Animals in Science Regulation Unit annual report 2022 (accessible version)
Published 25 April 2024
Foreword
UK life science research makes vital contributions to both the economy and cutting-edge scientific research outcomes. Some of this research requires the use of animals, and it is essential that we provide protections to those animals. It is also crucial to maintain and build public confidence that where animals are used in scientific research, that use is fully justified. It is the Animals in Science Regulation Unit’s (ASRU) responsibility to ensure that animals are only used in research where there are no alternatives; they are only used to the extent needed to meet the objectives of the research and harms are minimised. ASRU is committed to assuring that full compliance is maintained with the ‘3Rs’ (replacement, reduction and refinement of the use of animals), keeping it at the heart of our regulatory delivery, alongside maintaining compliance with the Animals (Scientific Procedures) Act 1986 (ASPA).
2022 marked the continuation of ASRU’s Bridging Ways of Working operating model, which was introduced in 2021 to align ways of working with leading regulatory practice. The new operating model separates compliance assurance and licensing functions, and inspectors are no longer assigned to specific establishments. ASRU continued embedding these new practices throughout 2022, demonstrating a commitment to continuous improvement and effective regulatory delivery. 2022 also saw the introduction of the Animals in Science Regulatory Policy Unit as a separate unit to ASRU.
Following the COVID-19 pandemic, onsite inspections resumed for the first full year since 2019. A new audit system was initiated at the end of 2021 and continued to be rolled out throughout 2022. This new system introduced documented audit reports for establishments to strengthen the systems in place to protect animals at establishments and improve compliance with ASPA. We look forward to making more improvements to our operating model through regulatory reform in 2023, and beyond, to deliver our purpose of protecting animals in science by maintaining compliance with ASPA.
Kate Chandler
Head of the Animals in Science Regulation Unit
Section 1: The Animals in Science Regulation Unit
Introduction
The purpose of the Animals in Science Regulation Unit (ASRU) is to protect animals in science by maintaining compliance with the Animals (Scientific Procedures) Act 1986 (ASPA).
ASRU regulates the use of animals in science according to ASPA. ASPA is UK law, approved by Parliament, which permits the use of animals in scientific research and provides the restrictions around which animals can be used and for what purpose. At the heart of ASPA is the requirement to:
- only use animals in research when there are no alternatives
- use the minimum number of animals needed
- only cause the minimum necessary pain, suffering, distress or lasting harm to animals
ASRU is part of the Home Office and is responsible for administration and enforcement of ASPA in England, Scotland and Wales. ASRU’s activities include:
- advising on the regulations
- operating the licensing system required by ASPA
- assuring the compliance of licence holders with ASPA and the terms of their licences
ASRU delivers these responsibilities through its licensing and compliance assurance functions, supported by additional functions delivering business support and overseeing processes and standards.
Licensing function
ASRU inspectors evaluate licence applications against the requirements of ASPA and use a harm-benefit analysis process to determine whether a licence should be authorised.
ASPA has a three-level licensing system (for the person, the project and the place):
- Those carrying out regulated procedures must hold a ‘personal licence’ (PIL), which authorises them to apply those procedures to specified animals, initially under supervision until they have demonstrated competence.
- The regulated procedures to be carried out must be authorised by a ‘project licence’ which specifies the programme of work within which the procedures are being performed.
- The place at which the work is carried out must normally be specified in an ‘establishment licence’.
Those breeding and/or supplying the species of animal listed in ASPA Schedule 2 must also hold an establishment licence.
The conduct of regulated procedures may be authorised at places other than licensed establishments when the nature of the work makes this necessary, and these places will be specifically identified in the relevant project licences.
The principles of replacement, reduction and refinement (the 3Rs):
- Replacement is the principle that, wherever possible, a scientifically satisfactory method or testing strategy not entailing the use of protected animals must be used instead of a regulated procedure.
- Reduction is the principle that, wherever a programme of work involving the use of protected animals is carried out, the number of protected animals used must be reduced to a minimum without compromising the objectives of the programme. On occasions, it may be necessary to use a greater number of animals than the absolute minimum scientifically justifiable if each individual animal will suffer less as a consequence of the greater number being used. The principle of reduction should apply to methods of breeding protected animals as well as their use in procedures.
- Refinement is the principle that, wherever a programme of work involving the use of protected animals is carried out (after rigorously applying the principles of replacement), the regulated procedures applied to those animals must be refined so as to eliminate or reduce to the minimum any possible pain, suffering, distress or lasting harm. As indicated above, refinement and reduction must be considered in balance. Refinement applies to the methods of breeding, accommodation and care of protected animals as well as the methods used in procedures.
How the 3Rs are applied:
Personal licence holders’ responsibilities for the 3Rs:
- The responsibilities conferred on PIL holders through standard licence conditions include the requirement that the licence holder shall act at all times in a manner that is consistent with the principles of replacement, reduction and refinement (Standard Condition 1).
Project licence holders’ responsibilities for the 3Rs:
- PPL holders are required to ensure that their programme of work does not involve any regulated procedures for which there is a scientifically satisfactory alternative method or testing strategy that does not entail the use of a protected animal. Such methods may include specific in vitro or in silico procedures as well as consideration of weight-of-evidence decision strategies. Such decision strategies may indicate that no animal tests, or no further animal tests, are reasonably justified in order to address the question posed (Standard Condition 2).
Establishment licence holders’ responsibilities for the 3Rs:
- The first standard condition of the establishment licence requires that the holder must put in place measures to ensure that the regulated activities carried on at the establishment are carried out in a manner that is consistent with the principles of replacement, reduction and refinement (the 3Rs) (Standard Condition 1).
In 2022, the Licensing Team’s work included:
- issuing establishment, personal and project licences, and amending these
- taking action in cases of non-compliance
- providing regulatory advice to licensed establishments
- leading on the technology for e-licensing
In 2022, the Licensing Team comprised one full-time equivalent (FTE) senior leader, 9.34 FTE inspectors, three FTE executive officers and two FTE administration officers.
Compliance assurance function
The compliance assurance function delivers all activities which provide oversight and assurance to the public of licence holders’ compliance with ASPA and their licence conditions, including:
In 2022, the compliance assurance function was responsible for:
- investigating potential non-compliance cases and the proportionate application of sanctions, as described in the published compliance policy
- managing the delivery of the 2022 audit activity undertaken by ASRU
- reviewing reports submitted to evidence compliance, such as Standard Condition 18 and other reporting requirements required by a specific licence
- requests to keep animals alive when the severity limits in a project licence and/or observance of any other controls appear to have been, or are likely to be, breached
- responding to compliance assurance related regulatory advice queries
It comprised one FTE senior leader, 5.75 FTE inspectors, one FTE senior executive officer and three FTE executive officers.
Processes and standards function
In 2022, ASRU launched a new function to oversee the development, refinement and implementation of procedural documents within ASRU. It is responsible for mapping out all processes for all ASRU business activities and ensures published regulatory guidance and advice is current.
In 2022, the team comprised two FTE members of staff.
Business support function
ASRU’s Business Support Team provides business support to all ASRU colleagues, including managers and leaders.
In 2022, the Business Support Team comprised the following specific functions:
- risk management activities, including health and safety
- all assurance and governance monitoring and reporting
- recruitment
- ASRU training, events, and conferences, including external stakeholder events
- providing a secretariat function and publication of newsletters
- administering and collecting the return of procedures for publication of the annual statistics
- managing procurement and all financial activities
- collecting licence fees
- maintaining our e-licensing system, ASPeL (Animals in Scientific Procedures e-Licensing), and IT resources within ASRU
In 2022, the team comprised 2.5 FTE members of staff. The team reported to the Head of Business Support, Assurance and Governance in 2022.
Section 2: Regulatory Reform Programme
Bridging Ways of Working
In 2020, a programme of transformational regulatory change was initiated to improve the performance of ASRU. The change programme would deliver alignment of the Regulator with the following expectations:
- improved ability for licensed establishments to comply with the Animals (Scientific Procedures) Act 1986
- greater protections for animals used in science
- improved assurances to the public
- greater openness and transparency of the Home Office in how it meets its regulatory obligations
- improved value for money
The extent of the reforms required initiated the transformational change. Three broad pillars of change were identified:
- The requirement for a policy function to which the Regulator would be structurally aligned.
- Delivery of a new regulatory operating model that is aligned to leading practice.
- Organisational redesign of the Regulator, mapped to the operating model.
On 5 July 2021, ASRU made changes to the regulatory operating model to align ways of working with leading regulatory practice and modern regulatory systems. The new operating model separates compliance assurance and licensing functions, and inspectors are no longer assigned to specific establishments.
In the new model, ASRU provides regulatory delivery through two teams – one covering licensing activities and the other, compliance assurance activities.
Requirement for a policy function
ASRU works in a policy landscape that includes policy regulating animals in science and broader government policies that influence the use of animals in science.
In April 2022, the Animals in Science Regulation Policy Unit was established in the Home Office, as a separate entity from ASRU.
The Policy Unit advises ministers on policy relating to regulating the use of animals in science, principally under ASPA. The Policy Unit:
- develops policy and advises the responsible minister on regulating animals in science
- engages with the regulated sector, and other life science and animal welfare stakeholders
- works with other government departments with relevant policy responsibilities
- commissions and considers advice from the independent advisory Animals in Science Committee
- sets policy requirements for implementation by ASRU
- sponsors ASRU by setting policy direction, ensuring operational independence and holding ASRU to account for delivery
More widely, a range of policies and legislation led by other government departments influence the use of animals in scientific procedures, and therefore the work of ASRU. These range from regulatory safety testing requirements to the funding of scientific research. The table below outlines the key departments that influence the use of animals in science and their areas of responsibility.
| Department | Areas of responsibility |
|---|---|
| Home Office | Regulation of the use of animals in science under ASPA, including licensing and compliance |
| Department for Science, Innovation and Technology | Policy on the development and validation of alternatives that cause less harm or do not use animals (under ASPA Section 20B) Government funding for alternatives (through UK Research and Innovation and the National Centre for the 3Rs (NC3Rs)) <Funding for basic and applied research Public attitudes to animal research survey Strategic support to the life sciences sector to promote research, innovation and the use of technology to improve health and care |
| Department for Environment, Food and Rural Affairs | Protection of the natural environment Chemical regulation (REACH) Precision breeding Health and preservation of species Veterinary medicine |
| Department for Health and Social Care | Medicines and healthcare products policy and regulation Training for surgeons |
Regulatory operating model
In 2021, ASRU identified that fundamental changes to the existing operating model were required to align with leading regulatory practice and in accordance with strategic shifts. ASRU launched a new operating model, ‘Bridging Ways of Working’, in July 2021, which was more aligned with modern regulatory requirements.
Bridging Ways of Working was embedded in ASRU throughout 2022, with the Regulatory Reform programme paused to gather feedback from establishments and the wider sector, alongside consolidating new processes and establishing a new Policy Unit separate to the Regulator. Further details on how ASRU’s functions were delivered under Bridging Ways of Working are in later sections of this report. The Regulatory Reform programme will resume in 2023.
Organisational redesign
The process of organisational design is scheduled for 2023, once the operating model has been finalised and embedded.
Section 3: Stakeholder engagement
Stakeholder engagement framework
ASRU engagement with the regulated community addresses the three tiers of requirements:
- relationship needs, e.g. establishment service delivery standards
- operational needs, e.g. compliance questions
- strategic needs, e.g. policy implementation
The principles of the various engagements that achieve each of these requirements are that they will be:
- focused and clear about outcomes
- differentiated and tailored to the needs of those in the regulated community to ensure value for those we engage with
Overall, the Regulator’s (ASRU) engagement with the regulated community is vital to ensure ASRU:
- reviews and issues licences and licence amendments in a consistent and timely way
- reviews compliance of licence holders in a consistent and timely way
- has a forum to inform the regulated community of regulatory changes that impact them
- understands the impact of the regulation on the regulated community
- has a forum to answer questions from the regulated community about the regulation and regulatory delivery
| Engagement mechanism(s) | Key areas for engagement | Output of engagement | |
|---|---|---|---|
| Individual Establishment engagement for relationship management, customer service and operational delivery | 1. Individual Establishment Discussion with Home Office Liaison Contact (HOLC)/ASRU liaison officer for review or concerns about individual issues Ad hoc and/or quarterly periodic 1:1 virtual meetings depending on business requirements |
Meeting expected service standards | Providing a consistent service |
| 2. Individual Establishment Email queries Queries triaged |
Compliance with ASPA | Operational – enabling the smooth functioning of the regulatory framework | |
| All Establishment operational engagement | 3. Home Office Liaison, Training and Information Forum (HOLTIF) Providing updates, clarification and information Quarterly |
Improving understanding of regulated community | Transparency and enabling regulated community to comply |
| All Establishment strategic engagement | 4. Regulator Engagement Forum ASRU engagement with the Establishment Licence Holders forum A representative establishment group for input and review of guidance and service standards Every four months |
Understanding impact on the regulated community | Avoiding unnecessary regulatory burdens |
Stakeholder meetings
The Home Office met three times with counterparts in establishments through HOLTIF in 2022. The meetings were an opportunity to discuss service delivery, for ASRU to receive feedback and to solve any associated issues. The main external attendees are the HOLCs, who undertake many of the administrative functions required under ASPA at each establishment, and support licence applicants and existing licence holders. Up to 60 HOLCs attend the HOLTIF and meetings have been held virtually since 2020.
Meetings took place in February, July and October, and were Chaired by ASRU’s Head of Unit. These meetings were used to update HOLTIF members on the change programme – specifically the development of the new Policy Unit and to and notify HOLCs that the change programme would shortly be resuming, and to discuss the value of 1:1 engagement with HOLCs, the quality of regulatory advice, the management of application amendments, and to introduce ASRU’s audit model.
ASRU also met with the Establishment Licence Holders (ELHs) forum twice in 2022, in March and October. These meetings included an update and explanation of the change programme, including governance expectations of ELHs, and discussion of the principles of successful engagement with ASRU.
Relationship management
In 2021, the role of operational relationship management lead was created with the purpose of engaging with the regulated community in a co-ordinated and centralised way. The operational relationship management lead conducted a series of 1:1 calls with all establishments in 2022 to give HOLCs an opportunity to share their feedback about the change programme and for ASRU to respond to any concerns. An operational relationship management mailbox was also set up in 2021 and continued to be used through 2022 as a first port of call for stakeholders to contact ASRU, including responding to any complaints. Additional mailboxes were created for each function, managing regulatory advice queries and potential non-compliance reporting.
Publications
In 2022, ASRU published:
- Bridging Ways of Working guidance PDF (updated)
- ASRU operational newsletter, 1 April 2022
- Standard genetically altered rodents protocols, Standard genetically altered zebrafish protocols, Guidance on the use of standard genetically altered animals
- ASRU operational newsletter, 12 September 2022
- Guidance: Notes for Project Licence Applications
- Animals in Science Regulation Unit annual reports 2019 to 2021
Correspondence
ASRU supports the Animals in Science Regulation Policy Unit to respond to Freedom of Information Act 2000 (FOI) requests or correspondence from the general public on issues related to the regulation of animals in science.
Section 4: Licensing
The framework
Under ‘Bridging Ways of Working’, the principles, processes and standards used in licence assessment, in accordance with ASPA requirements, remain unchanged.
The licensing service is delivered through a ‘taxi rank’ system with applications being assessed by an inspector in the order they are submitted through our electronic licensing system – ASPeL (Animals in Scientific Procedures e-Licensing). We prioritise licence applications using typical timelines that are aligned within the statutory timelines defined in ASPA. The typical timelines for the handling of licensing tasks are shown below and are based on the statutory requirements defined in ASPA. Licensing timelines can vary, based on the complexity of the application and level of incoming applications to the Regulator.
All days referenced are working days:
- New project application review and any returned project application review: 40 days/55 days for complex applications
- New PPL amendment review: 40 days
- Second and subsequent PPL amendment review: 40 days
The three-tier licensing system provides a framework for authorising research using animals.
The licensing system ensures that animal research and testing is only undertaken:
- where no practicable alternatives exist
- under rigorous controls where suffering must be kept to a minimum
ASRU administers the licensing function under ASPA, which comprises the following requirements:
- The place at which the work is carried out must hold an ‘establishment licence’ (PEL).
- The programme of work in which the procedures are carried out must be authorised in a ‘project licence’ (PPL).
- Those carrying out procedures must hold a ‘personal licence’ (PIL), which ensures that those working with the animals are qualified and suitable.
In 2022, ASRU licensed and regulated 135 establishments. These establishments include universities, pharmaceutical companies and contract research laboratories. At the end of 2022, there were 2,300 active PPLs and 13,483 active PILs.
Licensing activities
Establishment licences
During 2022, one PEL was granted, two were revoked, and 26 amendments were made. This shows a slight increase compared with 2021, predominantly due to administrative task changes within the e-licence establishment format.
Project licences
During 2022, 490 new PPLs were granted, and 987 amendments, a slight decrease compared with 2021.
Personal licences
During 2022, 2,319 new PILs and 696 PIL amendments were granted, a slight decrease compared with 2021.
Animals in Scientific Procedures e-Licensing
In 2019 ASRU rolled out ASPeL, a refreshed digital e-licensing system, to improve:
- consistency of approach
- the ability for establishments to be compliant
The ASPeL system ensures that licence and duty holders can easily access the information they need to do their work, helping to reduce instances of accidental non-compliance. It allows applicants to easily track the progress of their applications and see when mandatory actions are required, such as when a PIL is due for review.
ASRU recognises that the new project application form can continue to be improved. Further improvements to the form and ASPeL’s performance continued in 2022. For example:
- PELs are now easier to view and amend. This facilitates all authorised users to review the list of approved areas to view their contemporaneous authorisations. Similarly, all PILs are visible quickly and easily to all administrators and named people, enabling all duty holders to ensure that the appropriate authorisations are held. The time taken to authorise a PIL application or amendment has been reduced from up to 20 days to the next working day, with many applications processed on the same day.
- Further features have been added to ASPeL during 2022. Establishments are automatically alerted when the mandatory five-year PIL reviews are required and an improved workflow enables reporting of the completed reviews. The ability to submit and add a retrospective assessment to an expired or revoked PPL is now embedded for those licences that are required to supply them.
- Introducing financial and invoicing information for PELs and PILs, which began in January 2022, has provided establishments greater transparency over their financial data.
- The new ASPeL has passed all the Government Digital Service standard assessments required by the Cabinet Office and is seen as an exemplar of good service design. It has been built in such a way that it can be continually improved and upgraded as technology moves on. ASRU has committed to the ongoing development of ASPeL to ensure its continued development to meet user needs, both internally and externally.
Referrals to the Animals in Science Committee
The Animals in Science Committee (ASC) is an independent, non-departmental public body convened under sections 19 and 20 of ASPA. The ASC provides independent, balanced and objective advice to the Secretary of State on issues relating to regulating animals in science. At all times, the ASC must consider both the legitimate requirements of science and industry and protecting animals from avoidable suffering and unnecessary use in scientific procedures.
The ASC has a website detailing its activities.
The ASC also advises on specific categories of project licences, including those seeking authority for:
- using wild-caught non-human primates
- using cats, dogs, equidae or non-human primates in severe procedures
- using endangered species
- projects with major animal welfare or ethical implications
- projects of any kind raising novel or contentious issues, or giving rise to serious societal concerns
- projects involving the use of admixed embryos as advised in the ‘Guidance on the use of Human Material in Animals’
Section 5: Audit
Audit approach
In 2021 and 2022, audits were rolled out to replace the previous old-style inspection programme.
An audit is a process which verifies conformance to standards through a review of objective evidence. Audits provide assurance to ministers and the public that there are systems in place to ensure care of animals and that the experiments undertaken comply with the requirements of ASPA and the relevant conditions specified in licences. ASRU advises duty holders on how to comply with ASPA requirements and will enforce non-compliances.
ASRU audits establishments licensed to breed or supply animals, or to undertake regulated procedures on animals under ASPA in England, Scotland and Wales. The purpose of ASRU’s audit activity is to assess compliance against ASPA and associated licence conditions, and to objectively measure the risk of non-compliance within the establishment by assessing the robustness of governance systems.
More specifically, ASRU undertakes audits for the following purposes:
- Determine whether licence holders are compliant or to advise how to comply with the legal requirements of ASPA.
- Inspect areas included on the establishment licence where animals may be kept or used under ASPA to ensure that they comply with the standards laid down in the ‘Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes’.
- Determine whether animals are being or have been used in procedures, or being used for breeding or supply, in areas not included on establishment licences.
- Determine whether the breeding, supply and/ or use of animals in procedures complies with licence authorities and conditions on licences.
- Determine whether people named in the establishment licence understand and are fulfilling their required duties, and to advise on these roles.
The purpose of audit is primarily supportive and aims to recognise areas where systems are strong to maintain compliance, as well as identifying areas where improvements could be made. Although non-compliance may be detected during an audit, it is not primarily an enforcement activity but a monitoring and educational activity.
ASRU’s audit activity is risk-based, taking into consideration the factors specified in section 18 (2C) of ASPA, which are:
- compliance history of an establishment
- any information relating to potential non-compliance
- number and species of animals kept
- number and type of regulated procedures carried out
This was the first year in which audits fully replaced old-stye inspections as part of regulatory reform. The number of audits is not directly comparable to the number of inspections in previous years. The new audits are a more rigorous and comprehensive assessment of compliance compared to the previous inspections.
Description of audit types
In 2022, ASRU’s audit activity comprised:
- Full systems audits: evaluating governance systems within an establishment or a project to understand how robust they are at maintaining compliance.
- Facilities audits: to record evidence of the effectiveness of the governance systems in place to maintain compliance with standard conditions of the establishment licence and Code of Practice for the Housing and Care of Animals Bred, Supplied or Used for Scientific Purposes, and to decide about any regulatory actions required to reduce the risk of non-compliance.
- Facility assessment for establishment licence amendments: to assess new facilities and/or significant changes to existing facilities that cannot be confirmed remotely.
- For cause audits: for enforcement investigations when the cause of non- compliance cannot be confirmed and for other regulatory purposes such as investigation following a whistle-blowing report received by ASRU.
The criteria used to assess establishments is published online.
| Audit activity | Onsite visit | Number of inspectors |
|---|---|---|
| Full systems | 2 to 5 days | 2 to 4 inspectors |
| Facilities | 1 day | 1 to 3 inspectors |
| Facility assessment for establishment licence amendment | 1 day | 1 inspector |
| For cause audit | Minimum 1 day | Minimum 2 inspectors |
| Thematic audit | Conducted remotely | Variable |
Number of audits
In 2022, ASRU audited 56 establishments:
- 4 full systems audits
- 35 facilities audits
- 13 facility assessments for establishment licence amendments
- 4 for cause audits
Of these audits, ten were unannounced. Although no thematic audits were undertaken in 2022, these will be used in the future should the need arise.
Audit reports
Following an audit, an establishment receives a report detailing the findings of the audit, including timescales for confirming to ASRU that any required follow-up action has been completed. This allows any necessary action to be undertaken by the establishment and ASRU to monitor its completion in a timely manner.
Risk management
ASRU’s establishment risk management process comprises a review of the national risk profile and local establishment factors. ASRU undertakes reviews periodically throughout the year.
Evaluation of risk includes:
- the incidence and nature of non-compliance cases
- any significant low-level concerns
- procedures and species
- any other relevant information
ASRU takes these factors into account when planning audit activity.
Investigating allegations made to ASRU
ASRU periodically receives allegations about potential breaches of ASPA. These are taken seriously, and where sufficient information is provided, they are followed up by the most appropriate means, including a for cause audit, if appropriate. Where it appears there may have been a lack of compliance with ASPA, these are investigated in accordance with ASRU’s non- compliance policy.
Section 6: Management of non-compliance
Compliance policy
ASRU’s compliance policy focuses on the delivery of a proportionate, consistent, and outcome-based approach to incidents of non- compliance. Every establishment licensed under the Animals (Scientific Procedures) Act 1986 (ASPA) has a named person responsible for compliance (NPRC). This individual is responsible for ensuring compliance with the conditions placed on their establishment licence. A good culture of compliance at an establishment reflects evidence of effective governance over the use of animals in science. The NPRC must maintain robust systems and frameworks that support and assist all licensees to comply with their licence conditions.
Establishments notify ASRU of any incidents where there has been a potential breach of ASPA or a licence condition (which also includes the code of practice). Self-reporting indicates that an establishment is making efforts to ensure compliance. It demonstrates that role holders are aware of their responsibilities and are committed to building a compliant culture. ASRU expects self-reporting to be embedded within good governance frameworks and that employees are aware of the process for raising concerns within their establishment. This is set out in ASRU’s published compliance policy and guidance on the operation of the ASPA.
ASRU may identify potential breaches when auditing an establishment. When this occurs, the establishment is notified in the audit report that a potential non-compliance has been identified and may be investigated.
ASRU takes all reports of potential non- compliance seriously. An inspector gathers sufficient information to determine whether there is a case that merits further investigation. If the ASRU Enforcement Team determines that there is sufficient evidence for a breach, it will issue a suitable and proportionate remedy. The aim of this remedy is to prevent a recurrence of similar breaches.
Licensees and the establishment are notified in writing by ASRU when a non-compliance investigation is being conducted and are given an opportunity to provide any information that they wish to be considered before ASRU takes a decision regarding the appropriate remedy. There is also the opportunity for appeal against some types of remedy, which the licence holder will be notified of at the time the remedy is issued. Complex or serious cases may take some time to resolve. In rare cases, ASRU may take a view that an offence has been committed that is sufficiently serious to merit referral for prosecution.
Potential remedies for non-compliance
ASRU considers cases individually and applies the most appropriate remedy for the severity of the non-compliance and the aggravating and mitigating circumstances. ASRU takes the resulting measures and sanctions to deter or prevent a recurrence.
Factors considered when determining suitable remedy include:
- the extent of any unnecessary animal suffering
- evidence and extent of governance and systems failures
- the timeliness of any remedies applied by the establishment
- the risk of recurrence
- evidence of dishonesty or attempts to evade responsibility
The range of remedies available, as set out in the published compliance policy, benchmark and help to determine the outcome associated with each breach. These are briefly outlined below.
1. Inspector Advice
For a minor breach, an inspector will advise on what provision was breached and what is expected in the future to prevent a recurrence. A minor breach is one where:
- there are no or minor avoidable adverse animal welfare consequences
- the facts are agreed
- there was no intention to subvert the controls of ASPA
- the risk of a recurrence is judged by the inspector to be low
2. Compliance letters
Where provision of Inspector Advice is not considered sufficient, most cases of non- compliance are dealt with by a letter from ASRU, with or without a variation of the relevant licence(s). Where a breach has been committed by a licensee, a letter of reprimand is sent. Where a non-licensee has contributed significantly to the breach, a letter of censure may be sent.
Letters note the breach(es) that have occurred and summarise the evidence for those breaches. These letters are formal records of non-compliance and may be used as evidence should there be a further breach within five years. All letters are copied to HOLC so that local practices and processes can be reviewed, as appropriate.
3. Variation of licence
Requirement for re-training
Re-training is required where a licensee has demonstrated that they do not have the expected level of knowledge of their legal responsibilities or to undertake procedures.
Requirement for reporting
Where action is required to improve weaknesses identified by a breach, including poor record keeping, a report may be required to monitor progress. Reports are also useful for formally monitoring enhanced animal welfare, implementing refinements or improving scientific outcomes.
Suspension
Where a breach has been identified, ASRU may suspend the licence as a sanction. It may also suspend licences when there are urgent animal welfare concerns. Suspensions are appropriate where there is a risk to animal welfare and significant, urgent action is required to protect it. When a suspension is required, ASRU must ensure that the suspension itself does not result in an adverse impact on animal welfare.
4. Compliance Notices
ASRU will issue a Compliance Notice where it requires action to be taken to prevent further non-compliance. Such a notice will specify:
- the licence condition(s) or ASPA provision(s) that have been breached
- the action that must be taken to ensure that the failure does not continue or is not repeated
- any action that must be taken to eliminate or reduce any consequential risk of harms caused by the breach
The Compliance Notice will set out the consequences of failing to comply. In this eventuality, ASRU may sanction the licence holder with suspension, variation or revocation of their licence.
This type of remedy is particularly effective where specific actions are required to assure ASRU that the breach will not recur. ASRU usually specifies a timeframe for the actions to be completed; if not completed, it may sanction further, such as suspension, revocation or variation of the licence.
5. Revocation of a licence
ASRU will only revoke licences issued under ASPA in the most serious cases. It is appropriate where a licensee has shown a disregard for the controls of the ASPA and has caused avoidable suffering. It may also be appropriate where significant avoidable suffering has been caused through negligence or ignorance, or where the licensee otherwise appears to be unsuitable for the role. ASRU has a duty to ensure that the welfare of animals is not adversely affected by the revocation of a licence.
6. Prosecution
Extremely serious cases of non-compliance are referred to the prosecuting authorities to judge whether it would be in the public interest to prosecute. Prosecution could lead to a fine or imprisonment.
Summary of non-compliance cases in 2022
In 2022, 175 cases of non-compliance in 51 different establishments were confirmed and finalised. Of these, 97 (55%) were related to the failure to have or adhere to licence authorities, while the other 78 (45%) were related to the failure to provide appropriate care (including food, water, and suitable facilities). There were 123 cases (70%) for which the sole remedy was Inspector Advice.
Figure 1: Percentage breakdown of the type of non-compliance
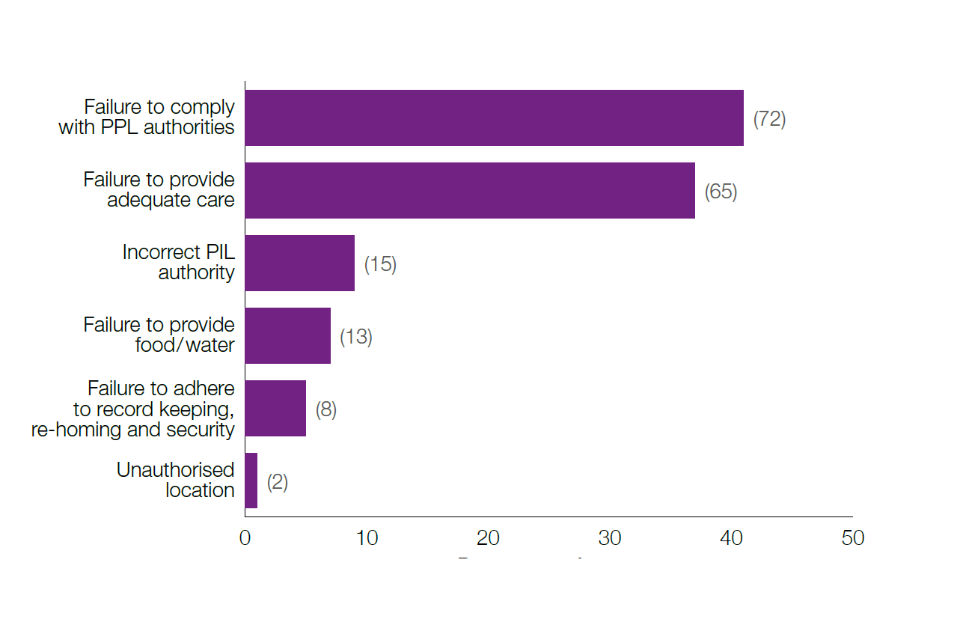
Notes: The number of non-compliance cases have been indicated in the brackets.
Number and type of animals
In 2022, of the 175 cases of non-compliance, animal numbers were reported in 163. These 163 cases involved a total of 16,062 animals.
Table 1: Number of animals involved in non-compliance cases, 2022
| Animal type | No. of animals | |
|---|---|---|
| Mouse | 11638 | |
| Fish | 2775 | |
| Chicken | 1100 | |
| Rat | 376 | |
| Frog/Xenopus | 64 | |
| NHP | 53 | |
| Hamster | 34 | |
| Guinea pig | 9 | |
| Sheep | 4 | |
| Cattle | 2 | |
| Dog | 2 | |
| Ferret | 2 | |
| Horse | 1 | |
| Pig | 1 | |
| Rabbit | 1 | |
| Total | 16062 |
In 12 cases, the number of animals involved was either not relevant or not known. The reasons for this are:
- four cases: administrative breaches – no animals were directly involved
- one case: security breach – no animals were directly involved
- five cases: breach was of the nature that it occurred over a prolonged time period, e.g. lighting issues in holding rooms
- two cases: the number of animals was not reported
Table 2: Number of animals involved in non-compliance involving over-breeding
| Animal type | No. of cases | No. of animals | % of animals |
|---|---|---|---|
| Mouse | 3 | 4810 | 41 |
| Fish | 1 | 1671 | 60 |
| Totals | 4 | 6481 | 40 |
Adverse welfare outcomes
An animal was assessed as having an adverse welfare outcome as the result of a non- compliance if they experienced more pain, distress, suffering or lasting harm than was authorised, which was greater than minor. Animals that were bred in excess of the authorised numbers, but that were required to achieve the scientific objectives, were not considered as having experienced an adverse welfare outcome.
In 2022, 1,063 animals experienced adverse welfare outcomes because of non-compliance.
Table 3: Number of animals with adverse outcomes by type
| Animal type | No. of animals | |
|---|---|---|
| Mouse | 242 | |
| Fish | 748 | |
| Chicken | 0 | |
| Rat | 18 | |
| Frog/Xenopus | 36 | |
| NHP | 7 | |
| Hamster | 3 | |
| Guinea pig | 7 | |
| Sheep | 0 | |
| Cattle | 0 | |
| Dog | 0 | |
| Ferret | 0 | |
| Horse | 1 | |
| Pig | 0 | |
| Rabbit | 1 | |
| Totals | 1063 |
Remedies
It should be noted that in a single case of non-compliance, there can be several different remedies applied to a variety of individuals. Therefore, the number of remedies is not the same as the number of cases.
Figure 2: Percentage and type of remedies issued
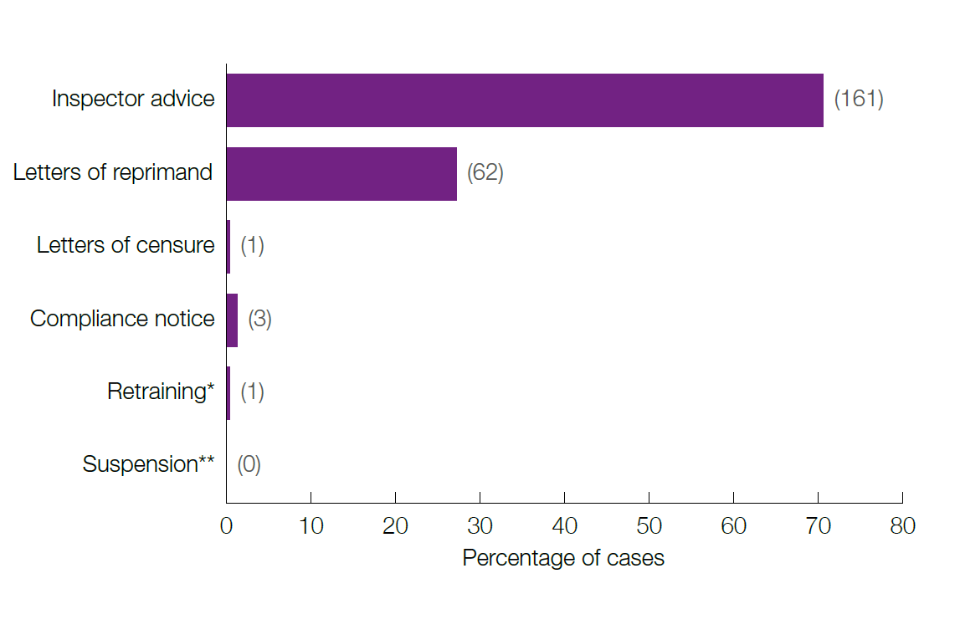
Notes:
*There was also a Compliance Notice with retraining requirements for a PIL holder, which has been counted here under Compliance Notice.
**For the same case mentioned above, the PIL was also suspended as part of the remedy, but has been recorded under the Compliance Notice remedy, as above, to avoid counting the remedy to that individual more than once.
The number of types of remedies have been indicated in the brackets.
Figure 3: Percentage of remedies issued to each type of licence holder
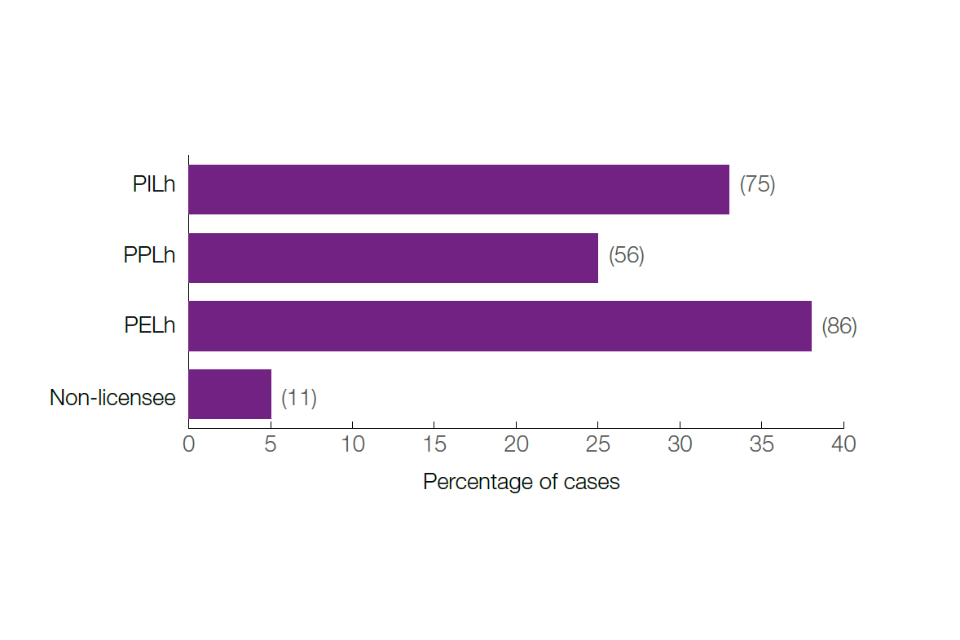
Notes: The number of licences against which remedies were issued have been indicated in the brackets.
Summaries of all the non-compliance cases completed in 2022 are in Annex A. Please note the exact number of cases and animals does not fully align between the appendices and this summary text due to:
- consolidation of case reports
- cases involving multiple species
- cases where exact species are not disclosed as it could identify the establishment
Trends in non-compliance cases over time
Figure 4: Number of non-compliance cases by principal breach of licence, by year, 2019 to 2022
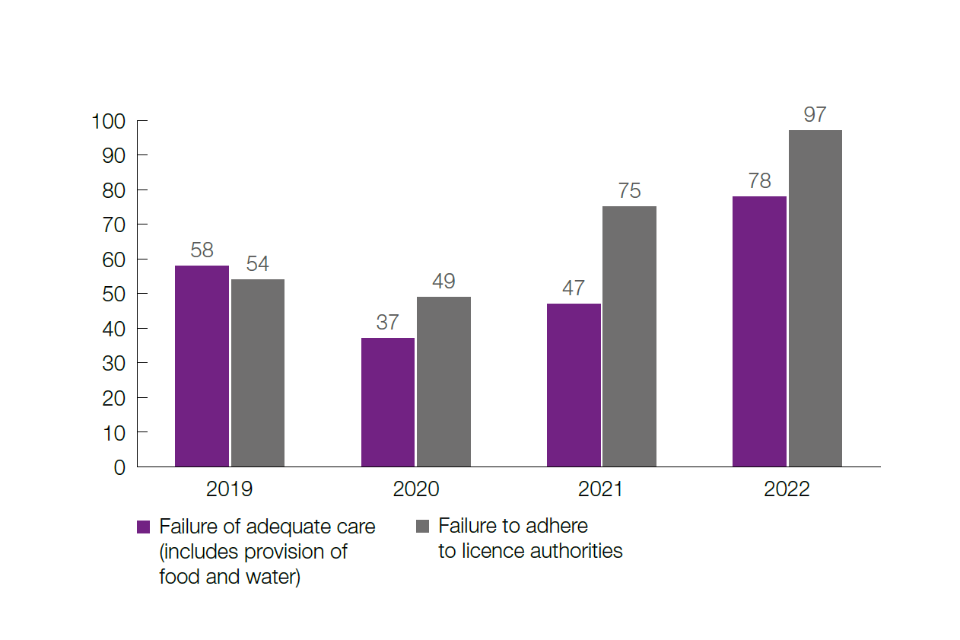
Except for 2020, the number of non-compliance cases has increased year on year from 2019 to 2022 (112 in 2019, 86 in 2020, 122 in 2021 and 175 in 2022). The decrease in 2020 may be attributable to the impact of the COVID-19 pandemic.
The reasons for the increase in the number of non-compliance cases in 2022 may include the introduction of the risk-based audit programme and the drive to improve governance within establishments, including self-reporting. ASRU will further evaluate trends in non-compliance as the regulatory reform programme develops. Every case is investigated and remedies and sanctions applied, using more rigorous sanctions in cases where animal welfare is impacted or there are significant systems failures.
Key learnings from 2022 non-compliance cases
Failure to comply with project licence authorities
This was the most frequent cause of non- compliance cases in 2022 – 72 (41%) of the 175 cases.
The number of animals involved was impacted by four cases of unauthorised over-breeding (see Table 2), as well as three cases involving regulated procedures:
- 3,572 mice not given analgesia after the procedure, as required by the controls in the PPL
- 415 more mice were used on an experimental protocol than was authorised by the PPL
- 1,100 chicken eggs that had undergone injections of a substance were kept beyond the age of protection, which did not have PPL authorisation
The main root causes of these types of non- compliances were:
- PPL and PIL holders failing to understand the authorities granted on the relevant PPL
- PPL and PIL holders failing to stay within the limits for procedures stipulated within the PPL (e.g. the number of procedures permitted or route of administration permitted)
- inadequate monitoring of animals in line with measures stipulated on the PPL
- PPL holders failing to be aware of and/or complying with the standard conditions on their PPL
- PEL holders failing to have adequate systems in place to prevent unauthorised procedures being undertaken
The following recommendations are made to reduce cases of failing to comply with PPL authorities:
- PPL holders must ensure that all individuals working under their PPL authority are fully aware of the exact authorities granted.
- PIL holders should be aware of the authorities of the PPL they are working under.
- PPL holders should have in place processes to review planned experiments to ensure compliance with PPL authorities.
- PEL holders must ensure they have taken reasonable steps to prevent unauthorised procedures from being conducted.
Failure to provide adequate care
In 2022, there were 65 cases (37%) of inadequate care. The numbers of animals involved were impacted by:
- A case involving 710 mice exposed to continuous light in holding rooms for up to approximately 12 days.
- Ten cases involving equipment failure or poor water quality/contamination, which resulted in the death of 719 fish.
Recommendations to reduce the number of cases where there was a failure to provide adequate care are as follows:
- PEL holders must ensure that fish facility equipment and tanks are properly maintained, and that the risks associated with manipulation of tanks are identified and provision is made to mitigate these.
- PEL holders must implement processes to ensure that animals are present in the cage and not trapped after any intervention inside the cage.
Failure to provide food and/or water
Failing to provide sufficient food and/or water to animals, as part of basic husbandry and care, is unacceptable. Establishments must always have robust procedures in place to ensure the adequate provision of food and water to animals kept under the provisions of ASPA.
In 2022, of the 175 cases of non-compliance, 13 (7%) were failure to provide adequate food and/or water, resulting in adverse welfare outcomes.
Cases in which there was a welfare impact involved the failure of establishment processes to ensure that the necessary daily checks were performed adequately, since if these were performed competently, the absence of food and water would be detected prior to adverse welfare outcomes occurring.
The following recommendations are made to reduce the number of cases where there was a failure to provide food and water:
- PEL holders must ensure adequate staffing levels to perform daily checks competently, especially at weekends.
- PEL holders should implement processes to ensure that the system of daily checks is robust.
- PEL holders should identify high-risk situations that may result in failing to provide adequate food and water and implement specific actions to mitigate these.
- PIL holders and staff performing husbandry duties must be explicitly trained and reminded to ensure that they check for the presence of food and water after any activities involving animals.
Failure to have appropriate PIL authority
Section 3(a) of ASPA requires that no person shall apply a regulated procedure as part of an authorised project to an animal unless they hold a relevant PIL.
In 2022, 15 (9%) cases were recorded where the breach was either failing to hold a PIL or to have the relevant authorities on their PIL to conduct the regulated procedures undertaken.
Recommendations to prevent this type of non-compliance:
- Providers of modular training should reinforce that, following the successful completion of the module training, a PIL must be applied for and held before they undertake regulated procedures.
- Establishments must ensure that processes are in place to ensure that appropriate PIL authorities are held by those undertaking regulated procedures. This includes appropriate checks of the PIL authorities of individuals visiting an establishment to perform regulated procedures.
Unauthorised location: Performing procedures or keeping animals in area not specified on PEL
In 2022, two (1%) cases were recorded where regulated procedures had been performed in a room not authorised on the PEL, or an animal had been kept in a room not authorised for overnight holding.
Recommendations to prevent this type of non-compliance are:
- Ensure all PIL holders and staff are aware of the authorities for each room on the establishment licence.
- Consider labelling rooms clearly with the authorities mentioned above.
Failure to adhere to licence conditions that mandate record keeping, re-homing and security
This category has been added for the 2022 Annual Report, to accurately capture the number of non-compliance cases that have arisen that cannot be placed into the five previously used categories. This reflects an increase in detection in these types of issues by the audit process. Some non-compliances in this category were self-reported.
This category contains eight cases (5%) and includes:
- four cases involving deficiencies in the establishment’s Killing Register
- three cases involving unauthorised re-homing
- one case involving a breach of security at an establishment premises
Section 7: Standard Condition 18
All project licences are subject to a set of standard conditions. Standard Condition 18 requires that the PPL holder notifies the Secretary of State as soon as possible if constraints on the severity limits, or observance of other controls described in the licence, have been breached or are likely to have been breached.
Licence holders are required to submit reports under Standard Condition 18 as a requirement of ASPA, ensuring that unexpected events are reported to ASRU so that advice can be provided or compliance action taken.
Notification to ASRU under PPL Standard Condition 18 relates to breaches or likely breaches of either severity limits or any other controls set in the licence. Notification provides an important opportunity for the licence holder, the establishment and ASRU to review whether any changes need to be made to licence authorities and is an important source of data for ASRU compliance assurance. Notification under PPL Standard Condition 18 is not the same as reporting potential non-compliance.
In 2022, ASRU received 3,232 Standard Condition 18 reports from establishments.
Section 8: Financial Report – income and expenditure for 2022
Financial Report
Since the year ending 31 March 2015, ASRU has been operating on a full cost recovery basis, meaning that the licence fee income should cover all expenditure incurred in delivering the service. As a full cost recovery unit, ASRU receives all its income from the licence fees it charges. It is only permitted to spend this income on its regulatory duties and associated business costs.
Table 10.1: Summary of income and fee-funded expenditure, by budgeting year, including capital spend, years ending March 2015 to 2023
| Year | Income | Expenditure (Running budget) | Expenditure (Capital(1)) | Variance |
|---|---|---|---|---|
| 2014-2015 | £4,380,206 | £4,378,929 | – | £1,277 |
| 2015-2016 | £4,692,833 | £4,207,503 | – | £485,330 |
| 2016-2017 | £4,482,578 | £4,467,404 | – | £15,174 |
| 2017-2018 | £4,421,361 | £4,777,455 | – | £356,094 |
| 2018-2019 | £4,752,912 | £4,579,303 | £1,625,492(2) | £173,609 |
| 2019-2020 | £4,943,224 | £4,947,844 | £1,800,230(3) | (£4,620) |
| 2020-2021 | £5,012,744 | £5,408,987 | – | (£396,243) |
| 2021-2022 | £5,067,060 | £5,163,588 | (£100,992)(4) | (£96,528) |
| 2022-2023 | £4,729,602 | £4,829,571 | – | (£99,969) |
Notes:
Some figures given above may differ from previously reported figures. This is due to final costs landing as the financial year closes and all accounting activities are recorded.
(1): In addition to the annual running budget of ASRU, there was additional capital expenditure which occurred for the replacement of our e-licensing system (ASPeL).
(2): In the year ending March 2019, £1,625,492 of agreed capital expenditure occurred for the replacement of ASPeL.
(3): In the year ending March 2020, £1,800,230 of agreed capital expenditure occurred for the replacement of ASPeL.
(4): In the year ending March 2022, ASRU received a credit of £100,992 for the replacement of ASPeL due to a previous administrative error.
ASRU income and expenditure for the years ending March 2021, 2022 and 2023
In the year ending March 2023, ASRU had a delegated budget from the Home Office in anticipation of the fee income of £4.3 million; by the close of the year, ASRU remained within 2.11% of the assigned budget.
Fee income
Increases in licence fees are necessary to ensure that fee income continues to cover all expenditure incurred in delivering the ASRU service.
Table 10.2: Annual licence fees, years ending March 2015 to 2022
| Annual fee(1) | 2015‑2018 | 2018‑2019 | 2019‑2020 | 2020‑2021 | 2021‑2022 |
|---|---|---|---|---|---|
| Personal licence | £242 | £257 | £275 | £299 | £299 |
| Establishment licence | £631 | £757 | £826 | £915 | £915 |
Notes:
(1): From 2018, fees are charged from 6 April each year, which is the common commencement date and is in line with practices in other government departments. Prior to 2018, fees were charged from 1 April.
Invoices are raised in arrears, so the income for the year ending March 2022 is collected in the following year.
The fees in the year ending March 2022 remained the same the following year.
Expenditure
Details of the expenditure for years ending March 2021, 2022 and 2023 are shown in Table 10.3.
Table 10.3: Summary of expenditure, by budgeting year, years ending March 2021 to 2023
| Category | 2020‑2021 | 2021‑2022 | 2022‑2023 |
|---|---|---|---|
| Pay & recharges | £3,397,001 | £3,187,412 | £2,775,603 |
| Consultancy | (£45) | ||
| Travel | £7,742 | £29,933 | £27,327 |
| Office supplies | £4,593 | £6,888 | £8,437 |
| Training & recruitment | (£3,626) | £13,696 | £17,0181 |
| Conferences | (£1,545) | (£29) | £31,755 |
| Estates | £56,903 | £1,771 | £214 |
| IT & comms | £1,231,632 | £775,639 | £793,171 |
| Marketing | £719 | (£719) | £5,353 |
| Legal | £12,143 | £14,453 | £869 |
| Special payments | |||
| Other | £3,427 | £8,034 | £113,207 |
| Direct costs | £4,708,987 | £4,037,032 | £3,772,954 |
| Overheads | £700,000 | £516,556 | £456,617 |
| Expenditure TOTAL | £5,408,987 | £4,553,588 | £4,229,571 |
| Depreciation | £600,000* | £600,000* | |
| Income | (£4,913,145) | (£5,067,060) | (£4,729,602) |
| Variance | £204,158 | (£86,528) | (£99,969) |
Notes:
*Financial year 2021 to 2022 is the first year that ASRU paid for depreciation for the ASPeL asset; this will be £600,000 for the next 5 years.
Some figures given above may differ from previously reported figures. This is due to final costs landing as the financial year closes and all accounting activities are recorded.
- In the year ending March 2021, approximately £3.40 million of the total pay costs were salary costs, of which £164,500 was transferred to other teams in the Home Office for the use of their staff on ASRU’s work, e.g. for the provision of statistical and legal advice.
- In the year ending March 2022, approximately £3.19 million of the total pay costs were salary costs, of which £202,695 was transferred to other teams in the Home Office for the use of their staff on ASRU’s work, e.g. for the provision of statistical and legal advice.
- In the year ending March 2023, approximately £2.77 million of the total pay costs were salary costs, of which £369,273 was transferred to other teams in the Home Office for the use of their staff on ASRU’s work, e.g. for the provision of statistical support. ASRU salary costs decreased that year due to a previous recruitment freeze, and as a result ASRU carried up to 15% vacancies.
- Central overheads are calculated on a headcount basis and cover core Home Office central functions and services such as central IT infrastructure, human resources and finance. They also cover an apportionment of the accommodation and facilities costs of the London Head Office at 2 Marsham Street and the Croydon Campus at Lunar House.
- The majority of IT and telecommunication costs for years ending March 2021, 2022 and 2023 include the hosting and support of ASPeL.
- Travel and subsistence costs were mostly incurred by inspectors during their visits to establishments. All travel occurred within Home Office policy guidance, which aims to balance speed and efficiency of travel against minimal cost. For the year ending March 2021, ASRU’s travel costs were greatly reduced following implementing national lockdown measures to control COVID-19, following which most inspection was undertaken remotely.
- For 2021, travel costs increased due to the easements of COVID-19 restrictions.
- In the year ending March 2021, ASRU paid other parts of the Home Office and other government departments for the use of office space in Glasgow, Dundee and Swindon.
Annex A: Non-compliance cases
| Glossary of terms | |
|---|---|
| ASPA | Animals (Scientific Procedures) Act 1986 |
| NVS | Named veterinary surgeon |
| PEL | Establishment licence |
| PIL | Personal licence |
| PPL | Project licence |
| SC | Standard condition |
Failure to comply with PPL authorities
| Description | Animal type involved | Animal numbers involved | Section of ASPA or SC breached | Regulator action taken |
|---|---|---|---|---|
| Multiple instances of lack of notification to the Regulator that severity/controls of the PPL had been exceeded, as required by PPL SC18 | Mouse, fish | 77 mice, 18 fish | PPL SC 18 | Inspector advice letter |
| Animals were not monitored in accordance with the PPL requirements | Mouse | 24 | PPL SC 1, PIL SC 2 | Letter of reprimand |
| Regulated procedure was performed without the use of anaesthesia, which contravened the PPL authorities | Mouse | 20 | PEL SC 1, PPL SC 1, PPL SC 17, PPL SC 18, PIL SC 9(b), PIL SC 12, PIL SC 19 | Letter of reprimand |
| Animal not given analgesia after procedure, as required by the controls in the PPL | Frog | 1 | PEL SC 1, PEL SC 21, PPL SC 1, PPL SC6, PIL SC 12, PIL SC 19 | Letter of reprimand |
| Pregnant animals unintentionally used for procedures, without PPL authority | Mouse | 4 | ASPA 3(b) | Inspector advice letter |
| Inadequate oversight of anaesthesia and monitoring regimen applied under the PPL | Mouse | 4 | PPL SC 1 | Inspector advice letter |
| Animals not given analgesia after procedure, as required by the controls in the PPL | Mouse | 3,572 | PEL SC 1, PEL SC 21, PPL SC 1, PIL SC 11, PIL SC 19 | Letter of reprimand |
| Substances administered that were not authorised by the PPL | Rat | 27 | PPL SC 1, PIL SC 19 | Inspector advice letter |
| Following a procedure, animals developed adverse effects that were not authorised on the PPL | Mouse | 28 | PPL SC 1, PIL SC 13, PIL SC 19 | Letter of reprimand |
| Animals were in metabolic cages for longer than authorised | Rat | 4 | PIL SC 2 | Inspector advice letter |
| Unauthorised buffer used for intraperitoneal injections, resulting in unauthorised adverse effects and ten deaths | Mouse | 14 | ASPA 3(b), PPL SC 1, PIL SC 19 | Letter of reprimand |
| Minor regulated procedures performed under general anaesthesia, which was not authorised on the PPL | Mouse | 70 | ASPA 3(b), PEL SC 15, PPL SC 1, PPL SC 4, PIL SC 1, PIL SC 19 | Inspector advice letter |
| Unauthorised post-surgical adverse effects were not reported and were treated without PPL authority | Horse | 1 | PPL SC 1, PPL SC 18 | Inspector advice letter |
| Procedures conducted by PIL holder without species authority | Frog | Unknown | ASPA 3(a), PEL SC 20, PPL SC 6, PIL SC 19 | Inspector advice letter |
| Inadequate monitoring of tumour size led to the authorised humane end-point being exceeded | Mouse | 1 | PIL SC 2, PIL SC 14 | Inspector advice letter |
| Regulated procedures were performed that were not authorised on the PPL; a specific surgical approach was authorised, but a different and less refined approach was used | Mouse | 2 | ASPA 3(b), PIL SC 19 | Inspector advice letter |
| Non-experimental companion mice were water restricted along with the experimental mice, which was not authorised by the PPL | Mouse | 5 | ASPA 3(b) | Inspector advice letter |
| After procedures had been performed under general anaesthesia, animals fully regained consciousness, which was not authorised by the PPL | Mouse | 8 | PPL SC 1, PIL SC 19 | Inspector advice letter |
| Animals were not killed promptly at the end of the regulated procedures | Mouse | 4 | PPL SC 11 | Inspector advice letter |
| Unnecessary blood sample taken, due to misidentification of the animal | NHP | 1 | ASPA 3(b), PEL SC20 | Inspector advice letter |
| Regulated procedure undertaken repeatedly, over a long period, without PPL authority | Fish | Unknown | ASPA 3(b), PIL SC 19 | Inspector advice letter |
| Intracerebral and intraperitoneal injections administered (under general anaesthesia) unnecessarily, in error | Guinea pig | 7 | PPL SC 2, PPL SC 4 | Letter of reprimand |
| A regulated procedure (ear clipping for genotyping) was conducted, which was not authorised by the PPL | Mouse | 1 | PEL SC 20, PPL SC 1, PIL SC 19 | Inspector advice letter |
| Animals under PPL authority that exceeded the severity limit were not reported promptly | Mouse | 6 | PPL SC 18 | Inspector advice letter |
| Animal not provided with minimum daily fluid requirement due to communication issue | NHP | 1 | PPL SC 1, PIL SC 2, PIL SC 14 | Inspector advice letter |
| Animal with an unexpected adverse effect was kept alive without authority | Mouse | 1 | PPL SC 1, PPL SC 6, PPL SC 18, PIL SC 1, PIL SC 2, PIL SC 14, PIL SC 15 | Letter of reprimand |
| Animals had vaginal smears taken in error | Rat | 59 | PIL SC 1 | Inspector advice letter |
| Animals exceeded the maximum age authorised by the PPL | Mouse | 15 | PPL SC 1 | Inspector advice letter |
| A substance was administered at a significantly higher dose than authorised by the PPL | Mouse | 1 | ASPA 3(b) | Inspector advice letter |
| Animal numbers used on an experimental protocol exceeded the number authorised by the PPL | Mouse | 289 | PPL SC 1, PPL SC 19 | Inspector advice letter |
| Procedures were conducted on animals solely for training, which was not authorised by the PPL | Mouse | 3 | ASPA 3(b) | Inspector advice letter |
| A neuromuscular blocking agent was administered to an anaesthetised pig without PPL authority | Pig | 1 | ASPA 3(b), ASPA 17, PIL SC 19 | Inspector advice letter |
| Anaesthetic solution was buffered inappropriately, which led to ten fish dying | Fish | 16 | PPL SC 4 | Letter of reprimand |
| Number of mouse embryos used for regulated procedures exceeded that authorised by the PPL | Mouse | 354 | PPL SC 1 | Inspector advice letter |
| Animals that had experienced greater actual severity than authorised by the PPL were not reported promptly | Mouse | 9 | PPL SC 18 | Inspector advice letter |
| Post-surgical wounds managed without PPL authorisation | Mouse | 1 | ASPA 3(b), PIL SC 15, PIL SC 19 | Inspector advice letter |
| Animal numbers used on a breeding protocol exceeded the number authorised by the PPL | Mouse | 1,462 | PPL SC 1 | Inspector advice letter |
| Animal numbers used on an experimental protocol exceeded the number authorised by the PPL | Mouse | 175 | PPL SC 1 | Inspector advice letter |
| Animal numbers used on a breeding protocol exceeded the number authorised by the PPL | Mouse | 2,743 | PPL SC 1 | Inspector advice letter |
| Monitoring and humane end- point not adhered to, as required by PPL | Mouse | 1 | PPL SC 1, PIL SC 2, PIL SC 14 | Letter of reprimand and Inspector advice letter |
| Water was removed for one hour per day without PPL authority | NHP | 36 | PPL SC 1 | Inspector advice letter |
| Animals were injected with a substance that was not authorised by the PPL | Mouse | 5 | PPL SC 1, PIL SC 19 | Inspector advice letter |
| Animal numbers used on an experimental protocol exceeded the number authorised by the PPL | Mouse | 415 | PPL SC 1 | Inspector advice letter |
| Animal was sedated unnecessarily due to mistaken identity | NHP | 1 | ASPA 3b | Inspector advice letter |
| Two intramuscular injections were administered to each animal, but the PPL only authorised one | Mouse | 20 | PIL SC 19 | Inspector advice letter |
| Monitoring and humane end-point not adhered to, as required by PPL | Dog | 1 | PIL SC 2 | Inspector advice letter |
| Animals received a second dose of a substance in error, which was not authorised by the PPL | Mouse | 10 | PIL SC 1, PIL SC 4 | Compliance Notice with re-training |
| Surgical wound was re-sutured more than 48 hours post-surgery, which was not authorised by the PPL | Mouse | 1 | PIL SC 19 | Inspector advice letter |
| Animals were administered a higher dose of a specified substance than was authorised by the PPL | Mouse | 28 | PPL SC 1, PIL SC 19 | Inspector advice letter |
| Animal was not euthanised when its weight loss exceeded the authorised humane end-point | Mouse | 1 | PIL SC 5 | Inspector advice letter |
| Animal kept beyond age authorised on the PPL | Mouse | 3 | PIL SC 19 | Inspector advice letter |
| Animals not monitored as required in PPL, and they exceeded the humane end-point for tumour growth | Mouse | 4 | PPL SC 1, PIL SC 2, PIL SC 19 | Letter of reprimand and Inspector advice letter |
| Animal was not euthanised when it reached the humane end-point that was authorised in the PPL | Mouse | 1 | PPL SC 1 | Inspector advice letter |
| Animals kept alive for longer than authorised | Fish | 17 | PPL SC 1 | Inspector advice letter |
| Animal moved from one PPL protocol to another without authority | Mouse | 1 | ASPA 3(b), PIL SC 2 | Inspector advice letter |
| Miscommunication led to animals not being monitored as required by the PPL or killed at the scientific end-point, and five subsequently died | Mouse | 10 | PIL SC 2, PIL SC 14 | Letter of reprimand |
| Animal numbers used on an experimental protocol exceeded the number authorised by the PPL | Rat | 72 | PPL SC 1 | Inspector advice letter |
| Animal numbers used on a breeding protocol exceeded the number authorised by the PPL | Fish | 1671 | PPL SC 1 | Inspector advice letter |
| Animals were not monitored as specified in the PPL | Hamster | 29 | PPL SC 1, PIL SC 2, PIL SC 19 | Inspector advice letter |
| Newborn pups were separated from their dams for a scientific purpose that was not authorised by the PPL; ten subsequently died | Mouse | 14 | PPL SC 1, PIL SC 19 | Inspector advice letter |
| Animals were re-used, which was not authorised by the PPL | Sheep | 2 | ASPA 14(1), PPL SC 1 | Inspector advice letter |
| Procedures on animals carried out for a purpose not authorised on PPL | Mouse | 48 | PPL SC 1 | Inspector advice letter |
| Regulated procedure performed that was not authorised by the PPL (suturing of a non-surgical wound) | Mouse | 1 | ASPA 3(b), PIL SC 15, PIL SC 19 | Inspector advice letter |
| Animal numbers used on a breeding protocol exceeded the number authorised by the PPL | Mouse | 605 | PPL SC 1 | Inspector advice letter |
| Controls specified by the PPL were not adhered to | NHP | 1 | PIL SC 2 | Inspector advice letter |
| Procedures performed that were not authorised by the PPL | Mouse | 30 | ASPA 3(b), PPL SC 1, PIL SC 19 | Inspector advice letter |
| Animals kept beyond the age authorised by the PPL | Fish | 64 | PPL SC 1 | Inspector advice letter |
| Animal kept for longer on a study than authorised by the PPL | Mouse | 1 | PPL SC 1 | Inspector advice letter |
| Animals not monitored as stated in the PPL | Mouse | 44 | PIL SC 19 | Inspector advice letter |
| Animals not monitored as stated in the PPL | Mouse | 2 | PIL SC 2, PIL SC 19 | Inspector advice letter |
| Control measure specified by PPL was omitted | Mouse | 16 | PPL SC 1, PIL SC 19 | Inspector advice letter |
| Hen eggs that had undergone injections of a substance were kept beyond the age of protection, which did not have PPL authorisation | Chicken | 1,100 | PPL SC 1, PIL SC 19 | Inspector advice letter |
Failure to provide adequate care
| Description | Animal type involved | Animal numbers involved | Section of ASPA or SC breached | Regulator action taken |
|---|---|---|---|---|
| A rabbit was injured when it escaped from its cage and was euthanised | Rabbit | 1 | PEL SC 4(1), PEL SC 4(4) | Compliance Notice |
| A number of fish died due to equipment failure | Fish | 118 | PEL SC 4(4), PEL SC 4(7) | Compliance Notice |
| Poor communication may have contributed to unnecessary suffering | Frog | 4 | PEL SC 21 | Letter of reprimand |
| A tank overflowed due to a blocked outflow, which resulted in fish fry escaping, which all died or were euthanised | Fish | 117 | PEL SC 4(1), PEL SC 4(4) | Letter of reprimand |
| Animals died as a result of water conductivity and temperature rising due to equipment malfunction | Fish | 198 | PEL SC 4(3), PEL SC 4(7) | Letter of reprimand |
| Fifteen mice died when their cage was flooded due to malfunction of the automated watering system | Mouse | 16 | PEL SC 4(1), PEL SC 4(4) | Letter of reprimand |
| Fish were accidentally exposed to water contaminated with saline due to a husbandry error | Fish | 200 | PEL SC 4(1) | Letter of reprimand |
| Poor communication processes led to animals that had been transferred to another room being left overnight without food or water | Mouse | 2 | PEL SC 21, PIL SC 14 | Inspector advice letters |
| Fish in two tanks died, due to poor water quality | Fish | 50 | PEL SC 4(1), PEL SC 4(7) | Letter of reprimand |
| Male study dog was accidentally singly housed for approximately 19 hours | Dog | 1 | PEL SC 4(2), PEL SC 4(4) | Inspector advice letter |
| Four mouse pups died during transport, due to excessive heat from a hot water bottle | Mouse | 9 | PEL SC 4(6) | Letter of reprimand |
| Eight rats died due to insufficient ventilation of their cages | Rat | 60 | PEL SC 4(1), PEL SC 4(4), PEL SC 5 | Letter of reprimand |
| Weight not monitored, and more weight lost than authorised | Mouse | 1 | PPL SC1, PIL SC 2, PIL SC 14 | Inspector advice letter |
| Rats under PPL authority were left without water for 19 hours due to lack of care by PIL holder | Rat | 4 | PIL SC 2 | Inspector advice letter |
| Rat was loose in room for up to 24 hours and missed at one check | Rat | 1 | PEL 4 | Inspector advice letter |
| An animal was accidentally left in a transport box for over 24 hours | Hamster | 1 | PEL SC 4(1) | Inspector advice letter |
| Immunocompromised animals not kept in suitable environment and became ill | Mouse | 11 | PIL SC 2 | Letter of reprimand |
| Animal placed in a warming cabinet for longer than standard practice and than was scientifically necessary | Rat | 1 | PIL SC 1 | Inspector advice letter |
| Animals experienced unauthorised adverse effects after surgical implantation of intraperitoneal device; lack of care taken in planning and communication | NHP | 3 | PEL SC 1, PEL SC 21, PPL SC 1, PPL SC 4, PIL SC 1, PIL SC 4 | Letter of reprimand |
| Oral dosing not performed in the most refined manner | NHP | 6 | PEL SC 1, PPL SC 1, PPL SC 4, PIL SC 1 | Letter of reprimand |
| Animal found in cage wash area 2 days after receipt and unpacking – absence not detected | Mouse | 1 | PEL SC 4(1), PEL SC 4(4), PEL SC 5 | Letter of reprimand |
| An animal overturned an insecure container it was in, which dropped to floor; the animal died almost immediately | Rat | 1 | PEL SC 4(4) | Inspector advice letter |
| An animal was trapped under a lid and died almost immediately | Mouse | 1 | PEL SC 4(4) | Inspector advice letter |
| Animal was injured when it fell to the floor, having been contained within an unstable container | Rat | 1 | PEL SC 4(4) | Inspector advice letter |
| Adequate care was not provided to an animal post-surgery | Rat | 1 | PEL SC 4(1) | Letter of reprimand |
| Rats and mice exposed to continuous light for many weeks in the holding rooms | Other | Unknown | PEL SC 4(3), PEL SC 4(5) | Inspector advice letter |
| Cage was flooded by the automatic watering system, resulting in the death of four mouse pups | Mouse | 8 | PEL SC 4(1), PEL SC 4(4) | Letter of reprimand |
| Mice exposed to continuous light for up to approximately three weeks in the holding rooms | Mouse | Unknown | PEL SC 4(3), PEL SC 4(7) | Inspector advice letter |
| Animal was found with missing limb, which had not been previously detected | Mouse | 1 | PEL SC 4(1), PEL SC 4(5) | Letter of reprimand |
| Animal incorrectly euthanised and offspring died as a consequence | Mouse | 8 | PIL SC 1 | Inspector advice letter |
| Pups drowned due to the automatic watering system flooding a cage | Mouse | 18 | PEL SC 4(1), PEL SC 4(4) | Letter of reprimand |
| Tadpoles died due to human error in care provision | Xenopus | 47 | PEL SC 4(1) | Inspector advice letter |
| Neonatal animals were left without their mother and died or were euthanised | Mouse | 4 | PEL SC 4(1) | Inspector advice letter |
| Cage was flooded by the automatic watering system, resulting in the death of nine mouse pups | Mouse | 12 | PEL SC 4(1), PEL SC 4(4) | Letter of reprimand |
| Daily check of animals not completed in an animal isolator | Mouse | Unknown | PEL SC 4(5) | Inspector advice letter |
| Animals died due to inadequate ventilation of experimental equipment | Rat | 2 | PIL SC 2 | Inspector advice letter |
| Four animals found dead after fin-clipping the previous day; probable cause was a drop in the water temperature | Fish | 53 | PEL SC 4(1) | Letter of reprimand |
| Animals died due to lack of adequate ventilation | Rat | 3 | PIL SC 2, PIL SC 14 | Letter of reprimand |
| Animal was injured when it fell to the floor | Rat | 1 | PEL SC 4(1), PEL SC 4(4), PEL SC 5 | Letter of reprimand |
| Schedule 1 killing of a rat pup was not performed competently | Rat | 1 | ASPA 15A(1)(b) | Inspector advice letter |
| Communication error led to lack of adequate care for three juvenile mice | Mouse | 3 | PEL SC 5 | Inspector advice letter |
| Fish became trapped in a piece of equipment in their tank and seven died | Fish | 9 | PEL SC 4(4) | Letter of reprimand |
| Nine cages of animals were not checked daily, for two consecutive days | Mouse | 14 | PEL SC 4(5) | Inspector advice letter |
| Animals jumped from cages in two separate incidents and died | Mouse, rat | 1 mouse, 1 rat | PEL SC 4(4) | Inspector advice letter |
| Two separate husbandry incidents, in which baffles were not placed in tanks to retain the fish, resulted in the death of animals | Fish | 15 | PEL SC 4(1) | Letter of reprimand |
| Animals escaped the tank and were found dead due to the baffle not being in place to retain fish | Fish | 6 | PEL SC 4(1) | Letter of reprimand |
| Animals suffered injuries after another animal managed to escape from its enclosure | NHP | 2 | PEL SC 4(1) | Inspector advice letter |
| Cage was flooded by the automatic watering system, resulting in the death of one mouse pup | Mouse | 15 | PEL SC 4(1), PEL SC 4(4) | Letter of reprimand |
| Animals left in inappropriate accommodation overnight, following miscommunication with courier | Mouse | 3 | PEL SC 4(1) | Inspector advice letter |
| Three mouse pups died after their cage was flooded by the automatic watering system | Mouse | 6 | PEL SC 4(1), PEL SC 4(4) | Letter of reprimand |
| Eight mouse pups died after their cage was flooded by the automatic watering system | Mouse | 11 | PEL SC 4(1), PEL SC 4(4) | Letter of reprimand |
| Animals died due to lack of ventilation; their cage was not placed back onto a ventilated rack | Hamster | 3 | PIL SC 2 | Letter of reprimand |
| Animals were left in the cage wash area and were subsequently found dead | Mouse | 5 | PEL SC 4(3), PEL SC 4(5) | Letter of reprimand |
| Animals were held for up to two weeks with less floor space than specified by the Code of Practice | Rat | 40 | PEL SC 4(3) | Inspector advice letter |
| Animals exposed to continuous light in holding rooms for up to approximately 6 months | Fish | Unknown | PEL SC 4(3), PEL SC 4(7) | Inspector advice letter |
| Animal was injured and died when it fell to the floor from a container while being weighed | Rat | 1 | PEL SC 4(4) | Inspector advice letter |
| Animals escaped tank and one was found dead; due to the baffle not being in place to retain fish | Fish | 8 | PEL SC 4(1) | Letter of reprimand |
| Animals exposed to continuous light in holding rooms for up to approximately 12 days | Mouse | 710 | PEL SC 4(3), PEL SC 4(7) | Inspector advice letter |
| Animal found dead, trapped between the food hopper and back of cage; daily checks failed to detect this | Mouse | 1 | PEL SC 4(5) | Inspector advice letter |
| Animals were left, unchecked, in a transport container for two days, due to administration errors | Rat | 6 | PEL SC 4(1) | Inspector advice letter |
| Manifold on water system found closed, which led to poor water quality, resulting in adverse outcomes for three fish | Fish | 150 | PEL SC 4(3), PEL SC 4(5) | Inspector advice letter |
| Cage label did not include all legally required information | Mouse | 1 | PIL SC 16 | Inspector advice letter |
| Cage label info missing | Marmoset | 2 | PIL SC 16 | Inspector advice letter |
| Power interruption stopped water system pumps running, which was not flagged by an alarm; five fish died | Fish | 33 | PEL SC 4(1), PEL SC 4(7) | Letter of reprimand |
| Animal escaped through a gap in the baffle at back of tank | Fish | 1 | PEL SC 4(1), PEL SC 4(4) | Inspector advice letter |
Failure to provide food/water
| Description | Animal type involved | Animal numbers involved | Section of ASPA or SC breached | Regulator action taken |
|---|---|---|---|---|
| Animals were without water for approximately 48 hours | Mouse | 8 | PEL SC 4(3) | Inspector advice letter |
| Animals were without access to water for approximately 96 hours, with adverse welfare outcomes | Mouse | 2 | PEL SC 4(3) | Letter of reprimand |
| Six mice left without access to water for four days, due to nozzle being removed and not noticed at daily checks | Mouse | 6 | PEL SC 4(1), PEL SC 4(3), PEL SC 4(5) | Letter of reprimand |
| Newly weaned mice did not have access to food for up to three days, and showed some clinical signs | Mouse | 7 | PEL SC 4(3) | Inspector advice letter |
| Animals were without food for approximately 36 hours | Mouse | 11 | PEL SC 4(3) | Inspector advice letter |
| A cage of animals was found with no access to food; one mouse had died and the others were euthanised due to clinical signs | Mouse | 7 | PEL SC 4(3), PEL SC 4(5), PEL SC 5 | Letter of reprimand |
| Animal found with injury to tail resulting in being culled by Schedule 1 | Mouse | 3 | PEL SC 4(3), PIL SC 16 | Inspector advice letter |
| No valves on two cages supplied by an automatic watering system; animals were found dead | Mouse | 4 | PEL SC 4(3) | Letter of reprimand |
| Animals were without water for ~1.5 days | Mouse | 2 | PEL SC 4(3), PEL SC 4(5) | Inspector advice letter |
| Animals were without food for approximately 40 hours | Rat | 2 | PEL SC 4(3) | Inspector advice letter |
| Daily checks failed to detect the lack of food for five to six days; mouse was found dead | Mouse | 1 | PEL SC 4(3) | Letter of reprimand |
| Animals were left in a transport box without water for 48 hours | Mouse | 4 | PEL SC 4(1), PEL SC 4(2), PEL SC 4(3), PEL SC 4(6) | Inspector advice letter |
| Animals supplied by the automatic watering system went without water for 24 to 48 hours (in two incidents) | Mouse | 7 | PEL SC 4(1), PEL SC 4(3) | Inspector advice letter |
Failure to have the appropriate personal licence (PIL) authority
| Description | Animal type involved | Animal numbers involved | Section of ASPA or SC breached | Regulator action taken |
|---|---|---|---|---|
| Regulated procedures were performed without PIL authority | Fish | 7 | ASPA 3(a), PPL SC 6 | Letter of reprimand, with re-training, and letter of censure |
| Regulated procedures performed without PIL authority | Mouse | 44 | ASPA 3(a), PEL SC 20 | Inspector advice letters |
| Procedures were carried out without PIL authority (killing by a non-Schedule 1 method) | Rat | 10 | ASPA 3(a), PEL SC 2, PEL SC 20, PPL SC 6 | Inspector advice letters |
| A regulated procedure (intranasal dosing) was carried out without PIL authority | Mouse | 1 | ASPA 3(a), PEL SC 20, PPL SC 6 | Inspector advice letters |
| Procedures (fin-clipping) carried out by four trainees without PIL authority and without PPL authority for training | Fish | 4 | ASPA 3(a), ASPA 3(b), PEL 15 | Inspector advice letters |
| Blood sampling performed without PIL authority (12 mice). Also, five mice underwent a regulated procedure for the sole purpose of training, which was not an authorised purpose on the PPL | Mouse | 17 | ASPA 3(a), ASPA 3(b) | Inspector advice letters |
| A regulated procedure was conducted when there was no PIL authority for that species | Other | 2 | ASPA 3(a), PIL SC 19 | Inspector advice letter |
| Procedures were carried out without the correct category of PIL authority | Cattle; sheep | 2 cattle; 2 sheep | ASPA 3(a), PIL SC 19 | Inspector advice letter |
| Performed surgery on live animals without PIL authority for surgical procedures | Mouse | 2 | ASPA 3(a), PIL SC 19 | Inspector advice letter |
| Procedures were carried out without the correct category of PIL authority | Mouse | 2 | ASPA 3(a), PIL SC 19 | Inspector advice letter |
| Injections performed without PIL authority | Mouse | 4 | ASPA 3(a) | Inspector advice letter |
| Regulated procedures (subcutaneous injections) were performed without PIL authority | Xenopus | 12 | ASPA 3(a) | Inspector advice letter |
| Procedures were carried out without the correct category of PIL authority | Mouse | 480 | ASPA 3(a) | Inspector advice letter |
| Regulated procedures (subcutaneous injections) were conducted when there was no PIL authority for that species | Rat | 72 | ASPA 3(a), PIL SC 19 | Inspector advice letter |
| Procedures were carried out without the correct category of PIL authority | Mouse | 9 | ASPA 3(a) | Inspector advice letter |
Unauthorised location: Performing procedures or keeping animals in area not specified on PEL
| Description | Animal type involved | Animal numbers involved | Section of ASPA or SC breached | Regulator action taken |
|---|---|---|---|---|
| An animal was kept in a room not authorised for overnight holding | Mouse | 1 | PIL SC 2 | Letter of reprimand |
| Regulated procedures (imaging) performed in a room not authorised on the establishment licence | Fish | 20 | ASPA 3(c), PEL SC 20 | Inspector advice letter |
Failure to adhere to licence conditions that mandate record keeping, re-homing and security
| Description | Animal type involved | Animal numbers involved | Section of ASPA or SC breached | Regulator action taken |
|---|---|---|---|---|
| A single stock animal was re-homed without authorisation | Hamster | 1 | ASPA 17A | Inspector advice letter |
| Live sentinel animals sent to diagnostic lab for diagnostic screening without authority | Mouse | Unknown | ASPA 17A | Inspector advice letter |
| Non-compliances in documentation discovered at audit | N/A | N/A | PEL SC 2, PEL SC 8 | Inspector advice letter |
| Killing register did not include all methods of killing | N/A | N/A | PEL SC 2 | Inspector advice letter |
| Establishment did not have a Killing Register | N/A | N/A | PEL SC 2 | Inspector advice letter |
| Unauthorised person could gain access to the establishment | N/A | N/A | PEL SC 17 | Inspector advice letter |
| Stock animals re-homed without authority | Rat; guinea pig | 6 rats; 2 guinea pigs | ASPA 17A(2) | Inspector advice letter |
| Killing register not legally compliant | N/A | N/A | PEL SC 2 | Inspector advice letter |
Annex B: Tables and figures
Table 1: Licence applications and amendments, 2022
| Totals | |
|---|---|
| PILs(1) Granted | 2319 |
| PILs Amended | 696 |
| PILs in force at year-end | 13483 |
| PELs(2) Granted | 1 |
| PELs Amended | 26 |
| PELs in force at year-end | 135 |
| PPLs(3) granted | 490 |
| PPLs amended | 987 |
| PPLs in force at year-end | 2300 |
Notes:
(1): PIL = personal licence. (2): PEL = establishment licence. (3): PPL = project licence.
Figure 2: Inspectorate staff, 2011-2022
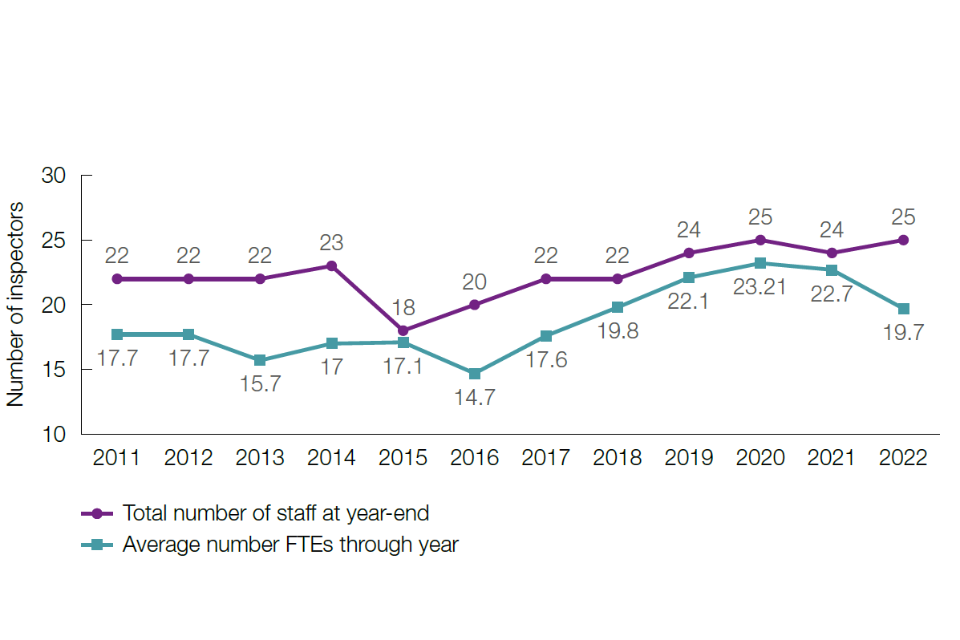
Notes:
FTE = full-time equivalent averaged across the year.
Figure 3: Project Licences granted, 2011-2022
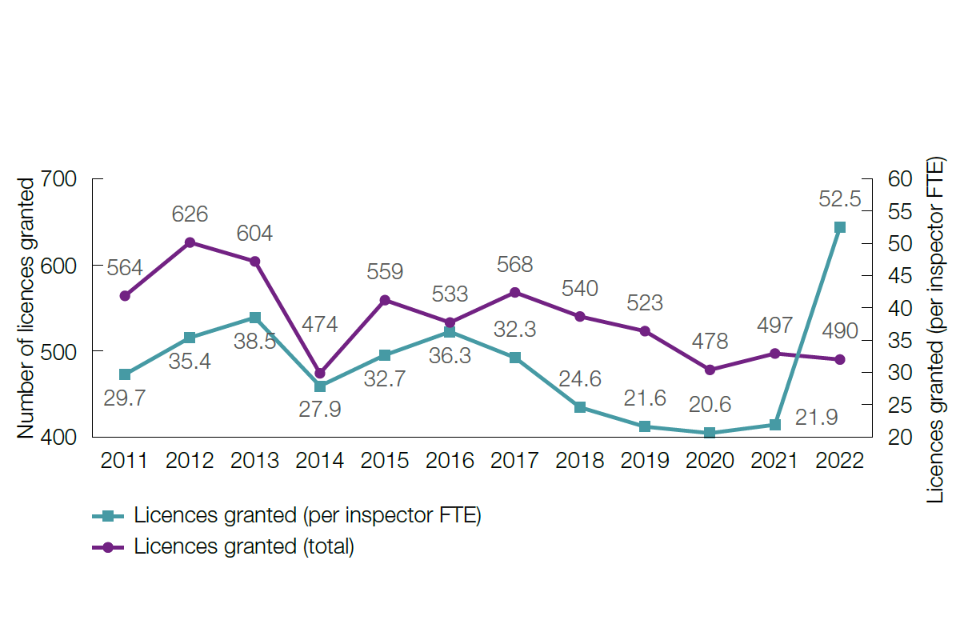
Notes:
Due to operational improvements productivity improved across licensing in 2022.
