Regulation 1223/2009 and the Cosmetic Products Enforcement Regulations 2013: Northern Ireland
Updated 4 May 2023
Guidance on the regulations as they apply to cosmetic products being supplied in or into Northern Ireland.
May 2023
Introduction
1) This Guide is for businesses placing cosmetics products on the market in Northern Ireland. Under the terms of the Windsor Framework, Northern Ireland aligns with relevant EU rules (in Annex 2) relating to the placing on the market of manufactured goods. This includes the EU regulation for cosmetic products.
2) This guide is designed to help you understand the European Union Regulation (EC) No 1223/2009 on Cosmetic Products as it applies in NI under the terms of the Windsor Framework (‘the Regulation’). The Regulation sets out requirements that must be met before cosmetics products can be placed on the NI market and European Economic Area (EEA) market.
3) Cosmetic products placed on the GB market (Great Britain comprises England, Scotland and Wales) must follow the rules for the GB market. If you are placing cosmetic products on the market in Great Britain, you should read the relevant separate guidance.
4) The government has committed to providing unfettered access for qualifying Northern Ireland goods to the rest of the UK market. Therefore, cosmetic products that can be placed on the market in Northern Ireland, in accordance with the Regulation, that are qualifying Northern Ireland goods, can be sold in the rest of the UK without any additional approvals. These arrangements are explained in detail in the separate guidance. Read guidance for placing cosmetic products on the market in GB.
Main changes to the existing processes and requirements for supplying products in Northern Ireland since 11pm on 31 December 2020
5) The main changes to note that took effect at 11pm on 31 December 2020 are:
- Responsible Person: Under the Regulation there must be a Responsible Person for each cosmetic product on the NI or EEA markets, regardless of when it was placed on the market. Responsible Persons established either in Northern Ireland or the EEA for cosmetic products can perform this role for the NI market and EEA market.
- Labelling: You must comply with the Regulation if you are placing your product on the NI market and this will also be valid in the EEA. Cosmetic products manufactured in Great Britain and placed on the Northern Ireland market are considered under the rules applied in Northern Ireland under the terms of the Windsor Framework as an imported cosmetic product. The country of origin must be specified for these imported cosmetic products. When a cosmetics product is manufactured in Great Britain and placed on the Northern Ireland market, it needs to be labelled with ‘Made in UK’.
- Safety Assessor: You must ensure that the product safety assessment is carried out by a safety assessor recognised by the EU.
- Notification: For goods placed on the Northern Ireland market or the EEA market, the Responsible Person must notify the EU Portal (the CPNP). Responsible Persons cannot be based in Great Britain. A Responsible Person, based either in Northern Ireland or the EEA, must be responsible for submitting a notification to the EU via the CPNP. For products that are also placed on the GB market, Responsible Persons (who need to be established in the UK) also need to notify UK authorities using the UK Submit cosmetic product notifications (SCPN) service. (see separate guidance on placing cosmetics on the GB market as well as the relevant section on unfettered access, which may be applicable, for qualifying Northern Ireland goods).
- Change of legal responsibilities: As of the end of the transition period on 31 December 2020, a Northern Ireland business that brings a cosmetic product from Great Britain into Northern Ireland automatically became the Responsible Person or they can designate in writing another person to be the Responsible Person (who must be established within the EEA or Northern Ireland and who must formally accept the role in writing). This is also the case if the cosmetic product is manufactured in another country, imported into Great Britain and subsequently moved into NI.
- Serious Undesirable Effects (SUEs): The requirement to notify the European Commission of SUEs remains unchanged (Read guidance and access to relevant forms.) The notification form for SUEs in NI should be sent to the Office for Product Safety and Standards (OPSS) at seriousundesirableeffects@businessandtrade.gov.uk, who will inform the Responsible Person and the European Commission.
- Products with nanomaterials: The Responsible Person for a product containing novel nanomaterials must notify the Commission by electronic means six months prior to the product being placed on the NI market or EU market. This is done through CPNP.
6) The following guidance provides detailed information on the articles in the EU regulation which are applicable to placing products on the NI market.
To note, for cosmetic products which are also aerosol products, the manufacturer will be required to follow the Aerosol Regulations, which includes the requirement to affix the reversed epsilon compliance mark.
Chapter I – Scope and Definitions
Article 1 – Scope and Objective
7) The Regulation applies to all cosmetic products made available on the Northern Ireland market or the EEA market. Because of the way ‘making available on the market’ is defined, the Regulation applies to any supply of cosmetic products in the course of a commercial activity, including situations where products are given away for free.
Article 2 – Definitions
Cosmetic Products
8) The definition of a cosmetic product comprises two parts: a function and a field of application. Both parts of the definition must be satisfied.
9) The Regulation specifies six functions in relation to external parts of the human body for products that may be cosmetic products, namely:
- to clean
- to perfume
- to change the appearance
- to protect
- to keep in good condition
- to correct body odours
10) The field of application of cosmetics is to the external parts of the human body; that is one or more of the following sites:
- the epidermis
- the hair system
- the nails
- the lips
- the external genital organs
- the teeth
- the mucous membranes of the oral cavity
11) A cosmetic product may be a substance or mixture of a number of substances, and it may come in one or more than one part to be combined by the user.
12) An illustrative list of cosmetics is given in Appendix 4 of this guide.
Borderline products
13) The status of many products on the borderline with cosmetics can be difficult to determine. The Medicines and Healthcare Products Regulatory Agency (MHRA) regulates medicinal products for human use in the UK, and has issued “A Guide to what is a Medicinal Product” see footnote 1. In case of doubt as to the status of a product, advice may be sought directly from the Borderlines Section of the MHRA.
14) Aromatherapy products supplied to consumers may fall within the scope of the General Product Safety Regulations 2005. Where they are intended to perform a medical or cosmetic function or are presented as performing such a function they may also fall within the cosmetics or medicinal products regulations. Both Local Authorities and The Aromatherapy Trade Council offer advice on this matter.
15) Products that are intended to be ingested, inhaled, injected or implanted are not classified as cosmetic products.
Manufacturer
16) The ‘Manufacturer’ is any person or business who manufactures a cosmetic product or has the product designed or manufactured and markets it under their name or trademark.
Importer
17) The ‘Importer’ is any person or business established in Northern Ireland or the EU who places a cosmetic product from a country other than Northern Ireland or the EU on the Northern Ireland or the EEA market. For the purposes of this legislation this includes products that are placed on the market in Northern Ireland from Great Britain. There are different rules that apply if a Northern Ireland business is placing cosmetic products on the market in Great Britain (See the GB guidance)
Distributor
18) A ‘Distributor’ is a person or business, other than the Manufacturer or the Importer, that supplies a cosmetic product on the Northern Ireland or the EEA market. It includes those businesses known as retailers or wholesalers. Professional suppliers such as hairdressers are also classed as ‘Distributors’ for those products sold or given to customers (consumers). There may be multiple ‘Distributors’ of the same product in the supply chain.
Making Available
19) ‘Making available’ on the market is any supply of a cosmetic product for distribution, consumption or use carried out in the course of a commercial activity. This applies regardless of any associated payment (i.e. whether in return for payment or free of charge). ’Making available’ will refer to supply on the Northern Ireland or the EEA market.
Placing on the Market
20) ‘Placing on the market’ means the first making available (supply) of a cosmetic product.
Nanomaterial
21) Nanomaterials are defined in the Regulation as materials that are ‘insoluble or biopersistant and intentionally manufactured material with one or more external dimensions, or an internal structure, on the scale from 1 to 100nm.’
Undesirable Effect / Serious Undesirable effect
22) An ‘Undesirable Effect’ is an adverse effect to human health that occurs following the normal or reasonably foreseeable use of a cosmetic product. There should be a demonstrable link between the affected person and the product.
23) A ‘Serious Undesirable Effect’ (SUE) is an undesirable effect which results in temporary or permanent functional incapacity, disability, hospitalisation, congenital anomalies or an immediate vital risk or death.
Competent Authority and Enforcement Authorities under the Cosmetic Product Enforcement Regulations 2013
24) The Secretary of State, and NI District Councils act as the competent authority. The Secretary of State may also authorise others to be the competent authority. NI District Councils are the enforcement authorities. Competent authorities which are enforcement authorities (i.e. District Councils) are not competent authorities for the purposes of the obligations to inform the Commission of various matters under Articles 23, 25 and 27 (see below).
Chapter II – Safety, Responsibility & Free Movement
Article 3 – Safety
25) A product must be safe under normal conditions of use and also under reasonably foreseeable conditions of use. Manufacturers, in the first instance, should consider the conditions of use which can be reasonably foreseen prior to placing a product on the market. They must look beyond what they consider to be the intended use of a product and put themselves in the position of the average user of the product – envisaging how they would reasonably consider using it. The safety requirement does not cover misuse of a cosmetic product (except where it is a reasonably foreseeable misapplication of the product). Ultimately it is for the Responsible Person (see below) to ensure that this obligation is complied with.
26) The Article provides that the presentation of the cosmetic product must take into account the requirements of The Food Imitations (Safety) Regulations 1989 (SR 1989 No. 1291) which concern dangerous imitations. The key provision in these Regulations is that no person shall supply, expose for supply or possess for supply any manufactured goods which are ordinarily intended for private use and are not food, but which:
- have a form, odour, colour, appearance, packaging, labelling, volume or size which is likely to cause persons, in particular children to confuse them with food and in consequence to place them in their mouths or suck them or swallow them
- where such action mentioned above is taken in relation to them, may cause death or personal injury
Article 4 – Responsible Person
27) Article 4 as it applies to Northern Ireland under the terms of the Windsor Framework provides that a cosmetic product cannot be placed on the Northern Ireland or EEA market unless there is a Responsible Person established in either Northern Ireland or the EU. Please note that Northern Ireland businesses that are supplied cosmetic products from GB and were distributors before 1 January 2021 took on importer duties under the EU law from 1 January 2021. They will, therefore, also be the Responsible Person for the specific cosmetic products they place on the NI or EEA market. It is possible for a Manufacturer or Importer to designate a third party to act as the Responsible Person via a written mandate. This person must be based in NI or the EU.
28) There are particular provisions for Northern Ireland businesses who place products from countries outside of the UK (including the EEA) on the market in Great Britain (see GB guidance).
29) Where the manufacturer is not based in NI or the EEA but the product is manufactured in NI or the EEA and remains in NI or the EEA between manufacture and placing on the market (i.e. it is not exported and imported back into NI or the EEA after manufacture but before being first supplied on the NI market) the manufacturer must ensure via written mandate that there is a third party based in NI or the EEA who agrees to be the Responsible Person in respect of that product.
Article 5 – Obligations of Responsible Persons
30) It is the duty of the Responsible Person to ensure compliance with the Regulation. While some of the requirements may not be directly undertaken by the Responsible Person, such as the safety assessment, it is their responsibility to ensure that all the requirements of the Regulation are fulfilled. If a Responsible Person has reason to believe that a cosmetic product is non-compliant, it is the duty of the Responsible Person to take corrective actions to bring the product back into compliance, withdraw it from the market or undertake a recall. The action taken should be commensurate with the degree of non-compliance. Further advice may be sought from the District Council or the Office for Product Safety and Standards.
31) The Article gives further provision on the duties of the Responsible Person for where a cosmetic product presents a risk to human health. The Responsible Person must immediately notify and cooperate with competent authorities, providing necessary information as set out in paragraphs 2 and 3 of the Article.
32) Responsible persons must also immediately take corrective measures necessary to bring a product into conformity, withdraw it or recall it, as appropriate when they consider or have reason to believe it is not compliant with the law. Where the cosmetic product presents a risk to human health, responsible persons shall immediately inform the competent national authorities (the Secretary of State via OPSS and the district council) in Northern Ireland if the products have been placed on the market in Northern Ireland and / or the competent national authorities of the EU Member States in which they made the product available. The responsible person should also inform the competent authority in Northern Ireland or in the relevant Member State in which the product information file is readily accessible, giving details, in particular, of the non-compliance and of the corrective measures taken. Responsible Persons must cooperate and provide information and documentation reasonably requested by competent authorities.
Article 6 – Obligations of Distributors
33) Distributors when making a cosmetic product available on the market have a general obligation to act with due care in relation to the applicable requirements.
34) Only compliant products should be made available on the market. Where Distributors have reason to believe the product is not compliant with the Regulation, they should not make the product available on the market until it is compliant. Where they have already made the product available, they should ensure that corrective measures are taken or that the product is withdrawn or recalled, as appropriate. In carrying out their responsibilities, Distributors should also keep the Responsible Person informed, and agree a course of action, while cooperating with competent authorities.
35) The Distributor is not responsible for ensuring that the product has been notified under Article 13 and is not entitled to check the Product Information File (PIF) of the product (unless they are also a Responsible Person).
36) The Distributor has a duty to store and transport products properly so that compliance with the Regulation is not compromised.
37) A Northern Ireland business placing a cosmetic product from GB on the NI market does so as an importer, not as a distributor under the Regulation, as they apply in NI, and automatically takes on the duties of the Responsible Person.
Article 7 – Identification within the Supply Chain
38) This Article concerns product traceability and requires the Responsible Person to identify the relevant Distributors. The Responsible Person must make this information available to competent authorities on request.
39) The Distributor has the responsibility to identify other Distributors in the supply chain, and also the Responsible Person who initially supplied the cosmetic product. This information must be made available to the competent authority on request.
40) In both instances the data must be kept for a period of three years following the date that the batch of cosmetic product was made available to the Distributor.
Article 8 – Good Manufacturing Practice
41) Manufacturing of cosmetic products should be carried out to cosmetic Good Manufacturing Practice (GMP). ISO Standard 22716 covers GMP, but there are other ways and guidance documents are available from trade associations.
Chapter III – Safety Assessment, Product Information File, Notification
Article 10 & Annex I – Safety Assessment
42) The Responsible Person is to ensure that a safety assessment is completed on a cosmetic product before it is placed on the market in order to demonstrate that the product complies with Article 3 (that is, that it is safe for human health when used under normal or reasonably foreseeable circumstances). The intended use of the cosmetic product – and the anticipated systemic exposure to individual ingredients in a final formulation – must be taken into account in the safety assessment. An appropriate weight–of–evidence approach must be used in the safety assessment for reviewing data from all existing sources.
43) The safety assessment should take the form of a Cosmetic Product Safety Report (CPSR) signed by a qualified safety assessor as recognised by the EU. The CPSR provides evidence of how the product is safe for its intended cosmetic use and takes account of reasonably foreseeable use. In addition, a specific safety assessment is required for cosmetic products intended for use on children under the age of three, and for cosmetic products intended exclusively for use in external intimate hygiene.
44) The format for the CPSR is detailed in Annex I of the Regulation and is divided into two parts: Part A (the cosmetic product safety information), and Part B (the cosmetic product safety assessment).
Part A – Cosmetic Product Safety Information
45) As a minimum, there are ten points that need to be considered:
- quantitative & qualitative composition
- physical/chemical characteristics and stability
- microbial quality
- impurities, traces and the packaging material
- normal and reasonably foreseeable use
- exposure to the product
- exposure to the substances
- toxicological profile of the substances
- undesirable effects and seriously undesirable effects
- other relevant information on the product
Part B – Cosmetic Product Safety Assessment
46) The cosmetic product safety assessment consists of the following sections:
a. an overall conclusion concerning the cosmetic product. This should indicate if the product is either safe for use or safe for use with restrictions. The conclusion should be based on the data presented in Part A of the Assessment.
b. Any mandatory labelling requirements should be listed. Advisory labelling requirements should also be included, for example:
- instructions for use if not obvious from the product / pack format;
- for a surfactant–based product, avoiding any eye contact;
- caution to avoid slipping when using bath foam / oils, and;
- avoid spraying on the face for aerosols / sprays.
c. Detailed reasoning for the final safety assessment should comprise a risk–based analysis, using ‘an appropriate weight of evidence approach for reviewing data from all existing sources’.
d. The safety assessment should include the name and address of the safety assessor including proof of qualifications; it should be signed and dated by the safety assessor.
47) The safety assessment should be reviewed and revised on a regular basis. In particular, this should be done when new data is available that might alter the safety conclusion outlined above.
Article 10 (2) – The Safety Assessor
48) The person responsible for the safety assessment is called a safety assessor. The safety assessor should be in possession of a diploma or other evidence of formal qualifications awarded on completion of a university course of theoretical and practical study in pharmacy, toxicology, medicine or a similar discipline, or a course recognised as equivalent under the Regulation as it applies in Northern Ireland.
Article 11 – Product Information File
49) Article 11 sets out the requirements relating to the Product Information File (PIF), and the detail of the information and data that should be contained concerning:
- description of the cosmetic product
- the Cosmetic Product Safety Report
- method of manufacture and GMP
- nature and proof of effect of the product
- animal testing
50) The PIF must be kept for a period of ten years after the date the last batch of the cosmetic product was placed on the market.
51) The Responsible Person must make the PIF readily accessible to a competent authority at the address notified, in accordance with Article 11.
52) The PIF should be a ‘living document’ and should be updated as necessary. For instance, it should be updated when changes are made to the Cosmetic Product Safety Report, such as the addition of new test data. If a product is significantly different from a product of the same name previously placed on the market, an update might not be sufficient and the Responsible Person will have to consider creating a new PIF.
Article 12 – Sampling and Analysis
53) Article 12 states that sampling and analysis of cosmetic products must be carried out in a reliable and reproducible way. Sampling and analysis are assumed to be reliable and reproducible if the method complies with the relevant harmonised standards see footnote 2.
Article 13 – Notification
54) It is the responsibility of the Responsible Person to notify the Commission of any cosmetic products placed on the NI and EEA markets. The Commission must be notified via the CPNP.
55) Separate notification requirements apply if the Responsible Person is established in NI and is placing a cosmetics product on the GB market, which will also require the Responsible Person to notify UK authorities using the UK Submit cosmetic product notifications (SCPN) service. Access the UK SCPN service.
Detailed guidance on placing goods on the GB market and the notification requirements under NI’s unfettered access to the rest of the UK can be found in the separate guidance for placing cosmetic products on the market in GB.
Chapter IV – Restrictions for Certain Substances
Article 14 – Restrictions for Substances Listed in the Annexes
56) Annexes 2 to 6 of the Regulation list prohibitions and restrictions on individual ingredients and how they may be used.
57) Annex 2 lists ingredients prohibited in all cosmetics.
58) Annex 3 lists ingredients that may only be used subject to the restrictions specified (e.g. they are not to be used in products for children under 3 years old). It can also set out wording of conditions of use and warnings for such products.
59) Annex 3 also includes certain ingredients commonly but not exclusively used in fragrances. These ingredients must be labelled individually if they exceed a certain threshold level, regardless of the function they perform in the product. This labelling requirement is in addition to normal perfume labelling requirements and does not replace them.
60) A number of these ingredients are also found in natural essential oils. To check the levels of these ingredients in their products, companies need to obtain information from their suppliers of essential oils and perfume compounds.
61) Only those colourants, preservatives and UV filters listed in the corresponding Annexes 4, 5 or 6 may be used, subject to any conditions specified in the Annex in which they appear.
62) For a substance in a cosmetic product to be permitted for use as either a colorant, preservative or UV–filter, the substance must be listed in the appropriate Annex.
Article 15 – Substances Classified as CMR Substances
63) Cosmetic products must not contain substances classified as category 2 or category 1A or 1B CMR substances under Regulation (EC) No 1272/2008, unless they are listed in one of the Annexes allowing their use (and so have been found safe for use in cosmetic products). Specific labelling to avoid misuse of the cosmetic product must be provided in accordance with Article 3, taking into account possible risks linked to the presence of hazardous substances and the routes of exposure.
Article 16 – Notification of Nanomaterials
64) Article 16 lays out the requirements relating to the notification of nanomaterials in cosmetic products. It does not apply where products contain nanomaterials that are colourants, preservatives, or UV–filters, and are listed in Annexes 3–6 (respectively); Article 14 covers these types of nanomaterial and these nanomaterials can only be used in accordance with the conditions laid down in the relevant Annex. All other nanomaterials must be notified in accordance with Article 16. Nanomaterials which are listed in Annexes 3–6 but are not intended to be used as colourants, preservatives or UV–filters must be notified in accordance with Article 16 and must comply with the conditions laid down in the relevant Annex. The Responsible Person must notify electronically a product containing nanomaterials to the Commission at least 6 months prior to the product being placed on the market. Article 16 (4) details the information that is required in this notification. This is to be done via the CPNP.
65) In certain circumstances, the notification information may be provided by a ‘Designated Person’ (defined in Article 16 (12)).
66) The European Commission will provide a reference for the toxicological profile.
67) Responsible Persons will need to work closely with their ingredient supplier of nanomaterials, and contractual arrangements should ideally reflect the high level of disclosure and cooperation necessary for the Responsible Person to comply with its obligations under Article 16.
Article 17 – Traces of Prohibited Substances
68) The non-intended presence of a small quantity of a prohibited substance is permitted, provided that the presence is in conformity with Article 3. Article 17 provides information on the circumstances under which this situation may arise.
Chapter V – Animal Testing
Article 18 – Animal Testing
69) Cosmetic products are not permitted on the NI or EEA market if the product’s ingredients, combination of ingredients or final formulation have been the subject of animal testing used to prove their safety for the purposes of this Regulation. However, historic animal testing data from animal testing that took place before such testing was banned at EU level may still be used in order to meet the requirements of the Regulation.
Chapter VI – Consumer Information
Article 19 – Labelling
70) Article 19 sets out the labelling requirements for cosmetic products. The Regulation requires all cosmetic products (except where specific exceptions apply – see below) to have clearly and indelibly marked on their container and packaging the following information:
- name and address of the Responsible Person
- country of origin
- nominal quantity of contents
- date of minimum durability (“Best Before” date)
- or (where the minimum durability is more than 30 months) a ‘Period After Opening’ (PAO)
- warning statements and precautionary information
- batch number
- product function, when not obvious from its packaging / presentation
- list of ingredients
Article 19 (1)(a) – Name and Address
71) The name and address required is that of the (NI or EU based) Responsible Person placing the product on the market.
72) The name and address allow identification of the Responsible Person to the consumers using the product. The details may be abbreviated, as long as the Responsible Person and their address can be identified. It is better to give as full an address as possible. The information must be given on both the container and the outer packaging.
73) If several addresses are listed the address through which the Product Information File (PIF) is readily accessible must be highlighted.
Article 19 (1)(a) – Country of Origin
74) The country of origin must be specified for imported cosmetic products, including products brought into NI from GB. The country of origin for a product made in GB and being brought into NI can say ‘made in UK’.
Article 19 (1)(b) – Statement of Contents
75) The Regulation requires the labelling of the nominal content at the time of packaging, given by weight or by volume. However, the following products are exempted:
- free samples
- where packing contains less than 5g or 5 ml
- single application, for example, sachets
- packs normally sold as a number of items, for which the details of weight or volume are not relevant, for example, bath balls, where the number of items appears on the packaging or is obvious, or if the product is normally sold individually
76) In addition to the requirements of the Regulation, compliance with the Weights and Measures (Packaged Goods) Regulations (Northern Ireland) 2011 see footnote 3 must be ensured where applicable.
77) The system of weight checking is known as ‘average quantity’. The three ‘Packers Rules’ for the average quantity system are:
- the average contents for a batch of product must not be less than the declared nominal quantity
- the proportion of packs which are short of the stated quantity by a defined amount – the ‘tolerable negative error’ or TNE must be sufficiently small to satisfy certain specified requirements
- no pack should be short by more than twice the TNE
78) Advice on Weights and Measures matters can be obtained from local weights and measures authorities in NI District Councils.
Article 19 (1)(c) – Minimum date of durability (Best Before Date)
79) A product which is likely to deteriorate up to and including 30 months from the date of manufacture so that it:
- ceases to satisfy the general safety requirement in Article 3 (being safe for human health under normal or reasonably foreseeable conditions of use), or
-
ceases to fulfil its intended function
-
must have a date of minimum durability marked on its container and packaging using either the words ‘best used before the end of’ or the ‘Hour–Glass’ symbol, given in Annex 7 (at point 3) to the Regulation, (reproduced in Appendix 3(3) of these Guidelines), immediately followed by either:
- the earliest date, in the form month, year or day, month, year, in which one of the matters set out in the bullet points above may occur, or
- an indication of where that date appears on the packaging.
80) The minimum durability date must appear on both the primary container and outer packaging in English. Best before November 2021, Best before Nov 21 and Best before 11/21 are all acceptable forms.
81) Any special precautions to be observed, such as storage conditions, must also be marked in English on both the primary container and outer packaging. This is in order to keep the product in a condition that satisfies the Regulation within the minimum durability date.
Article 19 (1)(c) – “Period After Opening” (PAO)
82) Dates of minimum durability (best before date) is not mandatory for products with a minimum durability of more than 30 months. Instead, for these products a Period after Opening (PAO) symbol is required it must appear on both the primary container and outer packaging.
83) The “Period After Opening” is the time after which the cosmetic product is safe and can be used without any harm to the consumer i.e. after this date it ceases to comply with the general safety requirement in Article 3.
84) By requiring labelling of a “Period After Opening”, the Regulation aims to provide useful information to consumers.
85) The PAO symbol must be used when, after opening, the deterioration of the product may lead to harm to the consumer.
86) Opening of the product may be considered as occurring when the consumer opens the product for use for the first time. The PAO symbol will not be necessary where there is no risk of harm to the consumer, as there is no risk of deterioration that could lead to damage to human health (in accordance with Article 3 of the Regulation) that is to say for single-use products, products not at risk of deterioration or products which do not open e.g. aerosols.
87) The PAO is indicated by a symbol representing an open cream jar, together with the period of time in months or years shown as a number i.e. 12 m. This is depicted in Annex 7 (2) of the Regulation and reproduced in Appendix 3 (1) of this guidance.
Article 19 (1) (d) – Warning Statements and Precautionary Information
88) Information must be provided on both the primary container and outer packaging in English. The additional presence of this information in other languages is not prohibited. Conditions of use and warnings for a range of ingredients are specified in the Annexes to the Regulation as follows:
| Annex Number | Column | |
|---|---|---|
| Chemical Substances | 3 | i |
| Colours | 4 | j |
| Preservatives | 5 | i |
| UV Filters | 6 | i |
89) If the ingredients are contained in these Annexes, any associated mandatory warnings must be provided in English. Additionally, any information deemed necessary for the safe use or safe disposal of the product must also be provided in English.
90) These requirements also apply to products intended for professional use (hairdressing in particular). Careful consideration should be given as to how the product is used and whether there is increased risk due to prolonged exposure or more unusual conditions of use. If judged to be necessary, special precautionary information must be provided in English.
Article 19 (1) (e) – Batch Number
91) A code which enables the manufacturer or supplier to identify the batch in which the product was manufactured must be marked on both the primary container and outer packaging. If the product is not made in a batch, then the code should enable the date and place of manufacture to be identified.
92) Where it is impossible for reasons of size for the batch number to appear on both the primary container and outer packaging, it may appear on the outer packaging alone.
Article 19 (1) (f) – Product Function
93) Unless this is clear from the presentation, the function of the product must be clearly stated on both the primary container and outer packaging in English. For example, the function of lipstick is obvious. However, a depilatory cream should not only be labelled as a ‘cream’.
Article 19 (1) (g) – List of Ingredients
94) A full list of ingredients. This may be given on the packaging alone and must be headed or preceded by the word ingredients. Where the product is not pre-packaged, the list must appear on the container or on a notice in immediate proximity to that container (see also paragraphs 108 to 126 on Labelling Difficulties); where the product is pre–packaged for immediate sale, the information must appear on an attached label, tag, tape or card or in an enclosed leaflet (where this is impossible for practical reasons this information must appear on a notice in immediate proximity to the container). This listing must:
- show all ingredients (‘ingredient’ means any substance or mixture intentionally used in the cosmetic product during the manufacturing process)
- use the name given in the glossary of Common Ingredient names; in the absence of a common ingredient name, a term as contained in the generally accepted nomenclature listed in Appendix 1 of this guidance note may be used
- for colourants (other than those intended to colour the hair), use the common ingredient name as detailed above. Colourants may be listed in any order after the other ingredients, using the Colour Index Number or denomination shown in Annex 4 of the Regulation (where applicable)
- perfume and aromatic compositions and their raw materials shall be referred to by the terms ‘parfum’ or ‘aroma’. For example, a flavour ingredient or mixture is an aroma. See also paragraph 104 concerning certain ingredients that must be labelled individually even if they form part of a perfume composition or essential oil. The threshold levels for declaration are 0.001% for leave-on products and 0.01% for rinse-off products
- show ingredients in descending order of weight (determined at the time the ingredients are added to the product)
- all ingredients present in the form of nanomaterials should be indicated in the list of ingredients immediately following the INCI name of the ingredient in question. This is done by adding ‘(nano)’ after the ingredient name
95) Additionally:
- ingredients in concentrations of less than 1% may be listed in any order after those in concentrations of 1% or more
- for decorative cosmetics see footnote 4 marketed in several colour shades, all colourants (other than those intended to colour the hair) used in the range may be listed preceded by the words ‘may contain’ or the symbol ‘+/–‘
- mixtures must be broken down into their individual components
96) For the purposes of labelling, the following are not regarded as cosmetic ingredients and do not need to be shown:
- impurities in the raw materials
- subsidiary technical materials used in the preparation of the cosmetic product but not present in the final product
Ingredient listing – Labelling issues
Variable Ingredients
97) The Regulation makes provision for the listing of all colourants used in a decorative range of cosmetics, although each product would only contain a selection of those colours. The intention is to simplify manufacture by allowing all of the colourants to be listed on one label in a market where fashions and colours change frequently. However, there is no specific provision made for other ingredients which are subject to change.
98) For example, minor formulation changes of non–colour ingredients are usually necessary to accommodate the different characteristics of colour pigments used within a range of colour cosmetics.
99) The Regulation does not make specific provision for this case and a strict interpretation would require separate labelling for each formulation. However, in this type of situation, it is the accepted industry practice to include the variable ingredients in the main body of the ingredients listing. The +/– (may contain) section of the ingredients listing is reserved for colourants. Colour ingredients which do not have a CI number (as listed in Annex 4 to the Regulation), but are closely associated with colour, might only be present in some products within the decorative range. The industry interpretation is to list these items under the +/– (may contain) section of the ingredient listing.
100) An example of an ingredient listing as it might appear on a cosmetic product is given in Appendix 2.
International Nomenclature
101) In developing the INCI system, Cosmetics Europe worked closely with the equivalent organisation in the USA, the Personal Care Product Council (PCPC). As a result, there is now a joint Cosmetics Europe / PCPC Ingredient Nomenclature Committee responsible for allocating labelling names, and recommending labelling rules for all ingredients used in cosmetics for the EU and US markets. In the interests of consumer safety, the UK fully supports the use of the INCI system, which has been adopted both in Europe and the USA and is widely accepted elsewhere.
102) Despite these efforts, there are still some geographical differences in the INCI system. These exist in the nomenclature of colours, botanicals and the so–called ‘trivial’ ingredients. This causes difficulties for importers and exporters. Where there are differences, the additional use of the alternative name, in brackets, is acceptable.
103) For example:
- water (aqua) or aqua (water)
- santalum album (sandalwood oil) or sandalwood (santalum album) oil
- CI 14700 (Red 4) or Red 4 (CI 14700)
Article 19 (2) – Labelling Difficulties
104) The variety and nature of cosmetic products and their packaging may pose difficulties when trying to include all of the information specified in the Regulation. Certain provisions have therefore been made to take into account the practical difficulties.
Warning statements and precautionary information
105) Particular precautions to be observed in use and at least those listed in Annexes 3–6 and special precautionary information will normally appear on both the primary container and outer packaging. Where this is impossible for practical reasons, the information may be given on a leaflet, label, tag, tape or card enclosed with the cosmetic product or attached to it.
106) When the information is given in an enclosed leaflet, label, tag, tape or card, the consumer must be referred to it. This can either be done by abbreviated information, or by a special symbol given in Annex 7(1) of the Regulation (the hand and book symbol). The symbol must appear on the container or the outer packaging. This symbol is reproduced in Appendix 3(1) of this guidance.
Ingredient listing
107) An ingredient listing, as detailed in Article 19, is required on the outer packaging only, or – in its absence – on the primary container. Where it is impossible (due to practical reasons) for the list to appear on the packaging or container, it must be given on a leaflet, label, tag, tape or card enclosed with the product or attached to it. The consumer must be referred to the text either by abbreviated information or by a special symbol, given in Annex 7 (1) of the Regulation (the hand and book symbol). This must be on both the container and outer packaging. The symbol is reproduced in Appendix 3(1) of this guidance.
Hotels
108) Cosmetic products made available to customers in their hotel rooms are subject to all of the requirements of the Regulation. However, full use can be made of the exemptions to ingredient labelling given for difficult shapes and small packs. For example, it is acceptable for the ingredient information to be given on a leaflet or card which can be placed close to the product where the Regulation allows this.
Public Conveniences and Toilet Facilities Open to the Public
109) Cosmetic products made available in public conveniences are subject to all of the requirements of the Regulation.
Vending Machines
110) The labelling requirements in the Regulation apply equally to products dispensed from vending machines.
Free Samples
111) Free samples, whether they are provided in–store, by direct mail or in magazines (for example shampoo samples), are considered to be within the definition of supply contained in the Regulation. Compliance with all of the applicable requirements of the Regulation is therefore required.
Article 20 – Product Claims
112) Article 20 requires that in the marketing of cosmetic product, every Responsible Person must ensure that the wording of any claim in relation to a cosmetic product does not imply that the product has a characteristic or function which it does not have. This covers claims made in the form of texts, names, trademarks and figurative or other signs that say or imply that the product has characteristics or functions in the labelling or making available or marketing of the product.
113) A Responsible Person must ensure that the wording of any claim complies with the common criteria set out in the Annex to Commission Regulation (EU) No 655/2013. This lays out the common criteria for the justification of claims used in relation to cosmetic products, which are as follows:
- legal compliance
- truthfulness
- evidential support
- honesty
- fairness
- allow informed decision-making
114) Legal compliance: Claims must comply with all applicable legal requirements and self–regulatory regimes and should meet the reasonable expectations of the average end user of the product. Claims of compliance with legal requirements or approval by competent regulatory authority are not allowed; neither are claims that convey the idea that a product has a specific benefit when this benefit is mere compliance with the minimum legal requirements.
115) Truthfulness: Claims should not be based on false or irrelevant information. If the presence of a specific ingredient is claimed, it must be deliberately added to the product and claims relating to the properties of an ingredient must not imply the finished product has that benefit when it does not. Claims must not imply that opinions verify claims unless the opinion reflects verifiable evidence.
116) Evidential support: All claims, whether implicit or explicit, must be supported by evidence that is adequate and verifiable. Studies must follow well-designed and well-conducted methodologies, and must respect ethical considerations and should be relevant to both the product and the benefit claimed. The level of evidence must be consistent with the type of claim being made – for example where a lack of efficacy may cause a safety problem (e.g. sun protection) more evidence may be required. Best practice is the key to the quality of evidence with the level of evidence being consistent with the type of claim. Statements of hyperbole or exaggeration not taken literally or of an abstract nature will not usually require substantiation.
117) Honesty: Claims must not go beyond supporting evidence, nor imply by action or omission that the product has characteristics or functions which it does not have. Extrapolation of ingredient properties to the finished product must be supported by adequate and verifiable evidence. Claims for ‘new and improved’ must not be overstated. Claims shall not attribute to the product concerned specific (i.e. unique) characteristics if similar products possess the same characteristics. If the claimed benefit is linked to specific conditions, these must be clearly stated.
118) Fairness: Claims should be objective, and not denigrate competitors nor denigrate ingredients that can be legally and safely used in cosmetic products. Claims must not create confusion with the products of a competitor.
119) Informed decision–making: By inclusion of the necessary information on function and characteristics of the product, claims should contribute to the ability of consumers and professionals to make informed decisions. They should be clear, precise, relevant and understandable to the average end-user in the target audience, taking into account the capacity of that end-user to understand the information.
Article 20 (3) – Reference to Animal Testing
120) The Regulation recognises that companies should be able to make claims that no animal testing was undertaken in the development of its cosmetic products. However, the Regulation is also clear that consumers must not be misled by these claims.
121) For any cosmetic product placed on the market, Article 20.3 states:
The Responsible Person may refer, on the product packaging or in any document, notice, label, ring or collar accompanying or referring to the cosmetic product to the fact that no animal testing tests have been carried out only if
- the manufacturer and the manufacturer’s suppliers have not carried out or commissioned any animal tests on the finished cosmetic product, or its prototype, or any of the ingredients contained in it, or
- used any ingredients that have been tested on animals by others for the purpose of developing new cosmetic products.
122) Data relating to the safety assessment of the product, including details of any animal testing, must be kept in the PIF which is open to inspection by the enforcement authority (see Article 11.2(e)).
Article 21 – Public Access
123) The information listed below must be made easily accessible to the public by the Responsible Person by any appropriate means:
- the qualitative and quantitative composition of the product
- the name and code number of the perfume and aromatic compositions
- the identity of the supplier; and
- information on existing data on undesirable or serious undesirable effects on human health (resulting from the use of the cosmetic product).
124) The qualitative information made accessible ought to be consistent with the ingredient list on the product’s package.
125) There is no obligation to provide a full declaration of the quantitative formula. However, for any cosmetic ingredients present in the product that fulfil the criteria listed in Regulation (EC) No 1272/2008 (on classification, labelling and packaging of substances and mixtures, Parts 2 to 5 of Annex I), the use concentration should be indicated.
126) Information on perfume or perfume compositions is generally subject to commercial secrecy and is part of a company’s intellectual property. They therefore do not need be made available to the public. However, the name and or code of the perfume together with the name of the supplier should be provided.
Chapter VII – Market Surveillance
Article 22 – In–Market Control
127) Article 22 outlines the responsibilities of enforcement authorities regarding market surveillance. They must monitor compliance by checking the PIF, how a business complies with GMP, and carry out physical product checks and laboratory analysis when necessary.
Article 23 – Communication of Serious Undesirable Effects
128) Information on Undesirable Effects (UEs) and Serious Undesirable Effects (SUEs) is included in the Safety Report which in turn is part of the PIF.
The requirement to notify the European Commission of SUEs remains unchanged. (Read guidance and access relevant forms.)
The notification form for SUEs in NI should be sent to the Office for Product Safety and Standards (OPSS) at seriousundesirableeffects@businessandtrade.gov.uk, who will inform the Responsible Person and the European Commission. Notification should take place without delay.
129) Where a Distributor reports the SUE of a cosmetic product to the Secretary of State, the Office for Product Safety and Standards acting on the Secretary of State’s behalf must inform the Responsible Person and the competent authorities of EU member States.
130) Consumers and health professionals may also report SUEs of a cosmetic product. If they report the SUE to any competent authority that is not the Secretary of State (ie a District Council), then that competent authority must immediately inform the Secretary of State via the Office for Product Safety and Standards. The Secretary of State must then inform the Responsible Person and the competent authorities of member States.
131) Competent authorities may use this data for in–market surveillance purposes, market analysis, evaluation and consumer information (in the context of Articles 25, 26 and 27 – see below).
Article 24 – Information on Substances
132) If a competent authority has serious concerns about the safety of a substance, it may request from the Responsible Person a list of all their products containing that substance and the concentration present in each product.
Chapter VIII – Non–compliance, Safeguard Clause
Article 25 – Non–compliance by the Responsible Person
133) Competent authorities are required to take action over a product that does not comply with the Regulation, primarily by requiring the Responsible Person to take corrective action.
134) A competent authority must request the Responsible Person to take all appropriate measures, proportionate to the nature of the risk, where there is certain non–compliance including corrective actions aimed at ensuring compliance, or withdrawal or recall, within an expressly mentioned time limit. Where applicable, the competent authority must inform the competent authority of the member State in which the Responsible Person is established. If the Responsible Person does not take the measures within the time limit, or where immediate action is necessary to prevent serious risk to human health, the competent authority must take all appropriate measures itself to stop the product going on the market, or to withdraw or recall products already on the market. Competent authorities have all the powers they need to prevent any further distribution or sale of the product if the Responsible Person is not taking the necessary actions.
135) The competent authority that has taken these above measures must inform all other competent authorities of the measures taken.
Article 26 – Non–Compliance by Distributors
136) Competent authorities must require distributors to take appropriate action over a product that does not comply with the Distributors responsibilities set out in Article 6. The actions available are similar to those for the Responsible Person.
Article 27 – Safeguard Clause
137) The safeguard clause allows an enforcement authority to take direct provisional action where it ascertains there may be a serious risk to human health, or where there are reasonable grounds for concern. An enforcement authority other than the Secretary of State must obtain authorisation from the Secretary of State prior to taking any provisional measures under Article 27.
Article 28 – Good Administrative Practices
138) Article 28 is intended to ensure that competent authorities do not act unreasonably by taking action under Articles 25 and 27. The competent authority must follow these procedures to ensure the Responsible Person and or Distributor concerned is kept fully informed of the situation and is allowed to input into the process.
Appendix 1
Identification of Ingredients
An ingredient should be identified by its common name (INCI name) or its CosIng name. In the absence of an INCI or CosIng name, any of the following means of identification may be used:
- chemical name
- European Pharmacopoeia name
- International non–proprietary name as recommended by the World Health Organisation
- Einecs, Iupac or CAS identification reference, or
- Colour Index number
Note: the Cosmetics Regulation refers to the INCI, published by the European Commission. The International Cosmetic Ingredient Dictionary and Handbook publish by PCPC is also referred to as INCI.
Unlisted Ingredients
Where an INCI name for an ingredient does not exist, then an application for a name should be made to the International Nomenclature Committee (INC) based in Washington, USA. Application for an INCI name should be made through PCPC (please see Appendix 6).
Appendix 2
Ingredient labelling example

Ingredient labelling example
Appendix 3
Symbols used on Packaging / Container from Annex VII of the Cosmetics Regulation
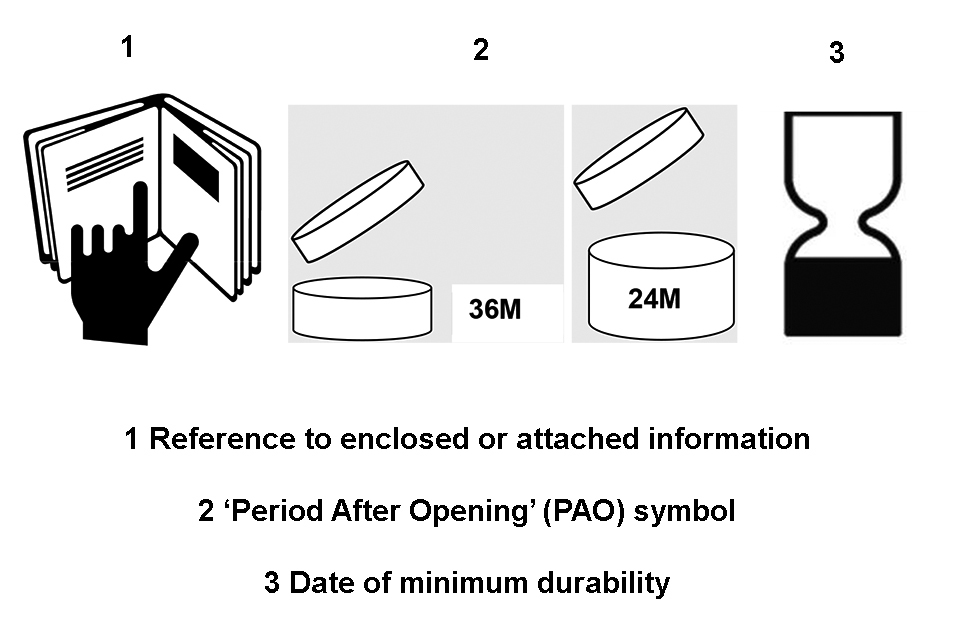
Symbols for reference to enclosed or attached information, Period After Opening (PAO), and date of minimum durability.
Appendix 4
Illustrative list of cosmetics (Recital 7 to the Regulation):
The following list is not exhaustive but is provided by way of example.
- Creams, emulsions, lotions, gels & oils for the skin
- Face masks
- Tinted bases (liquids, pastes, powders)
- Make-up powders, after-bath powders, hygienic powders etc.
- Toilet soaps, deodorant soaps, etc.
- Perfumes, toilet waters and eau de Cologne.
- Bath & shower preparations (salts, foams, oils, gels etc.)
- Depilatories
- Deodorants and anti-perspirants.
- Hair colorants
- Hair products for waving, straightening and fixing
- Hair–setting products
- Hair cleansing products (lotions, powders, shampoos)
- Hair conditioning products (lotions, creams, oils)
- Hairdressing products (lotions, lacquers, brilliantines)
- Shaving products (creams, foams, lotions)
- Products for making up and removing make-up
- Products intended for application to the lips
- Products for care of the teeth and mouth
- Products for nail care and make-up
- Products for external intimate hygiene
- Sunbathing products or sun protection products
- Products for tanning without sun
- Skin-whitening products
- Anti-wrinkle products
Appendix 5
Useful links
Read General European Commission guidance.
Read European Commission guidance on notifying serious undesirable effects.
Read European Commission guidance on the Cosmetics Product Safety Report.
Read European Commission technical document on cosmetics claims.
Read European Commission cosmetic ingredients database.
Read guidance on importing goods from the EU to Great Britain.
Footnotes
1: “A Guide to What is a Medicinal Product” may be ordered from the MHRA or downloaded from its website.
2: The UK has published a list of references to designated standards. These have the same function as harmonised standards and give presumption of conformity to legal requirements. These designated standards are the same as the harmonised standards.
3: R 2011 No. 331
4: Decorative cosmetics are taken to be cosmetic products intended to modify the appearance of the area to which they are applied, usually by the use of colour. Examples are: lipstick, eye shadow, blusher, eye pencil, liquid foundation, powder, mascara, nail polish, etc.
