland in May 2020 [Siczek et al., 2020).
7.11. The piperidinyl benzimidazolone opioid brorphine was first identified in US drug markets in June 2019 and in Europe (Belgium) in February 2020 [DEA, 2020; UNODC, 2020; Vanderputte et al., 2021]. It has also been reported by other countries including Canada, Sweden, Slovenia, Finland and the UK. It was detected in at least 7 fatalities in the US between June and July 2020. The National Forensic Laboratory Information System received 20 reports involving brorphine in 2019 and 2020 from three different states, with heroin, fentanyl, flualprazolam and diphenhydramine also identified in many cases. The evidence suggested that brorphine was used as a replacement for heroin or other opioids, with or without the knowledge of users [DEA, 2020]. By October 2020, exposure to brorphine had been identified in more than 100 US cases during forensic casework [Krotulski et al., 2021]. The increase in brorphine detections followed the scheduling of isotonitazene in the US in June 2020. Brorphine itself was scheduled in the US in March 2021, but worldwide interest in this compound, assessed using Google Trends, had already fallen substantially by that time [Vandeputte et al., 2021]. The CFSRE trend report for January to March 2022 demonstrates a peak in brorphine detections in the US in July to September 2020, with subsequent reductions. There has, however, been a small increase in recent detections [NPS Discovery, 2022].
7.12. The US’s DEA has reported that dealers have advertised brorphine as a replacement for fentanyls and that the population likely to abuse brorphine appears to be the same as those abusing heroin, prescription opioid analgesics and other synthetic opioids [DEA, 2021b].
8. Health harms
8.1. The most serious acute health risk from 2-benzyl benzimidazole and piperidinyl benzimidazolone opioids is likely to be respiratory depression, which in overdose could lead to apnoea, respiratory arrest and death [EMCDDA, 2020]. This is consistent with the effects of established potent opioids such as fentanyl. The unregulated nature of the drugs market may lead to the sale of products containing inappropriately high doses of potent compounds or preparations containing ‘hot spots’ of very potent material due to inadequate blending during the cutting process. These factors increase the risk of unpredictable and severe opioid toxicity.
8.2. Krutulsky et al. [2020] described 18 post-mortem cases in the US where isotonitazene had been detected in blood, urine, or vitreous fluid. In many cases other substances, including opioids and benzodiazepines, were also detected and may have contributed to death, but 9 were negative for other opioids. In these cases, median isotonitazene concentrations were 1.75ng/mL (range 0.4ng/mL to 9.5ng/mL) in blood and 2.7ng/mL (range 0.6ng/nL to 4.0ng/mL) in urine. Many of the cases were suspected heroin users, with visible needle marks consistent with intravenous drug use and signs of opioid toxicity, including pulmonary and/or cerebral oedema, commonly present. From August 2019 to February 2020, more than 100 cases were categorised as presumptively positive for isotonitazene [Krotulski et al., 2020; DEA 2020].
8.3. In their 2020 report, the EMCDDA listed 2 deaths involving isotonitazene reported from Europe, with the UK and Germany the reporting countries. Subsequently, 3 further fatal cases were reported from Switzerland [Mueller et al., 2021). Isotonitazene concentrations in femoral whole blood ranged from 0.74ng/ml to 2.28 ng/ml, but benzodiazepines were also detected in 2 cases and ethanol in the third; these substances may also have contributed to the fatal outcomes.
8.4. In the US, 8 post-mortem blood samples were found to contain N-pyrrolidino etonitazene between January and April 2021. In 7 cases, other substances were commonly identified, including benzodiazepines, fentanyl, and methamphetamine. In one case where it was the only drug of interest, the N- pyrrolidino etonitazene concentration was 8.3 ng/ml [NPS Discovery, 2021a).
8.5. A single case of severe toxicity, including cardiac and respiratory arrest, was reported in an 18-year-old male presenting in Birmingham, UK, after reported oral use of ‘Percocet®’ purchased via the dark web. Blood and urine specimens detected N-pyrrolidino etonitazene as well as flualprazolam, flubromazepam and methadone [Pucci et al., 2021].
8.6. Etodesnitazene [NPS Discovery, 2021b], protonitazene [NPS Discovery, 2021c] and N-piperidinyl etonitazene [NPS Discovery, 2021d] have also been detected during post-mortem death investigations in the US and Canada.
8.7. Other psychoactive substances were also present in many fatal cases in which 2-benzyl benzimidazole opioids were detected in biological samples, especially other opioids including heroin or benzodiazepines such as etizolam or flualprazolam [WHO, 2020]. It may be difficult in some cases to establish which substance made the most important contribution to the death. There have, however, been several documented fatal cases where only a benzimidazole opioid was detected, emphasising the severe toxicity that can occur with these compounds. In an American study comparing fatal overdoses involving isotonitazene with those involving other synthetic opioids, isotonitazene deaths involved a larger number of other substances detected, including the benzodiazepine flualprazolam [Shover et al., 2021].
8.8. Analytical findings from 20 fatal cases involving brorphine (13 male, 7 female, median age 49, range 29 to 61 years) have been reported, most arising from the US mid-west. Fentanyl was also present in all cases and flualprazolam in 80% [Krotulski et al., 2021]. The WHO reported that at the time of writing their report, screening had suggested the presence of brorphine in more than 200 US cases, with declining trends in the frequency of positive samples between July to September 2020 and January to March 2021 [WHO 2021].
8.9. A 45 year old man treated with sertraline for depression developed euphoria and dizziness after ingesting 4 tablets purchased via the internet as ‘oxycodone’ He subsequently developed unconsciousness associated with shivering and drooling, and later diffuse myalgia, ocular clonus, tremor, and hyperrefexia with elevated creatine kinase and acute kidney injury. Brorphine was identified in tablet and patient samples. The authors speculated that the combination of brorphine and sertraline might provoke serotonin syndrome [Razinger at al., 2021].
8.10. There is no information on the chronic health effects of 2-benzyl benzimidazole or piperidinyl benzimidazolone opioids, but these are likely to resemble those of established illicit opioids such as heroin and fentanyl, including dependence [EMCDDA, 2020].
9. Social harms
9.1. There is no information on the social harms that may be caused by 2-benzyl benzimidazole or piperidinyl benzimidazolone opioids, but these are also likely to reflect the substantial social harms of established illicit opioids, such as heroin and fentanyl.
10. UK prevalence
10.1. 2-Benzyl benzimidazole opioids had not been detected in samples collected in prisons up to March 2021 (Forensic Early Warning System annual report 2020-21), but more recent data are not available. Border Force had not identified any seizures involving 2-Benzyl benzimidazole opioids or brorphine up to November 2021. The Welsh Emerging Drug & Identification of Novel Substances (WEDINOS) study, funded by Public Health Wales, provides laboratory testing of UK drug samples (such as pills, powders) submitted anonymously. WEDINOS has recorded a single detection of butonitazene in a powder sent from Colchester in March 2022 but no other 2-benzyl benzimidazole or piperidinyl benzimidazolone opioids. TICTAC Communications Ltd, which provides drug identification services to the criminal justice and healthcare sectors, has not detected any compounds in either group.
10.2. The National Poisons Information Service (NPIS) has received only one enquiry from a clinician relating to 2-benzyl benzimidazole opioids or brorphine between January 2019 and March 2022 and this involved isotonitazene. It should be noted, however, that clinicians are unlikely to be aware of the involvement of novel synthetic opioids in cases of severe opioid toxicity as the necessary sample analysis is not performed as part of routine clinical practice,
10.3. The Identification of Novel Psychoactive Substances (IONA) study is collecting biological samples from patients attending approximately 30 participating emergency departments in England, Wales and Scotland after illicit drug use. These are currently analysed by LGC Ltd. A 2-benzyl benzimidazole opioid was identified in samples from 6 of 303 patients studied between January 2021 and March 2022, all males (age range 18 to 55 years). Isotonitazene was identified in 3 cases, all from London and all reporting heroin use by smoking. All were treated with naloxone and one required intubation and ventilation. N-pyrrolidino etonitazene was identified in 3 cases, one from Scotland reporting oral ‘oxycodone’ use, one from the West Midlands reporting use of ‘Percocet’ purchased via the dark net (as also described in paragraph 8.5) and one from the East Midlands where no details of the exposure were provided. All were treated with naloxone and one was treated by intubation and ventilation. In all 6 cases where a 2-benzyl benzimidazole opioid was detected, other substances of misuse were also identified, commonly benzodiazepines and other opioids. All subsequently recovered. IONA has not identified any cases with exposure to brorphine or other piperidinyl benzimidazolone opioids.
10.4. During August 2021 there was a significant increase in presentations to the Emergency Department at St Thomas’ Hospital London with acute opioid toxicity (36 during August 2021 compared to 13 to 17 monthly over the previous 7 months). Of these, 20 required naloxone and 8 were admitted to critical care. Comprehensive toxicological screening of serum samples taken at the time of presentation was available for two of the presentations. One had collapsed after smoking ‘heroin’ and was treated successfully with intramuscular naloxone (2mg). Isotonitazene was identified in a serum sample taken at the time of presentation (concentration 0.18ng/ml) with buprenorphine metabolites and cocaine. The second was found unresponsive and treated successfully with intramuscular naloxone 0.8mg. He reported smoking a new powder earlier that day. He deteriorated within an hour of presentation to hospital and was given further naloxone, including by infusion. A serum sample taken at presentation showed isotonitazene (concentration of 0.81ng/ml) with buprenorphine, cocaine and their metabolites [De Baerdemaeker et al., 2022].
10.5. Several sources of information on fatalities involving new synthetic opioids are available. As each provides limited anonymised information, it is not possible to exclude the possibility that some fatal cases feature in more than one of these data sets.
10.6. LGC Ltd provide specialist drug analytical services for general toxicology laboratories and coroners. Samples analysed between July and November 2021 identified at least one 2-benzyl benzimidazole opioid in samples from 15 fatal overdose cases (13 isotonitazene, 3 N-pyrrolidino-etonitazene, 1 both). For isotonitazene, estimated blood concentrations, where available, ranged from 0.06ng/ml to 0.57ng/ml. Estimated blood concentration was available for 1 N-pyrrolidino etonitazene-related fatality (9ng/ml). In 14 of the 15 fatal cases, other substances of misuse (or their metabolites) were identified, including heroin, cocaine, methadone or benzodiazepines. N-pyrrolidino etonitazene was also identified in 1 further case from the West Midlands where the clinical outcome was unknown. No cases with exposure to piperidinyl benzimidazolone opioids, including brorphine, have been identified.
10.7. Fatalities involving other 2-benzimidazole opioids have also been reported from other UK forensic analytical laboratories. Toxicology UK Ltd, which undertakes work for various coroners, reported the detection of metonitazene (19ng/ml) in post-mortem femoral blood taken from a person who died in November 2021. Analytical Services International Ltd (St. George’s, University of London) reported the apparent presence of etodesnitazene (awaiting reference standard for confirmation) in biological samples and a powder analysed in the investigation of a single fatal case, as well as the presence of isotonitazene in samples from 3 non-fatal cases presenting in London in the second half of 2021. N-pyrrolidino-etonitazene was detected in samples from 3 male fatalities in Birmingham between October 2021 and January 2022.
10.8. At the end of July 2021, the Toxicology Unit at Imperial College started to screen samples from all post-mortem cases with a history of, or toxicological results indicating, potential heroin use [Nahar et al., 2021]. Over a 2-month period (August to September 2021) 101 cases were screened of which 36 (36%) were positive for isotonitazene. Approximately 55% of the isotonitazene positive cases had morphine present in the blood at sub-therapeutic concentrations or below reportable limits. This indicates that in these cases heroin exposure may not have been sufficient to cause death and that isotonitazene is likely to have made an important contribution. Of note, cocaine (or its metabolite benzoylecgonine) was also present in 34 (94%) cases, raising the possibility of cocaine contamination with isotonitazene [Patterson 2021, unpublished]. The Toxicology Unit has also identified brorphine in a single fatal case from March 2021. Brorphine was detected in stomach contents and blood along with other psychoactive drugs.
10.9. In October 2021, the National Crime Agency (Operation ROPERY) reported on 31 suspected heroin overdoses in the UK where isotonitazene had been identified analytically, 24 of which were fatal. Most occurred in areas covered by the Metropolitan Police and Thames Valley Police, with clusters of episodes across England’s South East Region. Those affected were usually males aged between 35 and 45 years who were known drug users. In most cases it appeared that heroin use by smoking was involved. Some of these cases may be the same as those described in preceding paragraphs. Operation ROPERY also reviewed results of forensic analysis of seized drugs or associated paraphernalia. More than a quarter of cocaine samples and more than half of heroin/diamorphine samples were found to contain isotonitazene as an adulterant. Metonitazene and N-pyrrolidino etonitazene has also been detected in illicit tablets in the UK.
10.10. The Office for National Statistics has published data on substances involved in annual numbers of deaths related to drug poisoning in England and Wales up to 2020. Those where a new synthetic opioid was mentioned on the death certificate were 13 for 2018, 1 for 2019 and 1 for 2020. Details on the specific substances involved is not provided and data are not yet available for 2021. Data on drug-related deaths by substance is provided by the National Records of Scotland and by the Northern Ireland Statistics and Research Agency to 2020, but these sources do not include details of new synthetic opioids.
10.11. The National Programme on Substance Abuse Deaths (NPSAD) collates information from coroners about deaths related to drugs in England and Wales. To be included in the NPSAD database, there must be the presence of one or more psychoactive substance(s) directly implicated in the death, a history of dependence or abuse of drugs or the presence of controlled drugs at post-mortem. NPSAD receives drug-related death reports from over 88% of its coroners. Cases are recorded by year of death, so figures are subject to change as more reports are confirmed. As of June 2022, NPSAD had recorded 6 cases in which isotonitazene was detected (July 2019, June 2021, two in July 2021, two in September 2021) and two cases from July and September 2021 in which N-pyrrolidino etonitazene was detected. Other psychoactive compounds were also detected in all 8 cases. Brorphine was detected in a single fatal case from March 2021.
10.12. Anonymised case-level data on drug-related poisoning deaths received from the National Records of Scotland have been examined under an ongoing arrangement for the former EU-MADNESS project. No 2-benzyl benzimidazole or piperidinyl benzimidazolone opioids were identified in finalised registration data for the period 1 January 2013 to 31 December 2020 or in provisional data for 2021 and the first quarter of 2022 in the ‘cause of death’, ‘poisons implicated in death’ or ‘other substances present in toxicology’ fields.
11. Conclusions
11.1. 2-Benzyl benzimidazole and piperidinyl benzimidazolone opioids include compounds with very high potency. Although direct evidence of the health harms of these newly emerging groups of drugs is limited, these are likely to reflect those of other potent opioids and the clinical data described to date is consistent with this. Thus, the recent availability of these compounds presents a significant potential threat to public health.
11.2. There is evidence of the recent emergence of several new synthetic opioids from these groups into illicit drug markets internationally, including isotonitazene, N-pyrrolidino-etonitazene, butonitazene, metonitazene, protonitazene, etodesnitazene, flunitazene, metodesnitazene and brorphine. Many cases of severe or fatal toxicity involving these compounds have been described in Europe and North America.
11.3. 2-Benzyl benzimidazole opioids have also been detected in the UK, where there were at least 24 fatalities involving isotonitazene and 3 involving N- pyrrolidino etonitazene during 2021. In these cases, it cannot be established that 2-benzyl benzimidazole opioids were the sole cause of death, as other compounds were also commonly detected, but they are likely to have made an important contribution in many cases.
11.4. The total number of fatalities recorded as associated with NSO is likely to be an underestimate as not all drug-related deaths are investigated using methods sufficiently sensitive to detect the involvement of these compounds. For the same reason, it took several weeks for the involvement of isotonitazene in drug-related fatalities in the UK to be recognised. Further cases may emerge if retrospective analyses are conducted. More detailed sample analysis is needed to establish the role of emerging NPS in individual drug-related deaths, as well as to provide timely information on the cause of clusters of drug deaths. Such analysis, however, is costly and adequate funding would be required.
11.5. The involvement of NSO will not be recognised in most non-fatal cases of drug-related toxicity because detailed sample analysis is not a component of usual clinical care. Several non-fatal cases of exposure have, however, been reported involving isotonitazene and N-pyrrolidino etonitazene. In some cases, the opioid antagonist naloxone has been used with subsequent clinical improvement, consistent with experimental evidence that it antagonises the effects of 2-benzyl benzimidazole opioids as it does those of established opioids.
11.6. Evidence collected in the UK suggests that isotonitazene may be an adulterant in heroin or cocaine preparations and people using these are likely to be unaware of their exposure to isotonitazene. It remains uncertain if contamination is done deliberately to enhance the effects of the drug product or if in some cases it results from accidental cross-contamination, such as from using the same equipment for cutting different drug products.
11.7. The piperidinyl benzimidazolone opioid brorphine has been involved in multiple fatalities in other countries but has been infrequently detected in the UK, although numbers may be underestimates as analyses used to investigate fatal cases may not be able to detect this compound. There is currently no evidence of the misuse of other compounds from this group.
11.8. With the exception of etonitazene and clonitazene, 2-benzyl benzimidazole opioids are not controlled via the Misuse of Drugs Act 1971, although they are subject to the Psychoactive Substances Act 2016. This is also the situation for the piperidinyl benzimidazolone opioids including brorphine. This position is not consistent with that of other potent opioids including heroin, morphine and fentanyl, which are Class A drugs of misuse. The UK is obliged to control isotonitazene, metonitazene and brorphine now that they are listed in schedule I of the United Nations Single Convention on Narcotic Drugs of 1961, as amended by the 1972 Protocol, but control of other unlisted 2-benzyl benzimidazole or piperidinyl benzimidazolone opioids that have been detected in the UK or elsewhere should also be considered.
11.9. No legitimate medical uses of 2-benzyl benzimidazole opioids have been identified in the UK or internationally, so placement in schedule 1 of the Misuse of Drugs Regulations 2001 (as amended) would be consistent with the current legal status for other opioids that do not have a legitimate medical use, such as MT-45, U-47,700 and non-pharmaceutical fentanyl analogues. Etonitazene and clonitazene are currently listed in schedule 2 – this appears to be historic as they may have been considered as having possible therapeutic value in the past. No legitimate medical use has since been identified, however, so listing these in schedule 1 is appropriate and consistent with the position of other similar compounds.
11.10. To date almost all UK evidence implicates isotonitazene and N-pyrrolidino etonitazene, although metonitazene, etodesnitazene, butonitazene and brorphine have also been detected. Other 2-benzyl benzimidazole opioids detected internationally are very likely to appear in the UK and could cause substantial health harms, so monitoring for the emergence of these compounds and their health harms in the UK remains important. In view of the potential risks they pose to public health, pre-emptive control of these compounds should be considered. In view of the numbers of compounds identified to date, there is also a future risk of misuse of new examples of 2- benzyl-benzimidazole opioids. This could also occur with piperidinyl benzimidazolone compounds, although the risk is likely to be lower as only brorphine has been detected from this group so far.
11.11. Clinical advice on management of toxicity with isotonitazene is already available for health professionals via TOXBASE, including recommendations on the use of naloxone as an antidote. However, specific information on brorphine and some other NSO considered in this report is not yet provided. Public Health England (now the Office for Health Improvements and Disparities) also issued a national patient safety alert regarding isotonitazene in August 2021. Drug treatment services are also aware of 2-benzyl benzimidazole opioids. It would be useful to make those who may use drugs better aware of these compounds and the risks they carry, especially users of heroin and cocaine. Information on isotonitazene, brorphine and related compounds does not currently feature on the ‘Frank’ website (www.talktofrank.com) and this could usefully be updated to include these compounds in the synthetic opioids section.
11.12. The ACMD has considered two options for control, either listing specific 2- benzyl benzimidazole and piperidinyl benzimidazolone compounds known to have appeared anywhere in the world as NPS or generic controls intended to ‘future-proof’ the legislation by covering known and predicted variants which appear likely to present a significant risk to health.
11.13. Specifically listing currently identified variants for control is the simpler approach but risks being overtaken in the future by the development of further variants, as has been seen in other families of NPS. The NSO that have been identified in the UK or abroad at the time of preparing this report are as follows:
-
Metonitazene
-
Protonitazene
-
Isotonitazene
-
Butonitazene
-
Flunitazene
-
Metodesnitazene (metazene)
-
Etodesnitazene (etazene)
-
N-Pyrrolidino-etonitazene (etonitazepyne)
-
N-Piperidinyl-etonitazene (etonitazepipne)
-
Brorphine
11.14. Preparing generic controls is challenging, as these have to be designed so as to avoid inadvertently including materials of legitimate pharmaceutical interest which happen to include a 2-benzyl benzimidazole or piperidinyl benzimidazolone components within their chemical structure. The generic control developed in Germany (see Annex A) for 2-benzyl benzimidazoles provides a valuable model for these compounds and wording for a UK generic derived from this is presented below.
11.15. Currently the risk that 2-benzyl benzimidazole or piperidinyl benzimidazolone compounds other than those listed in paragraph 11.13 may be misused in the UK is unknown. In the absence of a generic control these new compounds would not be classified under the Misuse of Drugs Act, but examples with opioid activity would be captured by the Psychoactive Substances Act. The advice of the ACMD is therefore that legislation based on a generic chemical description should be developed, but introduction can await the following:
(a) the outcomes of work currently being done by the ACMD to reduce barriers to legitimate research with controlled drugs
(b) the outcome of a consultation with stakeholders, including academia and the chemical and pharmaceutical industries to ensure that any proposed legislation does not produce unintended barriers to research or legitimate commercial activity
In the meantime, monitoring should continue to detect any evidence of the misuse of new 2-benzyl benzimidazole or piperidinyl benzimidazolone opioids.
12. Recommendations
Recommendation 1
The following compounds should be added to Class A of the Misuse of Drugs Act 1971, consistent with the classification of other potent opioids. As these materials have no medical use it is recommended that they should be placed in schedule 1 of the Misuse of Drugs Regulations 2001 (as amended).
-
Metonitazene
-
Protonitazene
-
Isotonitazene
-
Butonitazene
-
Flunitazene
-
Metodesnitazene (metazene)
-
Etodesnitazene (etazene)
-
N-Pyrrolidino-etonitazene (etonitazepyne)
-
N-Piperidinyl-etonitazene (etonitazepipne)
-
Brorphine
Lead: Home Office
Measure of outcome: The inclusion of the listed compounds in Class A of the Misuse of Drugs Act 1971 and schedule 1 of the Misuse of Drugs Regulations 2001.
Recommendation 2
The following compounds should be deleted from schedule 2 and added to schedule 1 of the Misuse of Drugs Regulations 2001 (as amended).
-
Etonitazene
-
Clonitazene
Lead: Home Office
Measure of outcome: The inclusion of the listed compounds in schedule 1 of the Misuse of Drugs Regulations 2001.
Recommendation 3
The ACMD recommends that a consultation should be undertaken with stakeholders, including academia and the chemical and pharmaceutical industries on the introduction of a generic control on 2-benzyl benzimidazole variants, as new examples may be encountered and could present a serious risk of harm. Following this consultation, materials covered by the generic should be added to Class A of the Misuse of Drugs Act 1971, consistent with the classification of other potent opioids. As these materials have no medical use it is recommended that they should be placed in schedule 1 of the Misuse of Drugs Regulations 2001 (as amended).
The proposed wording for the generic for addition to the Misuse of Drugs Act is as follows:
Any compound (not being a compound for the time being specified in paragraph
(a) above) structurally derived from 2-[(2-benzyl)-benzimidazol-1-yl]ethanamine by modification in any of the following ways, that is to say:
(i) By substitution at the nitrogen of the ethanamine to any extent by alkyl substituents containing up to three carbon atoms or alkenyl substituents containing up to three carbon atoms or by inclusion of the nitrogen atom (and no other atoms of the side chain) in a cyclic structure.
(ii) By substitution in the phenyl ring of the benzyl system to any extent by alkyl containing up to four carbon atoms, trifluoromethyl, alkoxy containing up to four carbon atoms, trifluoromethoxy, acetyloxy, hydroxy, cyano, thioalkyl containing up to four carbon atoms, alkylsulphonyl containing up to four carbon atoms or halogen substituents.
(iii) By substitution at the 5- or 6- positions of the benzimidazole system by nitro, acetyl, cyano, methoxy, trifluoromethyl or halogen substituents.
(iv) By substitution at the benzylic carbon by a methyl group
(v) By replacement of the benzylic carbon by a nitrogen, oxygen or sulphur atom
These modifications are subject to a maximum molecular mass of any derived compound of 500 atomic mass units.
Note: Should evidence emerge of any variants of brorphine appearing, a further generic control, requiring a similar consultation, should be considered.
Lead: Home Office
Measure of outcome: The inclusion of the described compounds in Class A of the Misuse of Drugs Act 1971 and schedule 1 of the Misuse of Drugs Regulations 2001, following appropriate consultation.
Recommendation 4
In light of the continuing emergence of NPS and particularly synthetic opioid NPS, a working group should be established to consider and provide recommendations on a UK-wide minimum standard set of post-mortem toxicology tests for apparent drug-related deaths, to include testing for relevant novel psychoactive substances to improve consistency of analysis and detection. The best practice recommendations agreed would include standards for reporting. This working group should include (but not necessarily be limited to) representation from the following:
-
Chief Coroner’s Office for England and Wales
-
Coroners Service for Northern Ireland
-
Crown Office and Procurator Fiscal Service Scotland
-
UK and Ireland Association of Forensic Toxicologists
-
London Toxicology Group
-
Faculty of Forensic and Legal Medicine
-
Office for Health Improvement and Disparities
-
Home Office Forensic Early Warning System
-
Police
-
Local drug-related deaths review partnerships
-
The ACMD
Lead: Home Office
Measure of outcome: A report detailing best practice for forensic sample analysis in the investigation of apparent drug-related deaths
Recommendation 5
Adequate funding should be made available by government to allow coroners, procurators fiscal and forensic toxicologists to follow the best practice guidelines developed via Recommendation 4.
Lead: Home Office
Measure of outcome: Consistent and appropriate forensic analysis and reporting for suspected drug-related deaths across the UK.
Recommendation 6
Information for health professionals (such as TOXBASE) and the general public (such as Frank) on the health effects of NSO should be reviewed and updated, ensuring that information is available in an appropriate format on NSO compounds including benzimidazole and piperidinyl benzimidazolone opioids and the risks that result from the inclusion of compounds of varying and sometimes very high potency in heroin preparations and counterfeit medicines.
Leads: National Poisons Information Service, UK Health Security Agency, Office for Health Improvement and Disparities
Measure of outcome: Information available for health professionals and the general public, including those with lived experience.
Annex A: International legal controls of 2-benzyl benzimidazole opioids
United Nations controls
Signatories to the United Nations’ (UN’s) drug conventions are obliged to enact national controls on materials listed within the conventions in accordance with the UN’s requirements.
Two of the 2-benzyl benzimidazole opioids – etonitazene and clonitazene – have, since the 1960s, been listed within schedule 1 of the INCB’s List of Narcotic Drugs under International Control (the ‘yellow list’ of drugs controlled under the 1961 Convention on Narcotic Drugs). These materials have, therefore, been listed by name in each signatory country’s drug legislation, including the UK’s.
In spring 2021, following the widespread appearance of isotonitazene as an opioid NPS, this material was added to the UN’s yellow list as a schedule 1 material. In addition, the WHO’s Expert Committee on Drug Dependence has recently recommended to the UN Commission on Narcotic Drugs that metonitazene should also be added to schedule 1 of the Convention. This recommendation was ratified at the 65th Session of the UN Commission on Narcotic Drugs, held in March 2022 [UNODC, 2022].
European controls
Within Europe, a ‘fast-track’ system has been established to respond to the risks presented by NPS. This involves co-ordinated submission of NPS identifications to a European early warning system, assessment of risks and, where considered appropriate, pan-European control of materials not covered by the UN drug conventions.
A directive issued in September 2020 added isotonitazene to the list of substances controlled within Europe, in advance of the UN decision reported above. Under the European Commission system, this directive came into force in early December 2020 and member states then had 6 months to bring isotonitazene under their national controls.
In light of the risk presented by synthetic opioids, the European Monitoring Centre for Drugs and Drugs Addiction (EMCDDA) has decided that any benzimidazole opioids identified via the European early warning system should be placed under their intensive monitoring system, requiring member states to report any event involving materials on the intensive monitoring list to the EMCDDA. This is intended to ensure timely provision of evidence to underpin decisions on legislation to control within Europe.
The 8 other nitazene variants encountered within Europe and reported to the EMCDDA to date are:
-
Etodesnitazene (notification 12 of 2020, issued in June 2020, material seized in March 2020)
-
Metodesnitazene (notification 17 of 2020, June 2020, internet purchase in May 2020)
-
Metonitazene (notification 30 of 2020, September 2020, internet purchase in June 2020)
-
Flunitazene (notification 44 of 2020, December 2020, internet purchase in July 2020)
-
Etonitazepyne (notification 8 of 2021, February 2021, internet purchase in February 2021)
-
Butonitazene (notification 9 of 2021, February 2021, internet purchase in February 2021)
-
Protonitazene (notification 21 of 2021, May 2021, internet purchase in January 2021)
-
Etonitazepipne (notification 1 of 2022, January 2022, recovered from scene of unexplained death)
Germany
Germany has adopted a generic control on 2-benzyl benzimidazole synthetic opioids.
German controls on NPS are set out in the Neue psychoktive Stoffe Gesetz (NpSG). Revisions to the NpSG introduced in July 2021 include a generic control on 2-benzyl benzimidazole derived materials. This addresses modifications to 3 areas of the nitazene molecule: the attachments to the nitrogen atom of the diethylamino component, substitutions onto the phenyl ring of benzyl component and substitutions at the 5- and 6- positions of the benzimidazole core.
At each of the three sites, the structural variants which merit control are listed:
-
At the diethylamino nitrogen: hydrogen or alkyl groups containing up to three carbons, or ring formation so that the nitrogen atom forms part of a piperidinyl, pyrrolidino or morpholino ring.
-
On the phenyl ring of the benzyl group: hydrogen, alkyl groups containing up to 4 carbons, alkoxy groups containing up to 4 carbons, trifluoromethoxy, acetoxy, alkylsulphonyl containing up to 4 carbons, trifluoromethyl, hydroxy, cyano and halogens.
-
At the 5- and 6- positions of the benzimidazole core: hydrogen, nitro, trifluoromethyl, methoxy, cyano, and halogens.
In addition to specifying the modifications leading to controlled status and limiting the size of some of these, an overriding upper limit has been placed on the molecular mass of resulting variants of 500 atomic mass units (u).
This approach has the advantage of controlling a broad range of variants which are known or likely to have MOR potencies equalling or exceeding that of morphine, thereby addressing the risk of a series of variants emerging in response to specific legal controls, as has been seen with other NPS families.
Specifying the type and size of substituents, together with the overall mass limit on resulting structures, avoids the risk of the generic control unintentionally including pharmaceutical materials which happen to include a 2-benzyl benzimidazole structure.
United States
The US system of drug control is based on the Controlled Substances Act (CSA), supported by the Federal Analogue Act which addresses substances similar in structure and effect to materials listed in the CSA. In addition, there is a procedure for temporary emergency scheduling for a period of 2 (extendable to 3) years to enable novel substances which present an imminent hazard rapidly to be controlled under the requirements of the CSA.
The US has been severely affected by synthetic opioid NPS such as the fentanyls and it appears that such materials have now become embedded within the US’s illicit market for heroin and other opiates. Isotonitazene was the first of the nitazenes to be identified as a threat within the US and it was rapidly controlled by means of the temporary emergency scheduling procedure in August 2020. In June 2021 isotonitazene was added to the CSA.
Since international control was placed on isotonitazene, a series of other nitazene variants have been encountered within the US. In December 2021, the US’s DEA gave notice of its intention to place a further 7 nitazene variants under temporary control by means of the emergency scheduling process. These are:
-
Butonitazene
-
Etodesnitazene
-
Flunitazene
-
Metodesnitazene
-
Metonitazene
-
Etonitazepyne
-
Protonitazene
[Note: these are the same 7 nitazene variants which had been reported by the EMCDDA at the time of the publication of the US publication of intent to schedule, reflecting the international nature of the synthetic opioid problem.]
If the DEA’s emergency scheduling proposal is approved, these 7 materials will be brought under temporary control for a period of 2 years.
Other countries
Some other countries have enacted controls on nitazenes in addition to, or in advance of, the UN’s requirements. Examples include:
-
Japan, which added isotonitazene to the list of materials controlled under its Shitei Yakubutsu (designated substances) legislation in November 2020 (in advance of the UN requirement) and metonitazene in October 2021.
-
Sweden, which has recently added metonitazene to its list of controlled materials.
-
Canada, which includes within Part 13 of schedule 1 of its Controlled Drugs and Substances Act “benzimidazoles…their derivatives….including…” with etonitazene and clonitazene cited as examples, which is being interpreted to include other narcotic nitazene variants.
Annex B: International legal controls of brorphine
Following a review and recommendation from the WHO’s Expert Committee on Drug Dependence in October 2021, the UN Commission on Narcotic Drugs voted on 16 March 2022 to add brorphine to schedule 1 of the 1961 Convention on Narcotic Drugs.
Prior to the decision to place brorphine under international control, some countries had already acted to impose control under their national drug legislation.
These include the US where brorphine had begun to be identified in the illicit opioid market in mid-2019. In light of the perceived imminent hazard to public safety, the US’s DEA announced their intention to temporarily place brorphine in schedule 1 of the Controlled Substances Act on 3 December 2020 and this came into effect on 1 March 2021. This temporary control will be subsumed by the US’s response to the international control.
Other countries applying national controls in advance of the UN requirement include Japan which had placed brorphine under control as a Designated Substance on 19 January 2022 and Sweden which had controlled brorphine as a schedule 1 material.
On 1 June 2021 Italy added it to their list of controlled materials, as a material structurally similar to bezitramide [Ministero della Salute, 2022].
Annex C: Chemical structure of isotonitazene and related 2-benzyl benzimidazole opioids
Benzylic carbon
Can be substituted or replaced with N, O or S
Nitrogen tail
Typically diethylaminoethyl, but can be pyrrolidinylethyl (such as pyrrolidino etonitazene) or piperidinylethyl
C5-substituent
Typically nitro for greatest potency, but unsubstituted also seen (such as etodesnitazene)
Benzyl substituent
Greatest variety seen here with ethoxy replaced by chloro (clonitazene), fluoro (flunitazene), methoxy (metonitazene), i-propoxy (isonitazene), propoxy (protonitazene), butoxy (butonitazene) and hydrogen (nitazene)
| Nitrogen tail | Benzyl substituent | C5-substituent | |
|---|---|---|---|
| butonitazene | N(CH2CH3)2 | 4-O(CH2)3CH3 | 5-NO2 |
| clonitazene | N(CH2CH3)2 | 4-Cl | 5-NO2 |
| etonitazene | N(CH2CH3)2 | 4-OCH2CH3 | 5-NO2 |
| flunitazene | N(CH2CH3)2 | 4-F | 5-NO2 |
| isotonitazene | N(CH2CH3)2 | 4-OCH(CH3)2 | 5-NO2 |
| methylnitazene | N(CH2CH3)2 | 4-CH3 | 5-NO2 |
| metonitazene | N(CH2CH3)2 | 4-OCH3 | 5-NO2 |
| nitazene | N(CH2CH3)2 | H | 5-NO2 |
| protonitazene | N(CH2CH3)2 | 4-O(CH2)2CH3 | 5-NO2 |
| acetoxynitazene | N(CH2CH3)2 | 4-OCOCH3 | 5-NO2 |
| bronitazene | N(CH2CH3)2 | 4-Br | 5-NO2 |
| bronitazene | N(CH2CH3)2 | 4-CH2CH3 | 5-NO2 |
| bronitazene | N(CH2CH3)2 | 4-(CH2)2CH3 | 5-NO2 |
| bronitazene | N(CH2CH3)2 | 4-C(CH3)3 | 5-NO2 |
| methylthionitazene | N(CH2CH3)2 | 4-SCH3 | 5-NO2 |
| ethylthionitazene | N(CH2CH3)2 | 4-SCH2CH3 | 5-NO2 |
| N,N-dimethylamino etonitazene | N(CH3)2 | 4-OCH2CH3 | 5-NO2 |
| N-desethyl isotonitazene | NHCH2CH3 | 4-OCH(CH3)2 | 5-NO2 |
| etonitazepyne | N-pyrrolidino | 4-OCH2CH3 | 5-NO2 |
| etonitazepipne | N-piperidino | 4-OCH2CH3 | 5-NO2 |
| desnitazene | N(CH2CH3)2 | H | H |
| metodesnitazene | N(CH2CH3)2 | 4-OCH3 | H |
| etodesnitazene | N(CH2CH3)2 | 4-OCH2CH3 | H |
| 4-propoxydesnitazene | N(CH2CH3)2 | 4-O(CH2)2CH3 | H |
| 4-isopropoxydesnitazen e | N(CH2CH3)2 | 4-OCH(CH3)2 | H |
| 4-isopropoxydesnitazen e | N-pyrrolidino | 4-OCH2CH3 | H |
| 4-isopropoxydesnitazen e | N-piperidino | 4-OCH2CH3 | H |
| 5-cyanoetonitazene | N(CH2CH3)2 | 4-OCH2CH3 | 5-CN |
| 5-acetyletonitazene | N(CH2CH3)2 | 4-OCH2CH3 | 5-COCH3 |
| 3,4-dimethoxynitazene | N(CH2CH3)2 | 3,4-di-OCH3 | 5-NO2 |
| etoetonitazene | N(CH2CH3)2 | 4-O(CH2)2OCH2CH3 | 5-NO2 |
Annex D: Chemical structure of 4-piperidinyl benzimidazolones
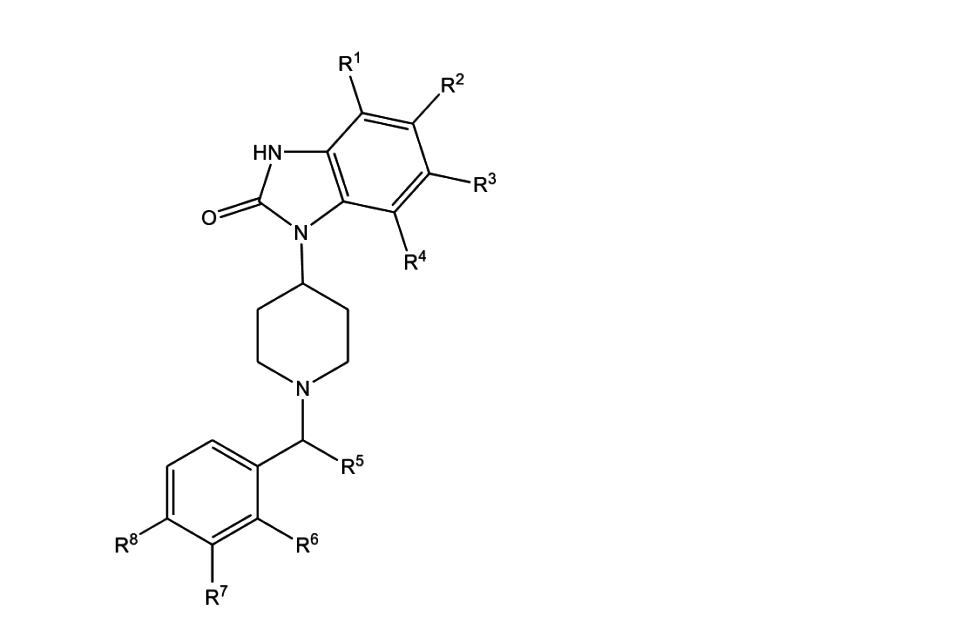
Brophine: R1-R4, R6, R7 all = H; R5=Me, R8=Br
Annex E: List of abbreviations used in this report
| Abbreviation | Name |
|---|---|
| ACMD | Advisory Council on the Misuse of Drugs |
| CFSRE | Centre for Forensic Science Research and Education |
| DEA | Drug Enforcement Agency (United States) |
| DHSC | Department of Health and Social Care |
| DOR | Delta opioid receptor |
| DSTL | Defence Science and Technology Laboratory |
| EU- MADNESS | European-wide, Monitoring, Analysis and knowledge Dissemination project |
| EMCDDA | European Monitoring Centre for Drugs and Drug Addiction |
| FEWS | Forensic Early Warning System |
| INCB | International Narcotics Control Board |
| IONA | Identification of Novel Psychoactive Substances study |
| KOR | Kappa opioid receptor |
| MDA | Misuse of Drugs Act 1971 |
| MDR | Misuse of Drugs Regulations 2001 |
| MOR | Mu opioid receptor |
| MHRA | Medicines and Healthcare products Regulatory Agency |
| NCA | National Crime Agency |
| NISRA | Northern Ireland Statistics and Research Agency |
| NPSAD | National Programme on Substance Abuse Deaths |
| NPIS | National Poisons Information Service |
| NPS | Novel Psychoactive Substances |
| NRS | National Records of Scotland |
| NSO | New Synthetic Opioids |
| OHID | Office for Health Improvement and Disparities |
| PSA | Psychoactive Substances Act 2016 |
| UK | United Kingdom |
| UNODC | United Nations Office of Drugs and Crime |
| US | United States |
| WEDINOS | Welsh Emerging Drug & Identification of Novel Substances |
| WHO | World Health Organization |
Annex F: ACMD membership at the time of publication
Dr Ann Sullivan - Consultant physician in HIV and sexual health
Dr Anne Campbell - Reader in substance use and mental health and co-director of the Drug and Alcohol Research Network at Queens University Belfast
Dr Carole Hunter - Lead pharmacist at the alcohol and drug recovery services at NHS Greater Glasgow and Clyde
Dr David Wood - Consultant physician and clinical toxicologist at Guy’s and St Thomas’ and Honorary reader in clinical toxicology at King’s College London
Professor David Taylor - Professor of psychopharmacology, King’s College, London
Dr Derek Tracy - Medical director of West London NHS Trust
Dr Emily Finch - Clinical director of the Addictions Clinical Academic Group and a consultant psychiatrist for South London and Maudsley NHS Trust
Professor Graeme Henderson - Professor of pharmacology at the University of Bristol
Mr Harry Shapiro - Director – DrugWise
Dr Hilary Hamnett - Senior lecturer in forensic science, University of Lincoln
Professor Judith Aldridge - Professor of criminology at the University of Manchester
Dr Kostas Agath - Consultant psychiatrist (addictions), Change Grow Live Southwark
Mr Lawrence Gibbons - Head of drug threat – National Crime Agency Intelligence Directorate – Commodities
Mr Mohammed Fessal - Chief pharmacist, Change Grow Live
Professor Owen Bowden- Jones - Chair of Advisory Council on the Misuse of Drugs,Consultant psychiatrist, Central North-West London NHS Foundation Trust
Dr Paul Stokes - Senior clinical lecturer in mood disorders, King’s College, London
Mr Rob Phipps - Former head of Health Development Policy Branch, Department of Health, Social Services and Public Safety, Northern Ireland
Dr Richard Stevenson - Emergency medicine consultant, Glasgow Royal Infirmary
Professor Roger Knaggs - Associate professor in clinical pharmacy practice at the University of Nottingham
Ms Rosalie Weetman - Public health lead (alcohol, drugs and tobacco), Derbyshire County Council
Professor Sarah Galvani - Professor of social research and substance use at Manchester Metropolitan University
Professor Simon Thomas - Consultant physician and clinical pharmacologist, Newcastle Hospitals NHS Foundation Trust and professor of clinical pharmacology and therapeutics, Newcastle University
Professor Tim Millar - Professor of substance use at the University of Manchester
Annex G: ACMD NPS Committee membership, at time of publication
Dr Ann Sullivan - Consultant physician in HIV and sexual health
Dr Anne Campbell - Reader in substance use and mental health and co-director of the Drug and Alcohol Research Network at Queens University Belfast
Dr Kostas Agath - Consultant psychiatrist (addictions), Change Grow Live Southwark
Mr Paul Bunt - Director of Casterton Event Solutions Ltd, Former Drug Strategy Manager for Avon and Somerset Constabulary
Mr Peter Cain - Drugs Scientific Advisor, Eurofins Forensic Services
Dr Caroline Copeland - Lecturer in Pharmaceutical Medicine at King’s College London, and the Director of the National Programme on Substance Abuse Deaths
Mr John Corkery - Senior Lecturer in Pharmacy Practice at University of Hertfordshire; mortality and epidemiological lead for EU-MADNESS project
Mr Lawrence Gibbons - Head of drug threat – National Crime Agency Intelligence Directorate – Commodities
Dr Hilary Hamnett - Senior lecturer in forensic science, University of Lincoln
Professor Graeme Henderson - Professor of Pharmacology at the University of Bristol
Professor Stephen Husbands - Professor of Medicinal Chemistry, University of Bath
Professor Roger Knaggs - Associate professor in clinical pharmacy practice at the University of Nottingham
Professor Fiona Measham - Professor and chair in criminology, University of Liverpool; co-founder and co-director, the Loop
Mr Harry Shapiro - Director – DrugWise
Dr Richard Stevenson - Emergency Medicine Consultant, Glasgow Royal Infirmary
Professor Simon Thomas - NPS Committee Chair, Consultant physician and clinical pharmacologist, Newcastle Hospitals NHS Foundation Trust and Professor of Clinical Pharmacology and Therapeutics, Newcastle University
Mr Ric Treble - Retired Laboratory of the Government Chemist (LGC) expert
Dr Mike White - Former Forensic Intelligence Adviser
Dr David Wood - Consultant physician and clinical toxicologist at Guy’s and St Thomas’ and Honorary reader in clinical toxicology at King’s College London
Annex H: Quality of evidence
Range of evidence
Evidence gathered was considered in line the ACMD’s standard operating procedure for quality of evidence [ACMD, 2020].
To evidence the identification and prevalence in the UK of the new synthetic opioids considered in this report, the ACMD’s NPS Committee wrote to stakeholders requesting available data on the 13 substances. Responses were received from the following (which include submissions of ‘no data held’ and anecdotal evidence:
External agencies:
-
EU-MADNESS project
-
NCA
-
NISRA
-
NPSAD
-
NRS
-
Tictac
-
Eurofins forensics
-
LGC forensics
-
WEDINOS Government departments:
-
Border Force Intelligence Analysis (Home Office)
-
FEWS (Defence Science and Technology Laboratory)
-
MHRA
This report also draws on evidence from peer-reviewed literature (UK and international publications) and government reports. The ACMD also considered international approaches when drafting its recommendations.
Quality of evidence (design, limitations, bias)
For the new synthetic opioids referred to in this report, evidence of their availability and harm has been sought, allowing the ACMD to make an informed recommendation on their classification and schedule.
Many agencies and departments returned ‘no data held’ for most of the compounds considered in this report. It is important to note that owing to the ‘novelty’ of all of these substances, forensic testing is limited and inconsistent across the UK and as a result, information being fed into reporting agencies that were approached will not be representative. This is supported by anecdotal reports. As reports have identified some of these substances elsewhere in Europe, there is potential availability of these substances in the UK.
References
Achat-Mendes, C, Valdez, GR, Platt, DM, Rowlett, JK, Spealman, RD (2009). ‘Intravenous self-administration of etonitazene alone and combined with cocaine in rhesus monkeys: comparison with heroin and antagonism by naltrexone and naloxonazine’, Psychopharmacology, 204(3), pages 489-498
Anonymous (1959). ‘Addictiveness of two benzimidazole derivatives; preliminary report’. https://archive.org/details/DOC_0000151874/mode/1up (viewed on 31 May 2022)
Bao Y, Meng S, Shi J, Lu L. (2019). ‘Control of fentanyl-related substances in China’, Lancet Psychiatry. 2019;6(7):e15
Barenholtz E, Krotulski AJ, Morris P, Fitzgerald ND, Le A, Papsun DM, Logan BK, Hahn WE, Goldberger BA, Cottler LB, Palamar JJ (2021). ‘Online surveillance of novel psychoactive substances (NPS): Monitoring Reddit discussions as a predictor of increased NPS-related exposures’, Int J Drug Policy. 98:103393. https://doi.org/10.1016/j.drugpo.2021.103393. Epub 2021 Aug 5. PMID: 34365124; PMCID: PMC8671170
Barnett, A, Goldstein, J, Fiedler, E, Taber, R. (1975). ‘Etonitazene-induced rigidity and its antagonism by centrally acting muscle relaxants’, European Journal of Pharmacology, 30(1), pages 23-28
Blanckaert P, Cannaert A, Van Uytfanghe K, Hulpia F, Deconinck E, Van Calenbergh S, Stove C (2020). ‘Report on a novel emerging class of highly potent benzimidazole NPS opioids: Chemical and in vitro functional characterization of isotonitazene’, Drug Test Anal. 2020 Apr;12(4): pages 422-430. https://doi.org/10.1016/j.drugpo.2021.103393. Epub 2020 Jan 8. PMID: 31743619.
Blanckaert, P, Balcaen, M, Vanhee, C, and others (2021). ‘Analytical characterization of “etonitazepyne”, a new pyrrolidinyl-containing 2- benzylbenzimidazole opioid sold online’, Drug Test
Anal. 2021; 13(9): 1627– 1634. https://doi.org/10.1002/dta.3113 (viewed on 31 May
2022)
Brishty SR, Hossain MJ, Khandaker MU, Faruque MRI, Osman H, Rahman SMA (2021). ‘A Comprehensive Account on Recent Progress in Pharmacological Activities of Benzimidazole Derivatives’, Front Pharmacol. 2021;12:762807. Published 2021 Nov 3. https://doi.org/10.3389/fphar.2021.762807
Bromig, G. (1958). ‘Über neue starkwirkende Analgetika und ihre klinische Erprobung’, Klinische Wochenschrift, 36(20), pages 960–963. https://doi.org/10.1007/BF01486702
Catalani, V., Arillotta, D., Corkery, J. M., Guirguis, A., Vento, A., Schifano, F. (2021). ‘Identifying new/emerging psychoactive substances at the time of COVID-19: a web-based approach’. Frontiers in Psychiatry, 11, 632405.
Cornelissen JC, Blough BE, Bohn LM, Negus SS, Banks ML (2021). Some effects of putative G-protein biased mu-opioid receptor agonists in male rhesus monkeys’, Behavioural Pharmacology; 2021, 32(5), pages 453-458
De Baerdemaeker, K. S., Dines, A. M., Hudson, S., Sund, L. J., Waters, M. L., Hunter, L. J., … & Dargan, P. I. (2022). ‘Isotonitazene, a novel psychoactive substance opioid, detected in two cases following a local surge in opioid overdoses’. QJM: An International Journal of Medicine.
Desert News (2003). Chemist charged in drug case. https://www.deseret.com/2003/6/3/19726544/chemist-charged-in-drug-case (viewed on 31 May 2022)
Drug Enforcement Administration (2020). Schedules of Controlled Substances: Temporary Placement of Brorphine in Schedule I. Federal Register / Vol. 85, No. 233
/ Thursday, 3 December 2020 2020-26301.pdf (govinfo.gov) (viewed 31 May 2022)
Drug Enforcement Administration (2021a). ‘Benzimidazole-opioids’, https://www.deadiversion.usdoj.gov/drug_chem_info/benzimidazole-opioids.pdf (viewed 31 May 2022)
Drug Enforcement Administration (2021b). ‘Brorphine’, https://www.deadiversion.usdoj.gov/drug_chem_info/brorphine.pdf (viewed 31 May 2022)
EMCDDA (2020). ‘EMCDDA technical report on the new psychoactive substance N,N-diethyl-2-[[4-(1-methylethoxy)phenyl]methyl]-5-nitro-1H-benzimidazole-1- ethanamine (isotonitazene)’, https://www.emcdda.europa.eu/publications/technical- reports/technical-report-isotonitazene_en (viewed 31 May 2022)
Gill H, Kelly E, Henderson G (2019). ‘How the complex pharmacology of the fentanyls contributes to their lethality’, Addiction. 2019;114(9): pages1524-1525. https://doi.org/10.1111%2Fadd.14614
Ginsburg BC, Lamb RJ (2018). ‘Frustration stress (unexpected loss of alternative reinforcement) increases opioid self-administration in a model of recovery’, Drug Alcohol Depend. 2018:182; pages 33-39
Grafinger KE, Wilde M, Otte L, Auwärter V (2021). ‘Pharmacological and metabolic characterization of the novel synthetic opioid brorphine and its detection in routine casework,’ Forensic Science International, 2021, 327, 110989
Gross F, Turrian H (1957). ‘Über Benzimidazolderivate mit starker analgetischer Wirkung’, Experientia. 1957;13(10):pages 401-403
Hughes, J, Kosterlitz, HW, Leslie, FM (1975). ‘Effect of morphine on adrenergic transmission in the mouse vas deferens. Assessment of agonist and antagonist potencies of narcotic analgesics’, British Journal of Pharmacology, 53(3), pages 371- 381
Hunger A, Kebrle J, Rossi A, Hoffmann K (1957). ‘Synthese basisch substituierter, analgetisch wirksamer Benzimidazol-derivate’, Experientia. 1957;13(10):pages 400- 401
Hunger, A, Kebrle, J, Rossi, A, Hoffmann, K (1960b). ‘Benzimidazole-Derivate und verwandte Heterocyclen III. Synthese von 1-Aminoalkyl-2-benzyl-nitro- benzimidazolen’, Helvetica Chimica Acta, 43(4), pages 1032-1046
International Narcotics Control Board (2021). ‘List of narcotic drugs under international control’ (Yellow list), https://www.incb.org/documents/Narcotic- Drugs/Yellow_List/60th_edition/60_Yellow_List_EN_rev1.pdf (viewed 31 May 2022)
Janssen PAJ (1965). ‘Benzimidazolinyl piperidines’, Research Laboratorium Dr. C. Janssen NV, Belgium. US3196157A, https://worldwide.espacenet.com/patent/search/family/026897320/publication/US319 6157A?q=pn%3DUS3196157A (viewed 31 May 2022)
Janssen, PAJ and Van der Eycken, CAM (1968). ‘The chemical anatomy of potent morphine-like analgesics’, In Drugs Affecting the Central Nervous System (Burger, A., Ed.), Vol. 2, pages 25-60, Marcel Dekker, Inc., New York
Jong L, Zaveri N, Toll L (2004). ‘The design and synthesis of a novel quinolizidine template for potent opioid and opioid receptor-like (ORL1, NOP) receptor ligands’, Bioorganic & Medicinal Chemistry Letters 2004, 14, pages 181-185
Kennedy NM, Schmid CL, Ross NC, Lovell KM, Yue Z, Chen YT, Cameron MD, Bohn LM, Bannister TD (2018). ‘Optimization of a series of mu opioid receptor (MOR) agonists with high G protein signaling bias’, Journal of Medicinal Chemistry 61 (19):8895-8907. https://doi.org/10.1021/acs.jmedchem.8b01136
Krotulski AJ, Papsun DM, Kacinko SL, Logan BK (2020). ‘Isotonitazene quantitation and metabolite discovery in authentic forensic casework’, J Anal Toxicol. 2020; 44: pages 520-30, https://doi.org/10.1093/jat/bkaa016 (viewed 31 May 2022)
Krotulski AJ, Papsun DM, Noble C, Kacinko SL, Logan BK (2021). ‘Brorphine- Investigation and quantitation of a new potent synthetic opioid in forensic toxicology casework using liquid chromatography-mass spectrometry’, J Forensic Sci. 2021 Mar;66(2): pages 664-676
Kudla, L, Bugno, R, Podlewska, S, Szumiec, L, FDAWiktorowska, L, Bojarski, AJ, Przewlocki, R (2022). ‘Comparison of an Addictive Potential of mu-Opioid Receptor Agonists with G Protein Bias: Behavioral and Molecular Modeling Studies’, Pharmaceutics 2022, 14, 55, https://doi.org/10.3390/pharmaceutics14010055 (viewed 31 May 2022)
Lamy FR, Daniulaityte R, Barratt MJ, Lokala U, Sheth A, Carlson RG (2021). ‘“Etazene, safer than heroin and fentanyl’: Non-fentanyl novel synthetic opioid listings on one darknet market’, Drug Alcohol Depend. 2021 Aug 1;225:108790. https://doi.org/10.1016/j.drugalcdep.2021.108790. Epub 2021 May 31. PMID: 34091156
Ministero della Salute, 2022. Act detail, Find Rules & Competitions - Health Regulations (salute.gov.it) (viewed 31 May 2022)
Mueller, F., Bogdal, C., Pfeiffer, B., Andrello, L., Ceschi, A., Thomas, A., Grata, E. (2021). ‘Isotonitazene: fatal intoxication in three cases involving this unreported novel psychoactive substance in Switzerland’. Forensic Science International, 320, 110686.
Nahar LK, Andrews R, Paterson S (2021). ‘Isotonitazene: a new synthetic opioid in the UK’, British Medical Journal, 2021 Dec 24;375:n3143. https://doi.org/10.1136/bmj.n3143. PMID: 34952838
NPS Discovery (2021a). New high potency synthetic opioid N-pyrrolidino etonitazene (etonitazepyne) linked to overdoses across United States (viewed 31 May 2022)
NPS Discovery (2021b). Etodesnitazene — new synthetic opioid identified during forensic death investigations in the United States and Canada, (viewed 31 May 2022)
NPS Discovery (2021c). New Synthetic Opioid protonitazene increasing in prevalence as ‘nitazenes’ gain traction across the United States and Canada, (viewed 31 May 2022)
NPS Discovery (2021d). N-Piperidinyl Etonitazene, (viewed 31 May 2022)
NPS Discovery (2022). Trend report: Q1 2022 – NPS opioids in the United States, (viewed 31 May 2022)
Pardeshi VAS, Chundawat NS, Pathan SI, Sukhwal P, Tejpal Pal Singh Chundawat TPS, Singh GP (2021). ‘A review on synthetic approaches of benzimidazoles’, Synthetic Communications, 51:4, pages 485-513
Pert CB and Snyder SH (1973). ‘Properties of opiate-receptor binding in rat brain’, Proceedings of the National Academy of Sciences, USA 70(8), pages 2243-2227
Pucci, M., Hudson, S., Hill, S. L., Thomas, S. H. L. (2022). ‘Severe toxicity involving N-pyrrolidino etonitazene in the United Kingdom - a case report’. Clinical Toxicology, 60(4), 533-534.
Razinger G, Grenc D, Pezdir T, Kranvogl R, Brvar M (2021). ‘Severe rhabdomyolysis and acute kidney failure due to synthetic opioid brorphine exposure in combination with chronic sertraline therapy’, European Journal of Clinical Pharmacology, 2021, 77, pages 1759-1761
Schmid CL, Kennedy NM, Ross NC, Lovell KM, Yue Z, Morgenweck J, Cameron MD, Bannister TD, Bohn LM (2017). ‘Bias Factor and Therapeutic Window Correlate to Predict Safer Opioid Analgesics’, Cell. 2017;171(5): pages 1165-1175
Schwienteck KL, Faunce KE, Rice KC, Obeng S, Zhang Y, Blough BE, Grim TW, Negus SS, Banks ML (2019). ‘Effectiveness comparisons of G-protein biased and unbiased mu opioid receptor ligands in warm water tail-withdrawal and drug discrimination in male and female rats’, Neuropharmacology, 2019, 150, pages 200- 209. https://doi.org/10.1016/j.neuropharm.2019.01.020 (viewed 31 May 2022)
Shover CL, Falasinnu TO, Freedman RB, Humphreys K. Emerging Characteristics of Isotonitazene-Involved Overdose Deaths: A Case-Control Study. J Addict Med. 2021 Sep-Oct 01;15(5):429-431.
Sorokin VI, Ponkratov KV, Drozdov MA. Etonitazene Encountered in Moscow. Microgram 1999; 32(9): 239-244
Siczek, M, Zawadzki, M, Siczek, M, Chłopaś-Konowałek, A, Szpot, P (2020). ‘Etazene (N,N-diethyl-2-{[(4-ethoxyphenyl)methyl]-1H-benzimidazol-1-yl}-ethan-1- amine (dihydrochloride)): a novel benzimidazole opioid NPS identified in seized material: crystal structure and spectroscopic characterization’, Forensic Toxicology, https://doi.org/10.1007/s11419-020-00552-9. ISSN 1860-8973
Tang AH (1982). ‘The etonitazene-dependent rhesus monkey as a model to study narcotic agonist and antagonist activities’, NIDA Res Monogr. 1982 Apr;41:200-7. PMID: 6126812
Ujváry, I., Christie, R., Evans-Brown, M., Gallegos, A., Jorge, R., de Morais, J., Sedefov, R. (2021). DARK classics in chemical neuroscience: etonitazene and related benzimidazoles. ACS Chemical Neuroscience, 12(7), 1072-1092
United Nations Office on Drugs and Crime (2020). ‘UNODC EWA: Brorphine, a newly emerging synthetic opioid detected in post-mortem cases’, https://www.unodc.org/LSS/Announcement/Details/21380025-9afb-494f-ad1f- d4a371489a65 (viewed 31 May 2022)
United Nations Office on Drugs and Crime (1961). ‘Single Convention on Narcotic Drugs’, 1961, https://www.unodc.org/unodc/en/treaties/single-
convention.html?ref=menuside (viewed 31 May 2022)
United Nations Office on Drugs and Crime (2021). ‘Current NPS threats’, Volume IV. https://www.unodc.org/documents/scientific/NPS_threats_IV_web.pdf (viewed 31 May 2022)
United Nations Office on Drugs and Crime (2022). ‘Three NPS “scheduled” at the 65th Session of the Commission on Narcotic Drugs’, https://www.unodc.org/LSS/Announcement/Details/71877433-6d31-4cea-949e- 198f8575f48d (viewed 31 May 2022)
Vandeputte MM, Van Uytfanghe K, Layle NK, St Germaine DM, Iula DM, Stove CP (2021). ‘Synthesis, Chemical Characterization, and μ-Opioid Receptor Activity Assessment of the Emerging Group of “Nitazene” 2-Benzylbenzimidazole Synthetic Opioids’, ACS Chem Neurosci, 2021 Apr 7;12(7): pages1241-1251
Vandeputte MM, Cannaert A, Stove CP (2020). ‘In vitro functional characterization of a panel of non-fentanyl opioid new psychoactive substances’, Archives of Toxicology 94 (11):3819-3830, https://doi.org/10.1007/s00204-020-02855-7 (viewed 31 May 2022)
Vandeputte MM, Krotulski AJ, Papsun DM, Logan BK, Stove CP (2021). ‘The rise and fall of isotonitazene and brorphine: two recent stars in the synthetic opioid firmament’, Journal of Analytical Toxicology – In press, https://doi.org/10.1093/jat/bkab082 (viewed 31 May 2022)
Verougstraete N, Vandeputte MM, Cannaert A, Stove C, Verougstraete N, Verstraete AG, Lyphout C, Hulpia F, Van CS, Verstraete AG (2021). ‘First report on brorphine: the next opioid on the deadly new psychoactive substance horizon?’, Journal of Analytical Toxicology, 44 (9): pages 937-946. https://doi.org/10.1093/jat/bkaa094 (viewed 31 May 2022)
Walker EA and Young AM (2001). ‘Differential tolerance to antinociceptive effects of mu opioids during repeated treatment with etonitazene, morphine, or buprenorphine in rats’, Psychopharmacology (Berl), 2001 Mar 1;154(2):pages 131-42
World Health Organisation (2020). ‘Critical review report: isotonitazene’, https://www.who.int/docs/default-source/controlled-substances/43rd- ecdd/isonitazene-43rd-final-complete-a.pdf?sfvrsn=c98d9c9_2 (viewed 31 May 2022)
World Health Organisation (2021). ‘Critical review report: Brorphine’, https://www.who.int/publications/m/item/brorphine-critical-review-report (viewed 31 May 2022)
Zhang, L, Walker, E, Sutherland II, J and others (2000). ‘Discriminative stimulus effects of two doses of fentanyl in rats: pharmacological selectivity and effect of training dose on agonist and antagonist effects of mu opioids’, Psychopharmacology 148, pages 136-145
