Acute hepatitis B: national enhanced surveillance report January to March 2023
Updated 24 April 2025
Applies to England
Background
Hepatitis B is a blood borne infection of the liver caused by the hepatitis B virus (HBV). The virus can cause an acute illness characterised by nausea, malaise, abdominal pain, and jaundice but can also result in a chronic persistent infection that is associated with an increased risk for chronic liver disease and hepatocellular carcinoma.
Surveillance of acute hepatitis B is essential as this represents recent transmission of HBV and therefore incidence. Surveillance guides targeted prevention and control activities such as the selective immunisation programme of people at higher risk of exposure and complications of HBV infection.The quarterly reporting of enhanced molecular surveillance of acute hepatitis B is based on clinical reports of acute cases to regional Health Protection Teams (HPTs) in UK Health Security Agency (UKHSA) and corresponding samples being submitted to the UKHSA Blood Borne Virus Unit (BBVU) in the Virus Reference Department (VRD) at Colindale.
Methods
Acute infectious hepatitis is a notifiable infectious disease, and clinicans suspecting an acute hepatitis B diagnosis are required to report the case to their regional HPT. Information on individuals reported with acute hepatitis B is capured through the UKHSA case management system (HPZone). Following the reporting of clusters of acute hepatitis B in 2015-2016, an HPZone Context ‘Acute hepatitis B’ was added for monitoring of acute cases.
For surveillance purposes an acute hepatitis B infection is defined as HBsAg positive and anti-HBc IgM positive with abnormal liver function tests plus a clinical pattern consistent with acute viral hepatitis. As acute hepatitis B flares can occur during chronic persistent infection, HBV core avidity testing may be done to differentiate between an acute and chronic infection.
In 2016, VRD re-introduced HBV anti-core avidity testing alongside genotyping of samples from patients diagnosed with acute hepatitis B, a service that is offered free of charge. Hospital microbiology and virology departments are requested to send samples to Colindale for confirmation, avidity testing and genotyping as part of the national enhanced surveillance of acute hepatitis B (see Acute hepatitis B: guide to national enhanced surveillance).
Records of any ‘Acute Hepatitis B’ reported between January and March 2023 were collated from HPZone, and linked to samples submitted to the UKHSA BBVU at Colindale, using a combination of Surname, First name, Date of birth, Sex, and NHS number.
Results
Between January and March 2023, 42 cases of acute hepatitis B were entered onto HPZone across England (confirmed, probable and possible). Overall, the cases entered on HPZone have been declining since 2018 from 297 that year compared to 213 in 2022. Monthly cases since 2018 in England are shown in figure 1. The number of cases observed during 2020 (199) and 2021 (201) are likely to be lower as an impact of SARS-CoV-2. The decline in the number of acute hepatitis cases is likely to be multifactorial including, but not restricted to: disruption to and/or reconfiguration of health services reducing access to testing; and the impact of social and physical distancing measures resulting in fewer opportunities for onward transmission.
Figure 1. Cumulative cases of acute hepatitis B in England entered on HPZone: 2018 to March 2023
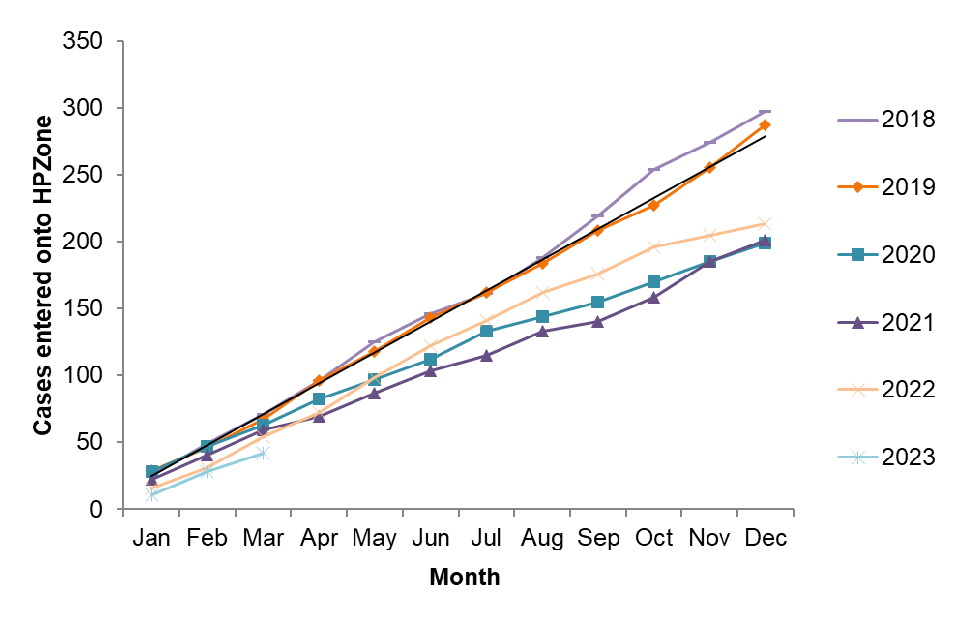
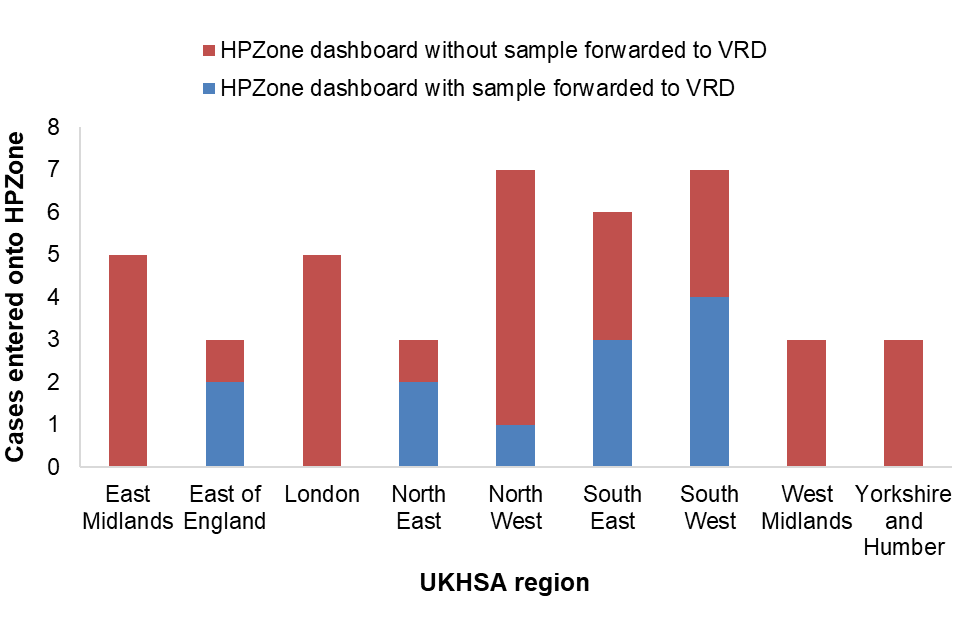
Figure 2 shows the number of cases entered onto HPZone by month and the number where a residual sample was recieved by the laboratory for avidity and molecular characterisation. Figure 3 shows this distribution by region.
Figure 2. January to March 2023 cases by month entered onto HPZone with or without a sample forwarded to VRD for avidity and molecular characterisation
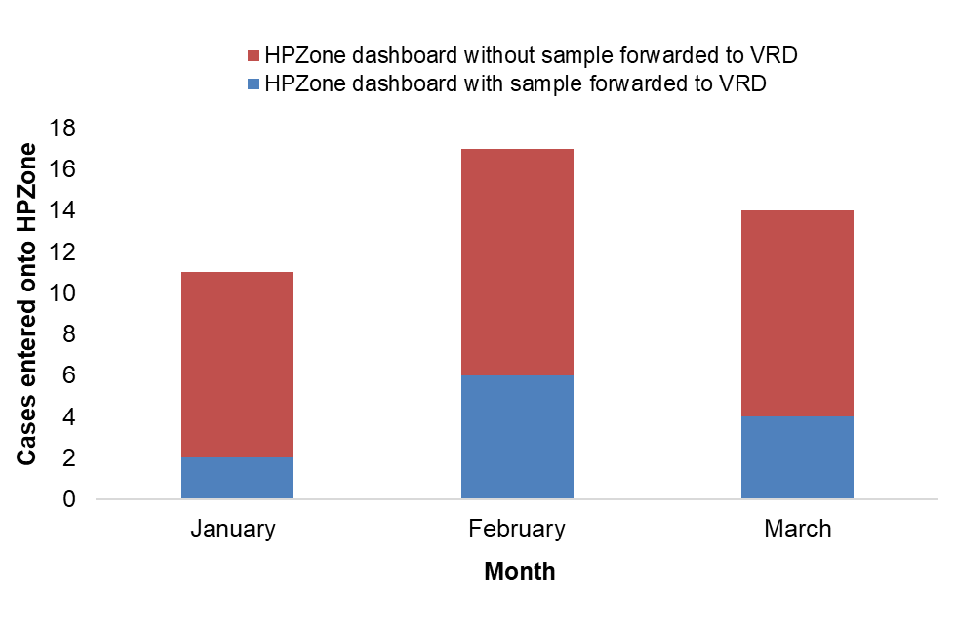
Figure 3. January to March 2023 cases by UKHSA region entered onto HPZone with or without a sample forwarded to VRD for avidity and molecular characterisation
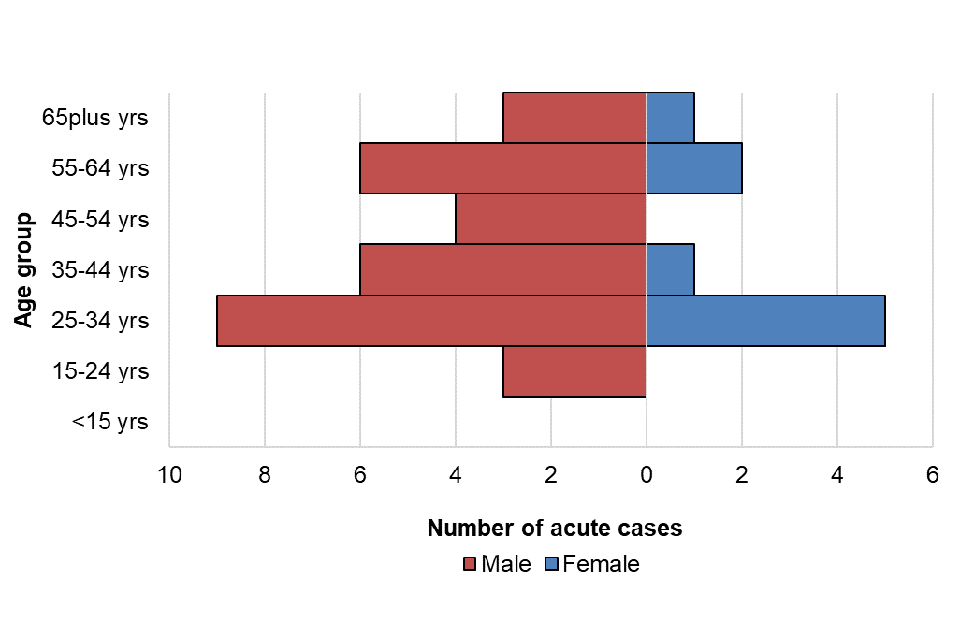
For cases entered onto HPZone between January and March 2023, age and sex was well reported (95%). Where sex was known, males accounted for 78% of cases (31 out of 40) and females 23% (9 out of 40). The median age of persons with acute HBV infection was 37 years (IQR: 30 to 59); 37 (IQR: 30 to 59) for males and 34 (IQR: 27 to 62) for females. The age distribution by sex is presented in figure 4; the highest proportion of cases was seen in the 25 to 34 age group followed by the 35 to 44 and 55 to 64 age groups. The highest proportion in males and females was seen in the 25 to 34 age group. Where reported, 45.2% (14/31) of persons with acute HBV infection were resident in the two most deprived quintiles. Where ethnicity was available (31), 55% (17) of persons with acute HBV were of white British ethnic origin, followed by those of Asian or Asian British ethnic origin (19%; 6) and black or black British ethnic origin (16%; 5).
Figure 4. Age/sex pyramid of acute HBV cases from HPZone: January to March 2023
Avidity testing and molecular characterisation investigations were undertaken on samples linked to cases to confirm the acute hepatitis B diagnosis with additional genotyping and phylogenetic analysis to inform on the diversity of the circulating viruses.
Of the 12 samples submitted to the VRD as part of the enhanced surveillance programme, 1 (8%) sample was confirmed to be from an individual with chronic hepatitis B and 10 (75%) were confirmed to be from individuals with acute hepatitis B infection. One sample was not tested. Not all cases with samples forwarded to the VRD could be matched to cases in HPZone; this could either be due to a case not being entered on HPZone or it could be due to the case being entered in a previous quarter.
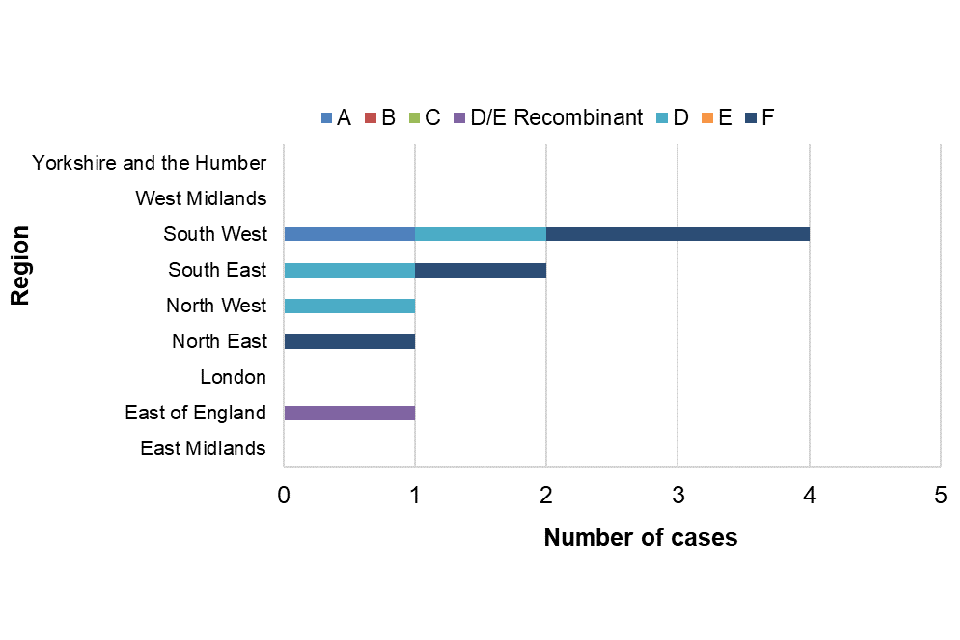
A total of 9 out of 10 confirmed acute cases could be genotyped between January and March 2023; the distribution of genotypes is shown in table 1. The distribution of genotypes seen in UKHSA regions is shown in figure 5.
Table 1. Genotype distribution and proportions of acute hepatitis B cases tested at VRD in January to March 2023
| Acute genotype | Number of cases | Proportion of cases |
|---|---|---|
| A [Note 1] | 1 | 11% |
| B | 0 | – |
| C | 0 | – |
| D/E Recombinant | 1 | 11% |
| D [Note 1] | 3 | 33% |
| E | 0 | – |
| F [Note 1] | 4 | 44% |
| Total | 9 | – |
Note 1. For January to March, sub-genotype breakdowns were: A2=1; D1=1, D2=1, D5=1; F=2, F1 = 2.
Figure 5. Genotypes of acute samples sent to VRD by UKHSA region: January to March 2023
Discussion
Quarterly publication of enhanced molecular surveillance using matched HPZone and reference laboratory confirmatory and typing data with a regional breakdown allows near real-time monitoring of acute hepatitis B transmission. The number of acute hepatitis B cases between January and March 2023 remained low and consistent with annual trends for the same timeframe. The number of cases observed during 2020 and 2021 are likely to be lower as an impact of SARS-CoV-2. Ascertainment of cases on HPZone through health protection investigations and HBV anti-core avidity testing provides assurances that this decrease in cases is likely to be real. Molecular analysis provides insight into the current hepatitis B genotypes circulating in England, although interpretation is limited by the small proportion of samples submitted to VRD. Of interest, a dominance of genotype F viruses was observed within this quarter; these viruses circulate through South Amercia and are rarely seen in HBV infections in England. Genotype characterisation can indicate a geographical origin which can contribute to understanding sources of infection and transmission routes. Improved submission of samples for molecular characterisation will allow for more comprehensive monitoring and interpretation of acute HBV infection trends in England.
