Carbapenemase-producing Gram-negative organisms in England since October 2020: quarterly update, Q2 2024
Updated 11 December 2025
Applies to England
Main messages
Main messages from this report are as follows:
- The quarterly rate of reported carbapenemase-producing organism (CPO) episodes in Q2 2024 was the highest since mandatory reporting began in Q4 2020, with 3.5 per 100,000 population (an increase from 2.7 per 100,000 population in Q1 2024). This increase was predominantly due to an increase in positive screening samples.
- Reporting of CPO from sterile site specimens remained stable in Q2 2024 compared with Q1 2024, with the latest three quarters seeing the highest numbers of positive sterile site specimens since mandatory surveillance began.
- The most commonly reported acquired carbapenemase in the last year (Q3 2023 to Q2 2024) was NDM (35.7%), followed by OXA-48-like (35.1%) and KPC (20.5%), however this varied by region; in London and the South East, NDM were the most commonly reported mechanisms, OXA-48-like was most common in the East of England, whereas KPC dominated reports in the North West.
- No changes were reported in the demographic groups (by age and sex, region, ethnic group and IMD decile).
Background
Since 1 October 2020, all diagnostic laboratories in England have a duty to notify the following via the UK Health Security Agency (UKHSA)’s Second Generation Surveillance System (SGSS) (1):
- acquired carbapenemase-producing Gram-negative organisms identified in human samples
- the results of any antimicrobial susceptibility test and carbapenem resistance mechanism in any of the causative agents listed in Schedule 2 of the Health Protection (Notification) Regulations 2020 (1).
This requirement was launched in conjunction with the national Framework of actions to contain carbapenemase-producing Enterobacterales (CPE), which sets out a range of measures that, if implemented well, will help health and social care providers minimise the impact of carbapenemase-producing organisms (CPOs).
The analyses below are based on data relating to notifications of confirmed acquired CPOs between 1 October 2020 and 30 June 2024 in England. The data was extracted on 14 August 2024 from both UKHSA’s voluntary surveillance database, SGSS, and the Antimicrobial Resistance and Healthcare-Associated Infections (AMRHAI) Reference Unit database.
Both annual and quarterly rates of acquired CPOs were calculated using mid-year resident population estimates for the respective year and geography. Geographical analyses were based on the patient’s residential postcode. Where this information was unknown, the postcode of the reporting laboratory was used. Cases in England were further assigned to one of 9 local areas, formed from the administrative local authority boundaries. The annual rates of acquired CPOs by geographical region included in this report differ slightly from those calculated in some previous reports due to a methodological adjustment following the identification of an inconsistency in previous reports’ methodology. Previous reports will be corrected accordingly.
Samples are split into three specimen types: sterile site specimens (eg blood or cerebrospinal fluid), screening site specimens (eg faeces or lower gastrointestinal tract) or ‘other’ specimen type (eg urine or lower genital tract). As patients may have more than one positive specimen taken, specimens taken from the same patient that yielded growth of the same bacterial species, had the same carbapenemase gene and were from the same specimen type, within a 52-week period from the initial positive specimen, were regarded as comprising the same episode and were de-duplicated. CPO isolates referred to the AMRHAI Reference Unit and local laboratory isolates were combined for this de-duplication process, with resistance mechanism results from the AMRHAI Reference Unit retained preferentially where patient specimen overlap occurred. (It should be noted that not all specimens in this report are confirmed by the AMRHAI Reference Unit). This method differs slightly from weekly causative agent notification data where data is not de-duplicated incorporating specimen type. In addition, the data presented in the weekly notification reports utilises SGSS reports only.
The index of multiple deprivation (IMD) is a way of summarising the level of deprivation within an area, based on a set of factors that includes their levels of income, employment, education and local levels of crime. Episodes were linked to IMD using patient postcode (and GP or laboratory postcode where patient postcode was unavailable) and the IMD decile score was identified by the lower super output area the patient resided in.
The Office for Health Improvements and Disparities developed a method for assigning ethnic group based on hospital admissions data. As different ethnicities may be recorded in different treatment episodes, the method selected a single ethnic group from a patient’s HES records. Episodes were linked to ethnic group using patient NHS number and date of birth.
The following report summarises trends and geographical distribution of carbapenemase mechanisms identified from Gram-negative bacteria in human samples. Bacterial species, mechanism, sample type, and age and sex of patients are also described. For the purposes of this report, quarters are calendar quarters, as such January to March is referred to as ‘Q1’, April to June is referred to as ‘Q2’, July to September is referred to as ‘Q3’ and October to December is referred to as ‘Q4’, alongside relevant years.
Note: Scientific names are not italicised in this report to ensure our content is inclusive for all users and in compliance with web accessibility legislation and associated guidelines.
Microbiology services
For reference services, including species identification and confirmation of susceptibility testing results, laboratories should contact UKHSA’s Antimicrobial Resistance and Healthcare Associated Infections (AMRHAI) Reference Unit in Colindale, London.
Table 1 summarises the carbapenemase gene families that are targeted – using the routine polymerase chain reaction (PCR) applied to referred Enterobacterales, Pseudomonas spp. and Acinetobacter spp. – because they are suspected of harbouring an acquired carbapenemase gene. UKHSA strongly recommends that all diagnostic laboratories are able to detect, at least, the 4 carbapenemase families shown in bold in this table (the ‘big 4’) using either PCR or immunochromatographic methods.
Where an ‘exceptional’ carbapenemase and genus combination result (cells without a ¥ symbol in Table 1) has been identified, or where an unusual organism has been identified with an acquired carbapenemase (that is, any bacterial genera other than a member of the Enterobacterales, Pseudomonas spp. or Acinetobacter spp.), isolates should be sent to the AMRHAI Reference Unit for confirmation.
Table 1. Distribution among bacterial pathogens of carbapenemase genes covered by AMRHAI Reference Unit’s molecular assay (based on AMRHAI data) [note 1]
| Carbapenemase family | Associated with common ‘host’ organism Enterobacterales | Associated common ‘host’ organism Pseudomonas spp. | Associated with common ‘host’ organism Acinetobacter spp. |
|---|---|---|---|
| KPC | ¥ | <10D | <10D |
| OXA-48-like | ¥ | <10D | 0 |
| NDM | ¥ | ¥ | ¥ |
| VIM | ¥ | ¥ | <10D |
| IMP | ¥ | ¥ | ¥ |
| IMI/NMC-A | ¥B | 0 | 0 |
| GES | ¥ | ¥ | <10D |
| FRI | <10 | 0 | 0 |
| SME | ¥C | 0 | 0 |
| DIM | 0 | <10D | 0 |
| GIM | <10D | <10D | 0 |
| SIM | 0 | <10D | 0 |
| SPM | 0 | <10D | 0 |
| OXA-23-like | <10D | 0 | ¥ |
| OXA-40-like | 0 | 0 | ¥ |
| OXA-51-likeA | 0 | 0 | ¥ |
| OXA-58-like | 0 | 0 | ¥ |
Notes to Table 1
Note 1. Table 1 uses the following symbols:
¥ = combinations of mechanism and genus are not considered exceptional.
A = intrinsic to A. baumannii and only expressed when associated with an insertion element.
B = almost exclusively reported in Enterobacter spp. with less than a handful of reports in other genera.
C = reported only in Serratia marcescens.
D = fewer than 10 in total ever referred to AMRHAI Reference Unit.
Recent developments
Unusual acquired CPOs referred to the AMRHAI Reference Unit in Q2 2024 included further isolates (n=1 each) of Proteus mirabilis and Escherichia coli positive for OXA-23 from urine and a rectal swab in the West Midlands and South East, respectively. Genomic analysis is underway to determine the clonality of OXA-23-positive isolates of these two genera and to assess the potential for further transmission of the OXA-23 gene. A further isolate of Pseudomonas aeruginosa positive for DIM metallo-carbapenemase was isolated in the East of England from an ear swab; this is only the tenth Pseudomonas spp. isolate referred to the AMRHAI Reference Unit since 2015. As neither OXA-23 nor DIM carbapenemase genes can be detected using diagnostic assays that target the ‘big 4/5’ carbapenemase families (‘big 5’ includes IMP), laboratories are reminded to refer isolates that meet AMRHAI referral criteria (2) to the Reference Unit for screening for rarer carbapenemase genes.
The most common dual carbapenemase combination observed amongst Reference Unit referrals is NDM + OXA-48-like. However, in Q2 2024 the following combinations were also identified: IMP + NDM (one P. aeruginosa), IMP + OXA-48-like (two Enterobacter cloacae complex and one Klebsiella oxytoca) and KPC + OXA-48-like (one Enterobacter cloacae complex). Isolates with these combinations are currently rare amongst AMRHAI submissions and should be referred for confirmation (free-of-charge for NHS laboratories) and inclusion in the AMRHAI strain collection.
Locally-confirmed unusual combinations of organism and mechanism should be referred to the AMRHAI Reference Unit for confirmation. Follow up of these unusual combinations has identified some have been due to mixed cultures or reporting errors.
Specimen type
Between October 2020 and June 2024, there were 16,623 acquired CPO episodes reported. The majority were identified in screening samples, accounting for 71.6% of notifications, with only 4.5% reported from sterile site specimens (Table 2).
Table 2. Number and percentage of acquired CPO episodes by specimen type (England): October 2020 to June 2024
| Specimen type | All reports number | All reports % [note 3] | From AMRHAI number | From AMRHAI % [note 4] |
|---|---|---|---|---|
| Sterile site samples | 740 | 4.5 | 253 | 10.3 |
| Screening samples | 11,901 | 71.6 | 1,179 | 48.2 |
| Other samples [note 2] | 3,982 | 24.0 | 1,014 | 41.5 |
| All samples | 16,623 | 100.0 | 2,446 | 100.0 |
View this table in the data tables file.
Notes to Table 2
Note 2. Samples that do not fall into either ‘sterile site’ or ‘screening’ samples, for example, urine and lower genital tract specimens.
Note 3. The percentages presented in this table are column percentages, with the breakdown of specimen types shown for all reports and AMRHAI reports separately.
Note 4. The AMRHAI Reference Unit actively encourages submission of sterile site isolates for carbapenemase confirmation; the distribution of specimen type will reflect this.
Quarterly trends
The overall annual rate of CPO episodes in England was 11.9 per 100,000 population. In Q2 2024, the highest quarterly rate of CPO episodes was observed since mandatory reporting began in Q4 2020, with 3.5 episodes per 100,000 (Figure 1). This represents a 29.6% increase in rate since the previous quarter (2.7 CPO episodes per 100,000 in Q1 2024). This increase was predominantly due to an increase in positive screening samples, from 1,053 in Q1 2024 to 1,416 in Q2 2024. The number of sterile site infections remained stable in Q2 2024 compared with Q1 2024. However, this followed a steady increase in the rate of CPO episodes in sterile site samples since Q2 2022 (Figure 1), with the number of reported sterile site infections doubling from Q4 2020 to Q1 2024 (see accompanying data tables).
Changes to screening policies, outbreaks, and/or an improvement in national reporting of CPO episodes can have a substantial impact on overall CPO trends; it is therefore important to note that this may not necessarily represent a change in clinical infections as screening policies change and national reporting improves over time. Furthermore, as there are only 15 quarters of notification data, it is too early to discern whether there may be any seasonality, particularly in light of the COVID-19 pandemic; quarterly changes may have been affected by the pandemic during this period, or may have been associated with local CPO outbreaks.
Figure 1. Quarterly rate of acquired CPO episodes by specimen type and quarter (England): October 2020 to June 2024

Epidemiology of CPO episodes over the last year (Q3 2023 to Q2 2024)
The subsequent data summaries in the rest of this report consider all sample types grouped together and only for the past rolling year (July 2023 to June 2024), using both annual and quarterly rates.
Geographic distribution
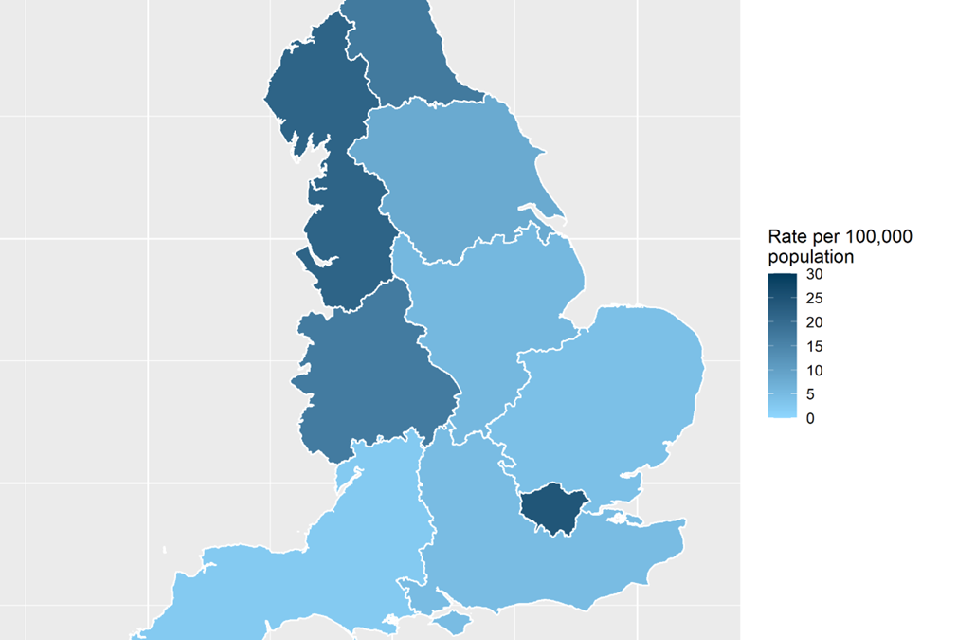
The annual rate of acquired CPO episodes varied by region (Office for National Statistics [ONS] regions) (Figure 2), with the highest overall rate between July 2023 and June 2024 reported in London (24.3 episodes per 100,000 population), followed by the North West region (21.5 episodes per 100,000 population). The lowest annual incidence across the last year was reported in the South West region (2.3 episodes per 100,000 population). Regional variation may be due to differences in incidence, variation in screening policies, outbreaks or reporting to national surveillance.
Figure 2. Geographical distribution of acquired carbapenemase-producing organism annual incidence rates per 100,000 population (England): April 2023 to June 2024 [note 5]
Notes to Figure 2
Note 5. The region geography is based on the patient’s residential postcode (if unknown, the laboratory location was used) and linked to the ONS data for regions.
The number and quarterly rate of reported acquired CPO episodes for each ONS region by calendar quarter is shown in Table 3. The quarterly rate of reported CPO episodes in ONS regions increased from Q1 2024 to Q2 2024 in most regions. The largest increase in rate was in the North East, where the quarterly rate increased from 2.9 per 100,000 population in Q1 2023 to 7.1 per 100,000 population in Q2 2024.
Table 3. Number of acquired CPO episodes and quarterly rate per 100,000 population for all specimen types by ONS region (England): July 2023 to June 2024
| ONS region | Q3 2023 number | Q3 2023 rate | Q4 2023 number | Q4 2023 rate | Q1 2024 number | Q1 2024 rate | Q2 2024 number | Q2 2024 rate |
|---|---|---|---|---|---|---|---|---|
| East Midlands | 51 | 1.0 | 79 | 1.6 | 65 | 1.3 | 80 | 1.6 |
| East of England | 55 | 0.8 | 71 | 1.1 | 74 | 1.1 | 76 | 1.1 |
| London | 523 | 5.9 | 483 | 5.4 | 482 | 5.4 | 669 | 7.5 |
| North East | 101 | 3.8 | 92 | 3.4 | 77 | 2.9 | 190 | 7.1 |
| North West | 404 | 5.4 | 424 | 5.6 | 365 | 4.9 | 421 | 5.6 |
| South East | 75 | 0.8 | 140 | 1.5 | 109 | 1.2 | 117 | 1.3 |
| South West | 34 | 0.6 | 37 | 0.6 | 30 | 0.5 | 34 | 0.6 |
| West Midlands | 253 | 4.2 | 284 | 4.7 | 203 | 3.4 | 272 | 4.5 |
| Yorkshire and The Humber | 93 | 1.7 | 133 | 2.4 | 127 | 2.3 | 96 | 1.7 |
Geographical differences in carbapenemase family distribution
Between July 2023 and June 2024, the most common carbapenemase families reported across all regions were NDM (35.7%), OXA-48-like (35.1%), and KPC (20.5%), although the distribution of carbapenemase families identified varied regionally (Figure 3).
In London, which had the highest annual incidence rate, the most reported carbapenemase families were NDM (47.9%) and OXA-48-like (39.4%). However, in the North West, which also had a high annual incidence rate, KPC accounted for 42.9% of episodes. KPC was not as common in any of the other regions, accounting for fewer than a third of episodes in each region; in London, KPC accounted for only 3.3% of episodes.
In the North East and Yorkshire and Humber, IMP was more commonly reported compared to any of the other regions, accounting for 17.0% and 11.6% of episodes, respectively (other regions reporting ranged between 1.3% and 8.4% with the national average being 5.6%). However, given the small number of CPO episodes reported in some regions, the diversity of carbapenemases reported in these regions is likely to be strongly impacted by individual outbreaks.
The distribution of carbapenemase families varied by quarter in some ONS regions. For example, the most reported carbapenemase family in the East Midlands was OXA-48-like between Q4 2020 and end of Q4 2022 (representing 68.1% of episodes in this period) but switched to NDM in Q1 and Q2 2024, accounting for 59.6% of episodes during these quarters.
The East of England has also seen a recent increase in the proportion of CPOs with NDM, which represented 43.4% of episodes in Q2 2024 compared with 28.4% of episodes in Q1 2024.
In the North West, the proportion of reported KPC episodes has been decreasing since the beginning of mandatory reporting , however, this is due to an increase in the number of NDM and OXA-48-like episodes, not a decrease in the number of KPC reports.
Figure 3. Geographical distribution of acquired CPO episodes by carbapenemase family (England): July 2023 to June 2024
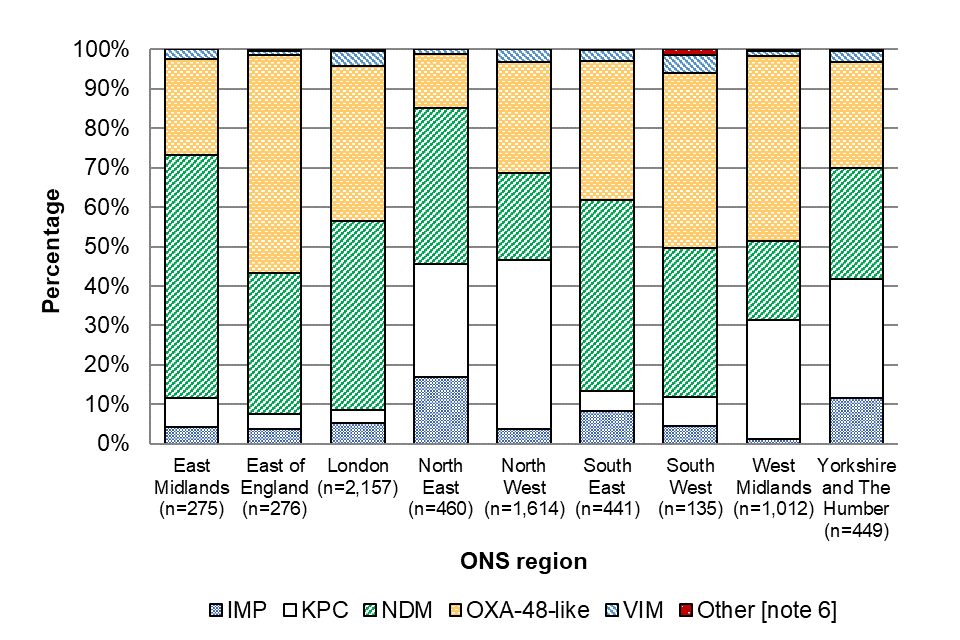
Notes to Figure 3
Note 6. Other carbapenemase families included DIM, GES, GIM, IMI and SME.
Distribution of species and carbapenemase family
Between July 2023 to June 2024, the most frequently isolated Gram-negative bacterial species with an acquired carbapenemase was Escherichia coli, accounting for 33.8% of all CPOs. This was followed by Klebsiella pneumoniae, and Enterobacter spp., which accounted for 31.0% and 19.1% of all CPOs, respectively (Table 4).
The carbapenemase family most frequently identified in E. coli and K. pneumoniae isolates was OXA-48-like (44.4% and 37.5%, respectively), followed by NDM (42.4% and 31.1%, respectively) and KPC and (10.1% and 27.7%, respectively). In Enterobacter spp. isolates the most common carbapenemase family was NDM (37.3%), followed by KPC and OXA-48-like (23.0% and 21.2%, respectively).
Aside from the ‘big 5’ carbapenemase families (KPC, OXA-48-like, NDM, VIM and IMP), the AMRHAI Reference Unit also screens for rarer carbapenemase families, and it is recommended that all isolates suspected to produce an acquired carbapenemase but that test negative for the ‘big 4/5’ carbapenemase families (depending on the testing capabilities of the local laboratory) are referred to the AMRHAI Reference Unit for further testing. Between October 2020 and June 2024, DIM, GES, GIM, IMI, and SME carbapenemases were identified in small numbers and represented less than 0.5% of all reports.
Table 4. Acquired CPO episodes by species and carbapenemase family (England): July 2023 to June 2024
| Species | IMP number (%) | KPC number (%) | NDM number (%) | OXA-48-like number (%) | VIM number (%) | Other number (%) | Total number | % of Total per species | |
|---|---|---|---|---|---|---|---|---|---|
| Acinetobacter spp. [note 7] | 4 (6.3) | 1 (1.6) | 53 (84.1) | 4 (6.3) | 1 (1.6) | 0 (0.0) | 63 | 0.9 | |
| Citrobacter spp. | 26 (8.1) | 66 (20.5) | 80 (24.8) | 136 (42.2) | 13 (4.0) | 1 (0.3) | 322 | 4.7 | |
| Enterobacter spp. | 197 (15.2) | 298 (23.0) | 484 (37.3) | 275 (21.2) | 33 (2.5) | 11 (0.8) | 1,298 | 19.1 | |
| Escherichia coli | 58 (2.5) | 233 (10.1) | 975 (42.4) | 1022 (44.4) | 13 (0.6) | 0 (0.0) | 2,301 | 33.8 | |
| Other Escherichia spp. | 1 (5.3) | 11 (57.9) | 3 (15.8) | 3 (15.8) | 1 (5.3) | 0 (0.0) | 19 | 0.3 | |
| Klebsiella oxytoca | 6 (2.4) | 144 (58.5) | 14 (5.7) | 78 (31.7) | 4 (1.6) | 0 (0.0) | 246 | 3.6 | |
| Klebsiella pneumoniae | 63 (3.0) | 584 (27.7) | 657 (31.1) | 754 (35.7) | 54 (2.6) | 0 (0.0) | 2,112 | 31.0 | |
| Other Klebsiella spp. | 4 (4.3) | 25 (26.9) | 18 (19.4) | 45 (48.4) | 1 (1.1) | 0 (0.0) | 93 | 1.4 | |
| Morganella spp. | 0 (0.0) | 1 (6.7) | 3 (20.0) | 9 (60.0) | 2 (13.3) | 0 (0.0) | 15 | 0.2 | |
| Pseudomonas aeruginosa [note 7] | 14 (9.5) | 3 (2.0) | 68 (45.9) | 8 (5.4) | 48 (32.4) | 7 (4.7) | 148 | 2.2 | |
| Other Pseudomonas spp. [note 7] | 0 (0.0) | 1 (3.6) | 6 (21.4) | 0 (0.0) | 21 (75.0) | 0 (0.0) | 28 | 0.4 | |
| Serratia spp. | 1 (2.6) | 3 (7.7) | 15 (38.5) | 20 (51.3) | 0 (0.0) | 0 (0.0) | 39 | 0.6 | |
| Other Enterobacterales [note 8] | 6 (4.7) | 28 (22.0) | 56 (44.1) | 36 (28.3) | 1 (0.8) | 0 (0.0) | 127 | 1.9 | |
| Other Gram-negative bacteria [note 9] | 0 (0.0) | 0 (0.0) | 1 (50.0) | 1 (50.0) | 0 (0.0) | 0 (0.0) | 2 | 0.0 | |
| Total | 380 (5.6) | 1398 (20.5) | 2433 (35.7) | 2391 (35.1) | 192 (2.8) | 19 (0.3) | 6,813 | 100.0 |
View this table in the data tables file.
Notes to Table 4
Note 7. KPC and OXA-48-like in Pseudomonas spp. and OXA-48-like in Acinetobacter spp. are extremely rare, and positive results should be interpreted with caution. The numbers reported here have not all been confirmed by the AMRHAI Reference Unit and laboratories identifying these unusual combinations should refer such isolates to AMRHAI.
Note 8. Includes coliform, Cronobacter spp., Hafnia spp., Kluyvera spp., Leclercia adecarboxylata, Lelliottia amnigena, Mixta calida, Pantoea spp., Phytobacter ursingii, Pluralibacter gergoviae, Proteus spp., Providencia spp., Raoultella spp., and Shigella spp.
Note 9. The isloates reported here have not all been confirmed by the AMRHAI Reference Unit and laboratories identifying these unusual combinations should refer such isolates to AMRHAI.
Distribution of demographic risk factors
Between July 2023 to June 2024, the annual rate of reported acquired CPO episodes was highest among the oldest and youngest members of the population. A similar age pattern was noted for both sexes, although overall the annual rate was higher in males compared to females (overall rates of 13.2 and 10.6 episodes per 100,000 population, respectively: Figure 4). This aligns with the age group and sex distribution noted in previously published reports on Gram-negative bacteraemia such as E. coli, Klebsiella spp., P. aeruginosa and Enterobacter spp.
Figure 4 shows the reported acquired CPO annual incidence rates by age group between July 2023 and June 2024, with the highest annual rate reported in those 85 years and over (71.0 per 100,000 population) followed by those aged 75 to 84 years (41.7 per 100,000 population). For infants less than 1 year old, the CPO annual incidence rate was 9.6 per 100,000 population
Figure 4. Annual rate of acquired CPO episodes per 100,000 population by age and sex [note 10] (England): July 2023 to June 2024
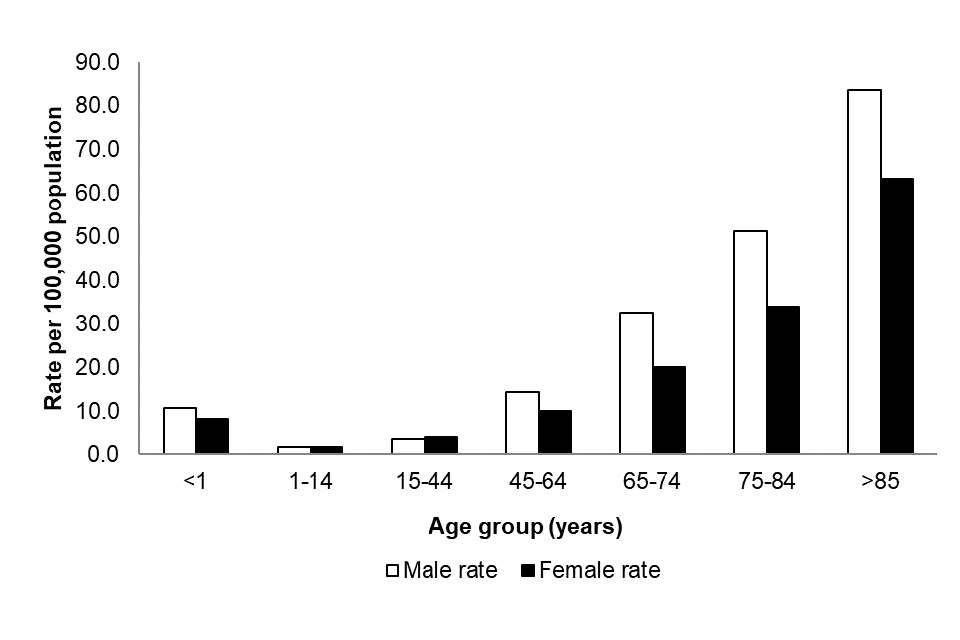
Notes to Figure 4
Note 10. Information about patient sex is recorded in 99.4% of cases.
Figure 5 shows the reported acquired CPO annual incidence rates by ethnic group and sex between July 2023 to June 2024, with the highest annual rate reported in those of Asian or Asian British ethnicity (20.1 per 100,000 population), followed by those of Black, Black British, Caribbean or African ethnicity (14.4 per 100,000 population). A similar pattern was noted for both sexes, although overall the annual rate was higher in males compared to females (overall rates of 11.4 and 9.5 episodes per 100,000 population, respectively: Figure 5).
Figure 5. Annual rate of acquired CPO episodes per 100,000 population ethnic group [note 11] and by sex [note 10] (England): July 2023 to June 2024
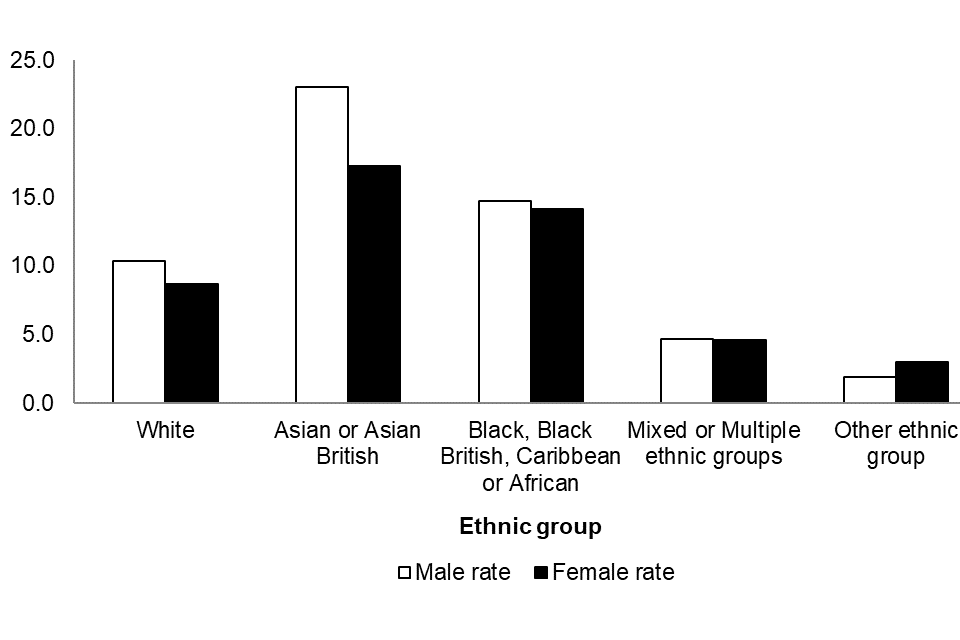
Notes to Figure 5
Note 10. Information about patient sex is recorded in 99.4% of cases.
Note 11. Information about patient ethnicity is recorded in 86.5% of cases.
The most common carbapenemase families identified in individuals of Asian or Asian British ethnicity were NDM (55.8%) and OXA-48-like (34.9%) (Figure 6). KPC was not as common in patients of Asian or Asian British ethnicity (4.9%) as it wasin those of White ethnicity, where KPC accounted for 27.3% of episodes.
Figure 6. Distribution of acquired carbapenemase families by ethnic group [note 11] and carbapenemase family (England): July 2023 to June 2024
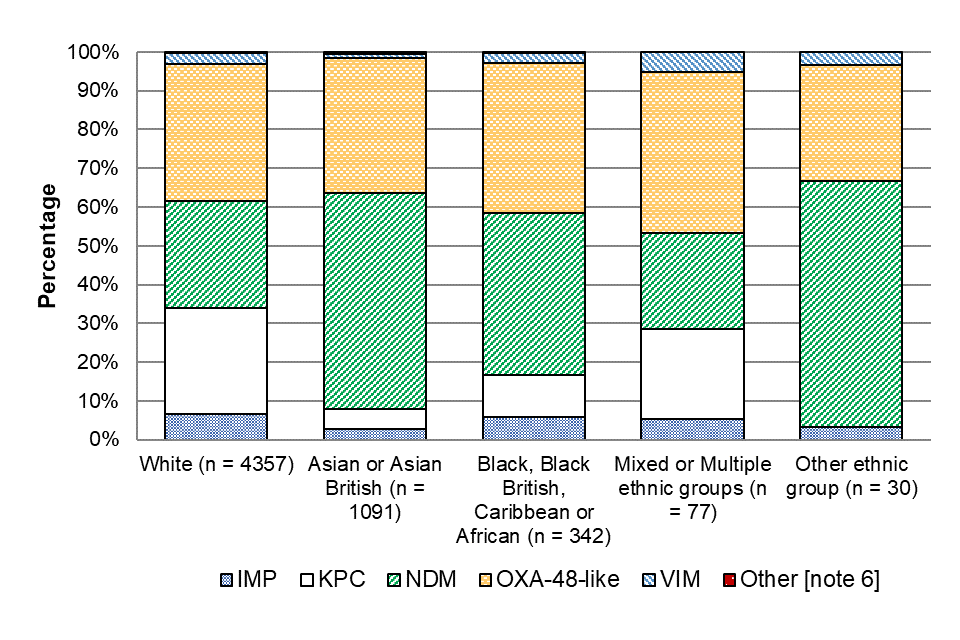
Notes to Figure 6
Note 6. Other carbapenemase families included DIM, GES, GIM, IMI and SME.
Note 11. Information about patient ethnicity in recorded in 86.5% of cases.
Figure 7 shows the acquired CPO annual incidence rates by IMD decile and sex between July 2023 to June 2024. The highest annual rate was reported in those in the most deprived decile (17.4 per 100,000 population). The annual rate generally declines as the IMD decile increases, with the lowest annual rate reported in the least deprived IMD decile (7.1 per 100,000 population). A similar pattern was noted for both sexes, although overall the annual rate was higher in males compared to females (overall rates of 13.2 and 10.6 episodes per 100,000 population, respectively: Figure 7).
Figure 7. Annual rate of acquired CPO episodes per 100,000 population by index of multiple deprivation decile and sex [note 10] (England): July 2023 to June 2024
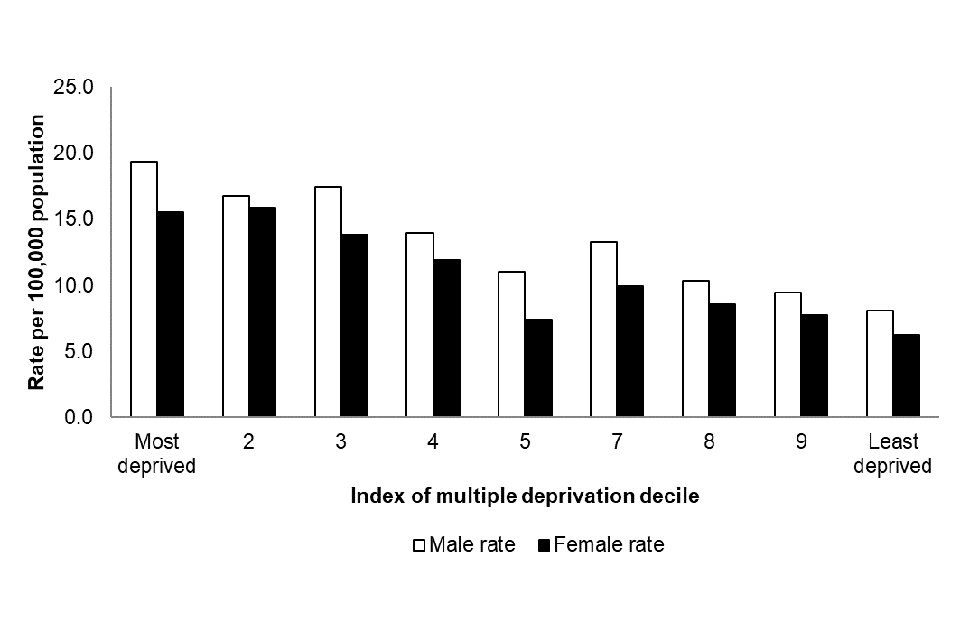
Notes to Figure 7
Note 10. Information about patient sex is recorded in 99.4% of cases.
Figure 8 shows the acquired CPO incidence annual rates by IMD decile and carbapenemase family between July 2023 to June 2024. Overall, the rate of CPO in the most deprived IMD decile is more than double that of the least deprived decile; 17.4 episodes per 100,000 population in decile 1 (least deprived) compared with 7.2 episodes per 100,000 in decile 10 (most deprived). The three carbapenemase families with the highest annual rates across all IMD deciles are OXA-48-like, KPC and NDM, where the annual rate generally declines with increasing IMD decile (least deprived).
Figure 8. Annual rate of acquired CPO episodes per 100,000 population by index of multiple deprivation decile and resistance mechanism (England): July 2023 to June 2024

Notes to Figure 8
Note 6. Other carbapenemase families include DIM, GES, GIM, IMI and SME.
Quarterly mandatory laboratory return reporting (July 2023 to June 2024)
Table 5. Quarterly mandatory laboratory returns (QMLR) for the total number of rectal swabs and faecal screening specimens taken for CPO screening by acute trust type [note 12] (England): July 2023 to June 2024
| Trust type [note 12] | Q3 2023 reported screens (%) | Q3 2023 total screens | Q4 2023 reported screens (%) | Q4 2023 total screens | Q1 2024 reported screens (%) | Q1 2024 total screens | Q2 2024 reported screens (%) | Q2 2024 total screens |
|---|---|---|---|---|---|---|---|---|
| Small (n=21) | 15 (71.4) | 8,159 | 15 (71.4) | 7,127 | 15 (71.4) | 7,133 | 13 (61.9) | 6,564 |
| Medium (n=21) | 20 (95.2) | 7,573 | 19 (90.5) | 7,079 | 20 (95.2) | 7,956 | 18 (85.7) | 9,747 |
| Large (n=24) | 20 (83.3) | 18,639 | 20 (83.3) | 20,444 | 20 (83.3) | 20,410 | 19 (79.2) | 19,325 |
| Multi-service (n=7) | 7 (100.0) | 6,291 | 6 (85.7) | 7,025 | 6 (85.7) | 7,280 | 6 (85.7) | 10,818 |
| Specialist (n=16) | 12 (75.0) | 5,067 | 12 (75.0) | 5,127 | 12 (75.0) | 6,457 | 12 (75.0) | 7,783 |
| Teaching (n=48) | 42 (87.5) | 124,529 | 39 (81.3) | 96,350 | 40 (83.3) | 117,930 | 35 (72.9) | 100,739 |
| Total (n=137) | 116 (84.7) | 170,258 | 111 (81.0) | 143,152 | 113 (82.5) | 167,166 | 103 (75.2) | 154,976 |
View this table in the data tables file.
Notes to Table 5
Note 12. Trust type obtained through NHS Digital Estate Return Information Collection (ERIC).
Reporting of quarterly totals of rectal swabs and faecal specimens taken for CPO screening was added to the mandatory quarterly laboratory returns (QMLR) section of the HCAI DCS in October 2019, and reporting became mandatory in October 2020. In Q2 2024, there were 154,976 screens reported by 103 NHS Trusts, representing an overall Trust reporting rate of 75.2% (Table 5). As of the writing of this report, 34 (24.8%) Trusts have not reported any screens and 2 (1.9%) Trusts reported zero screens for Q2 2024. The lower report rate for Q2 2024 may be due to late reports as the report rate for Q1 2024 has changed since the previous report (3).
The full list of reporting, including those that did not submit a return, is available in the data tables by individual NHS Acute Trust.
References
1. Department of Health and Social Care (2020). The Health Protection (Notification) (Amendment) (No.2) Regulations 2020/674
2. UKHSA (2023). Bacteriology Reference Department User Manual
3. UKHSA (2024). Carbapenemase-producing Gram-negative organisms in England since October 2020: quarterly update, Q1 2024
Acknowledgements
These reports would not be possible without the weekly contributions from microbiology colleagues in laboratories across England. The support from colleagues within UKHSA, and the AMRHAI Reference Unit in particular, is valued in the preparation of the report. Feedback and specific queries about this report are welcome via hcai.amrdepartment@ukhsa.gov.uk
