[Withdrawn] Novel coronavirus (COVID-19) standard operating procedure: running a medicines reuse scheme in a care home or hospice setting
Updated 2 September 2020
Applies to England
Purpose
This standard operating procedure (SOP) supports timely access to essential prescribed medicines during the COVID-19 pandemic for patients who are being cared for in a care home[footnote 1] or hospice setting.[footnote 2] In England, care homes can offer nursing and personal care or personal care only. The latter may not employ any registered nurses. This guidance is applicable in England and for use during the COVID-19 pandemic only.[footnote 3]
Care homes and hospices in Northern Ireland, Scotland and Wales should refer to guidance and SOPs produced by the governing bodies and regulators in their devolved administration.
Background
COVID-19
Public Health England (PHE) has issued guidance on managing COVID-19 in a residential care setting.
Managing medicines
The National Institute for Health and Care Excellence (NICE) has issued good practice for managing medicines in care homes. The guidance promotes safe and effective use of medicines in care homes by advising on processes for prescribing (including remote prescribing), handling and administering medicines. It also recommends how medicines (including controlled drugs) should be received, stored and disposed of within a care home setting. That guidance includes a recommendation that care home providers must ensure that medicines prescribed for a resident are not used by another resident.
Although this remains good practice, this new SOP is designed to help providers manage situations where, during the COVID-19 pandemic, the best interest of patients mean that it is not appropriate to follow this recommendation.
Recycling or reuse of unused medicines
The Human Medicines Regulations 2012[footnote 4] are the legislation that underpins the dispensing and supply of medicines, supplemented in the case of controlled drugs by the Misuse of Drugs Regulations 2001. Part 12 of the Human Medicines Regulations 2012 limits the supply of prescription-only medicines (POMs) to supply in accordance with a prescription of an authorised prescriber, subject to various exceptions including supply under a patient group direction (PGD).
Provided that a supply is, in fact, in accordance with the prescription, for the specific purposes of Part 12 of the Human Medicines Regulations 2012, it will generally not be relevant how that medicine is sourced.
Accordingly, if at each stage of the supply chain the legal requirements relevant to that stage have been adhered to, the possibility exists that providers may have in their possession medicines that they are lawfully entitled to have in their possession for one purpose which they may be able to use for another purpose. This new guidance is to support making appropriate use of that recognised possibility in care homes and hospices.
When a patient is prescribed a medicine, once the final supply of the medicine is completed and it is in the patient’s safe keeping, it is their property (although not their exclusive responsibility). If the medicine is still in the safe custody of the care home or hospice care provider, whether or not the final supply to the patient has been completed is the subject of differing legal views. Some would say that it becomes the patient’s property as early as when it leaves the pharmacy.
This guidance does not seek to resolve these complex legal issues. Rather, it presents an agreed line through them, in the current very unusual circumstances, and that agreed line is strictly for the limited purposes which the guidance addresses.
Under usual circumstances, the reuse or recycling of another patient’s medicine is not recommended by the Department of Health and Social Care (DHSC) as the quality of any medicine that has left the pharmacy cannot be guaranteed. Any unused medicines would normally be disposed of by returning them to a contracted external company or community pharmacy.
However, there are increasing concerns about the pressure that could be placed on the medicines supply chain during the peak of the COVID-19 pandemic. A medicines reuse scheme for care homes and hospices could potentially ease some of that pressure in the coming weeks.
Medicines reuse schemes already operate successfully in NHS hospitals across the UK. In addition, hospices and care homes generally have good procedures in place to store medicines in an appropriate way. We can therefore be more confident of the quality of any unused medicines in these settings.
Due to the current unprecedented impact of COVID-19, DHSC and NHS England and NHS Improvement are recommending a relaxation of previous recommendations and the NICE recommended good practice guidance to accommodate reuse of medicines, under very specific circumstances and only in a crisis situation as outlined.
First and foremost, the quality, integrity and safety of medicines are paramount and the best way to assure this is for pharmacies to supply medicines obtained through the regulated supply chain, appropriately labelled for individual patients.
However, in the unprecedented COVID-19 situation, DHSC and NHS England and NHS Improvement recognises that the reuse of medicines may be appropriate in certain circumstances. It is recommended that medicines should only be reused in accordance with a medicines reuse scheme, set out in a SOP.
This SOP has been developed to support care home and hospice providers. It offers a framework to run a safe and effective medicines reuse scheme that is in the best interest of patients.
Medicines reuse scheme SOP for care homes and hospices
When would this apply?
This is time limited and would only apply during this period of emergency (that is, during the COVID-19 pandemic).
What might constitute a crisis?
Each individual care home or hospice must carry out a risk assessment on an individual medicine basis.
Three key indicators should inform the risk assessment and the subsequent decision:
- no other stocks of the medicine are available in an appropriate timeframe (as informed by the supplying pharmacy) and there is an immediate patient need for the medicine
- no suitable alternatives for an individual patient are available in a timely manner (that is, a new prescription cannot be issued, and the medicine(s) supplied against it in the conventional manner quickly enough)
- the benefits of using a medicine that is no longer needed by the person for whom it was originally prescribed or bought outweigh any risks for an individual patient receiving that unused medicine
Is a medicine suitable for reuse?
The medicine must be checked against the criteria in tables 1 to 3 (see below) by a registered healthcare professional.[footnote 5]
Where no registered healthcare professional is on site (for example, in a care home that only offers personal care and has no registered nurses on site), registered healthcare professionals (for example, pharmacists, pharmacy technicians, general practitioners, community nurses) from other local organisations, such as clinical commissioning groups, general practices or community settings, can perform that check (this may be done virtually) and confirm that the medicine is suitable for reuse.
All medicines no longer needed by the person for whom they were originally prescribed and intended for reuse must be under the supervision of a registered healthcare professional and appropriate records should be kept, including details of the registered healthcare professional who performed the check on suitability for reuse.
If the medicine suitable for reuse is a controlled drug, then it must remain in the control (possession) of an organisation authorised to do so. Further information can be found in the Home Office guidance on domestic controlled drug licensing in healthcare settings. Appropriate records (for example, a controlled drugs register) must be maintained in respect of controlled drugs.
This SOP applies to medicines that have been supplied to patients while in a care home or a hospice, have not been removed from that setting (other than for short periods, such as an outpatient appointment) and have been stored in accordance with good practice guidance on storing medicines in a managed setting. It applies to all medicines, including liquid medicines, injections (analgesics, insulin), creams and inhalers that are in sealed or blister packs and when the criteria in table 1 (below) are met.
Providers should also consider, in the case of medicines that they have had difficulty accessing, whether the normal assumption of allowing patients to keep their own supplies of medicines for self-administration is appropriate, or whether other storage arrangements would better facilitate their reuse, if that patient no longer needs them.
Reuse should only be within a single care home or hospice setting. Medicines identified for reuse should not be transferred to another care home or hospice, even those within the same parent organisation.
Tables 1 to 3 below provide supporting prompts to assist the registered healthcare professional with their decision-making. It is advised that medicines for reuse are proactively assessed prior to them being needed in an emergency situation.
Table 1: criteria to be considered before the medicine can be reused
| Yes | No | Notes | |
|---|---|---|---|
| Is the medicine in an unopened pack or blister that has not been tampered with? | In an unopened, unadulterated and sealed pack (including sub-pack) or blister strip. If any doses have already been used, the remainder of that blister strip should be destroyed. If the contents (including blister strips and sealed individual units such as ampoules) are completely intact, then as long as they match the description on the packaging they were retrieved from (including check of batch numbers) they can be considered for reuse. |
||
| Is it in date? | Medicines should be in date. If expired, they will need to be returned to a pharmacy to be safely destroyed. | ||
| Has it been stored in line with the manufacturer’s instructions, including any need for refrigeration? | Any medication that requires refrigeration, or that has a reduced shelf-life once removed from refrigerated storage, should be destroyed if it has not been stored appropriately. Medicines left in unsuitable conditions (for example direct sunlight, near radiators) or where appropriate storage cannot be confirmed, should be destroyed. |
||
| Is the medicine a licensed medicine that has either been prescribed by a registered healthcare professional with prescribing rights or bought ‘over the counter’? | For some medicines, ‘homely remedies’ are an option in care homes and should be considered in line with guidance. |
If the answer to all of the above questions is yes, then the risk of reuse may be judged to be minimal. If the answer to any question is no then the medicine should not be reused. If doubt remains, discuss with appropriate registered healthcare professionals and local networks to get a wider perspective on the decision.
Table 2: minimise risk of cross-contamination
| Yes | No | Notes | |
|---|---|---|---|
| Is the medicine from a patient with a diagnosis of COVID-19 or showing symptoms of COVID-19? | Ensure that adequate infection prevention and control precautions have been taken. Medicine that has been retrieved from a patient infected with COVID-19 should be sealed (double bagged) and quarantined for 3 days. A ‘do not process before’ date should be fixed to the bag before the bag is stored safely and away from any other medicines. |
Table 3: ensuring permission is obtained and patients, families and/or carers are fully involved
| Yes | No | Notes | |
|---|---|---|---|
| If a medicine is thought to be suitable for reuse, permission should, if possible, be obtained for reuse from the patient for whom it was prescribed, or (if the patient lacks capacity) from a person with power of attorney, or (if the patient has died) from their next of kin. | If the patient has become responsible for the safe keeping of the medicine, it is the property of the patient (although not their exclusive responsibility), but if the medicine is still in the safe custody of the care home or hospice care provider, whether the final supply to the patient has been completed is the subject of differing legal views. Reflecting this uncertainty, if possible, ensure the patient or their next of kin agrees for the medicine to be reused. See annex B. |
To ensure reuse of medicines is an option that can be used as flexibly as possible we suggest that care homes and hospices proactively seek written permission from all patients for:
- their medicines (if no longer needed) to be made available for other patients
- them to receive a reused medicine, provided it is deemed safe for reuse
Further information to inform discussions is available in annex B.
Once a decision has been made to reuse a medicine, then the following processes (summarised in the chart in the section medicines reuse pathway) should be followed:
All medicines
- A log should be maintained of reused stock. The log should include the generic drug name, batch number, strength, formulation, expiry date, quantity and details of the registered healthcare professional who assessed the medicine, as a minimum. When the stock is reused, the quantity used should be entered. An example log returns sheet is given in annex B.
- Any medicines that might be reused should be placed in a sealed container and marked as ‘patient returns’, to make it clear that the stock should only be reused when stock cannot be obtained from the legitimate supply chain. The additional obligations in respect of storage of controlled drugs must be adhered to.
- Once a medicine has been assessed as being suitable for reuse, the usual processes and governance, as recommended by NICE guideline SC1: Managing medicines in care homes, apply.
- Any reused medicine would need to be administered according to the direction of a relevant prescriber[footnote 6] and recorded by care home or hospice staff in the relevant administration chart.
- Unless the product is being supplied under a PGD or a patient-specific direction, a new prescription must be obtained prior to supply to the new patient. If it is for a controlled drug, the extra requirements in relation to controlled drugs prescriptions must be satisfied. New remote prescriptions should be scanned and emailed before the first dose is given, and a copy of the prescription kept with the patient’s records in line with current processes.
- The administration chart (paper or electronic) should be updated by the care home or hospice, in line with the direction from the prescriber (in most cases this would be the prescription). The new record should be checked for accuracy and signed by a second trained and skilled member of staff before it is first used. The prescriber does not need to sign the medication administration record (MAR) chart.
Controlled drugs (CDs)
-
Any stock of medication schedule 2 or 3 controlled drugs (CDs) should only be retained if it can be stored securely with controlled and limited access (in line with safe storage requirements for controlled drugs). Lawful possession of such drugs is generally predicated on a prescription or direction being in place, so continuity of prescriptions is important for these particular products, having regard to the normal timeframes for safe disposal of these products where they are no longer needed.
-
Any schedule 2 CDs must be entered into a separate section of the CDs register and then an entry made when they are reused, as is usual practice.
Records
- All records (CDs register entries and returned medicines stock, risk assessments) must be kept in line with legislation.
Prescribers
-
When medicines are out of stock and there is an immediate need for them, an alternative preparation should be prescribed and dispensed, as is usual practice where possible.
-
Where stock is not available, the supplying pharmacy will contact the care home or hospice to establish whether a medicines reuse scheme is in place and stock of the required medicine is available in the home.
-
Reused medicines may be administered to residents in a care home or hospice under the direction of a prescriber, and in line with this SOP, where an appropriate medicines reuse scheme is in operation.
-
In this situation, the direction would normally be in the form of a prescription. If a prescription is issued remotely, it should be scanned and emailed to the care home by the prescriber (for known medicines shortages) or the community pharmacy as appropriate in each individual case.
Community pharmacy
-
When medicines are out of stock and there is an immediate need for them, an alternative preparation should be prescribed and dispensed, as is usual practice where possible.
-
Where there is no suitable alternative or a prescription cannot be written for the alternative medicine (for example, out of hours), the community pharmacy team should ask the care home or hospice whether they run a medicines reuse scheme and whether they have any stock of the required medicine.
-
If stock of a reused medicine is available in the care home or hospice, the community pharmacy team must share a copy of the prescription for that medicine with the home and update the corresponding MAR chart as necessary.
The supply of the medicine by the care home or hospice will need to be in accordance with that prescription. They cannot rely on a report of its contents.
Medicines reuse pathway
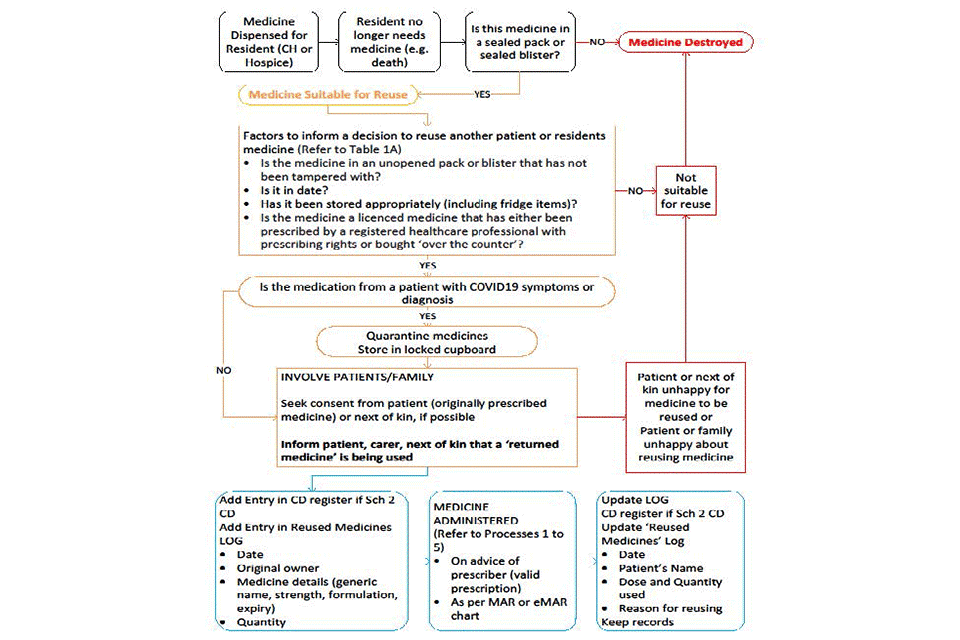
Medicines reuse pathway flow diagram
Text alternative to the flowchart
A medicine is dispensed for a resident (in a care home or hospital). The resident no longer needs that medicine (for example, because they have died).
Is this medicine in a sealed pack or sealed blister?
No: medicine destroyed.
Yes:
- medicine suitable for reuse
- see next section
Factors to inform a decision to reuse another patient or resident’s medicine (refer to table 1)
- Is the medicine in an unopened pack or blister that has not been tampered with?
- Is it in date?
- Has it been stored appropriately (including fridge items)?
- Is the medicine a licensed medicine that has either been prescribed by a registered healthcare professional with prescribing rights or bought ‘over the counter’?
No:
- not suitable for reuse
- medicine destroyed
Yes: see next section.
Is the medication from a patient with COVID-19 symptoms or diagnosis?
No: see next section
Yes:
- quarantine medicines
- store in a locked cupboard
- see next section
Involve patients and family
- Seek consent from patient (that was originally prescribed the medicine) or next of kin, if possible
- Inform the patient, carer, next of kin that a ‘returned medicine’ is being used
Is the patient that was originally prescribed the medicine, their family or next of kin happy for the medicine to be reused?
No:
- not suitable for reuse
- medicine destroyed
Yes: see next section.
Is the patient receiving the medicine or their family happy about reusing medicine?
No:
- not suitable for reuse
- medicine destroyed
Yes: see next section.
Add entries
- Add entry in CDs register if schedule 2 CD
- Add entry in reused medicines log:
- date
- original owner
- medicine details (generic name, strength, formulation, expiry)
- quantity
See next section.
Medicine administered (refer to processes 1 to 5)
- On advice of prescriber (valid prescription)
- As per MAR or eMAR chart
See next section.
Update log
- CDs register if schedule 2 CD
- Update reused medicines log:
- date
- patient’s name
- dose and quantity
- reason for reusing
- Keep records
Pharmacovigilence
- Report all adverse events and problems to clinical commissioning group, COVID-19 triumverate
- Report clinical adverse via the Yellow Card Scheme (note that medicine was reused)
- Log all errors via National Reporting and Learning System or equivalent
Annex A: other sources of supporting information
- UK government guidance for care of the deceased with suspected or confirmed COVID-19
- NHS England and NHS Improvement COVID-19 standard operating procedure for community pharmacy
- Marie Curie provide links to supporting guidance and resources
- Hospice UK has brought together links to official guidance and resources to provide information on COVID-19
- Palliative Drugs: collated COVID-19 resources
- Health Education England e-learning module: End of Life Care
- Hospice UK: caring for your dying relative at home
- Care Quality Commission letter to providers (16 March 2020)
- Regulatory approach in challenging circumstances: General Pharmaceutical Council and the Pharmaceutical Society of Northern Ireland’s joint statement
- Joint statement from chief executives of statutory regulators of health and care professionals
-
The Care Standards Act 2000 defines a ‘care home’ as accommodation that provides nursing or personal care. ↩
-
Hospice care aims to improve the quality of life and wellbeing of adults and children with a life-limiting or terminal condition. It helps people live as fully and as well as they can to the end of their lives, however long that may be. ↩
-
The up-to-date status of the COVID 19 pandemic is confirmed at gov.uk/coronavirus. ↩
-
A healthcare professional should be registered with one of the UK’s professional regulatory bodies regulated by the Professional Standards Authority. ↩
-
This can be a verbal direction initially with a written prescription to follow either by email or hard copy. ↩
