Assessment of swabs available for testing children under 5 years of age
Published 27 February 2023
Applies to England
Summary
This report summarises the findings of 2 assessments of swabs used for coronavirus (COVID-19) testing of children under 5 years of age, conducted to ensure safe implementation of testing for this age group, from June to December 2020.
At the outset of NHS Test and Trace (initially known as the COVID-19 National Testing Programme, and referred to at times in this report as ‘the testing programme’), expert advice was sought from the NHS COVID-19 clinical cell on the appropriate sample collection swabbing technique for children under 5 years of age. The advice received was that a parent or guardian should take the sample using ‘standard’ COVID-19 polymerase chain reaction (PCR) sample collection swabs (oropharyngeal or nasal swabs with a 5mm tip) but only where they felt comfortable to do so. Swab specifications can be found in appendix A.
To provide assurance that this testing approach for children under 5 years of age was safe and provided an optimal experience, 2 assessments were conducted in 2020.
The first assessment comprised enhanced surveillance of incidents in those under 5 years of age across the whole testing programme together with some focussed follow-up with parents attending a subset of regional testing sites (RTS). Participating RTSs recorded subjective response observations for 434 children in this age group during June and July 2020 using a brief questionnaire administered by staff.
Results from this assessment were presented on 31 July 2020 to the NHS Test and Trace Clinical Issues Group – a forum where public health and clinical topics were discussed in order to provide advice and oversight across the testing programme (this was later replaced by the Public Health and Clinical Oversight team). The level of incidents of harm or distress experienced within children under 5 years of age as a result of the use of standard swabs across the testing programme, was low, especially when balanced against the benefits of being able to test young children. This led to the enhanced surveillance being stood down. Incidents affecting all age groups continued to be monitored via incident management and clinical governance systems.
In August and September 2020, a ‘mini-tip’ swab (an oropharyngeal or nasal swab with a 2 to 3mm tip) was validated for use in the testing programme as part of standard procurement with a view to implementation across all age groups. This presented the opportunity to innovate with the bespoke use of this type of swab for children under 5 years of age. Swab specifications can be found in appendix A.
In the absence of evidence of specific additional benefit of using the mini-tip swab for those under 5 years of age, it was decided to conduct a focussed roll out of the mini-tip swab alongside a second assessment. The purpose of this assessment was to determine if there was sufficient evidence of benefit compared to the standard swab, that could justify the routine supply of mini-tip swabs in the testing programme for swabbing children under 5 years of age.
Due to the logistical and procurement challenges, as well as opportunity cost to other aspects of the programme to ensure every child under 5 years of age had access to mini-tip swabs, implementation of this option could only be considered if there was clear proven user benefit. As the mini-tip swabs were already validated for use in the programme, a performance evaluation of the mini-tip swabs was not required.
The mini-tip swabs were initially made available for testing those under 5 years of age at a single RTS. In October and November 2020, observational monitoring using the same questionnaire, which included user feedback on testing experience, and enhanced incident reporting were also implemented for this period.
The results from the observation of 128 participants in group of children under 5 years of age were presented to NHS Test and Trace Clinical Issues Group in December 2020. The potential benefits were not sufficient to support implementing a system to make bespoke mini-tip swab kits available for children under 5 years of age, so the recommendation was made to continue with standard swabs. Incidents for all age groups continued to be monitored via incident and clinical governance systems (details in addendum 1).
Introduction
The global SARS‑CoV‑2 coronavirus outbreak in early 2020, led to the rapid implementation of the testing programme. PCR testing was initially offered in designated ‘drive-through’ RTSs for symptomatic key workers, such as NHS staff, with the dual public health aim of minimising onward spread of the virus and allowing critical national infrastructure to remain functioning.
Government policy at that time was that if one person within a household was symptomatic for COVID-19, all other individuals within that household had to self-isolate in an attempt to stop the chain of transmission. This resulted in key workers having to self-isolate if their families were symptomatic and unable to access testing.
Maintaining critical national infrastructure during periods of lockdown was crucial. This was particularly the case for healthcare services, as the NHS was adapting to the high demand created by the pandemic. Self-isolation of key workers who were household contacts represented a great cost to society and put essential services under strain. Testing family members enabled those whose contacts tested negative to return to work.
Initially, only people aged over 5 years were able to access testing. This meant that if a key worker’s family member aged under 5 years living in the same household had COVID-19 symptoms, the key worker and their family were obliged to self-isolate and the key worker became unavailable for work. To alleviate this problem, access to testing was expanded to children under 5 years of age in regional and local testing sites.
Expert clinical consideration and advice on the appropriate swabbing methodology was sought from the NHS COVID-19 clinical cell, supported by the Royal College of Paediatrics and Child Health. The recommendation was clear that standard swabs were appropriate for use in testing the group with children under 5 years of age.
NHS Test and Trace’s PCR sample collection instructions recommended that for the majority of cases in all age groups, the sample collection was performed via throat and single mid-turbinate nasal (nose and throat) swabbing, using the standard swab included in the SARS-CoV-2 PCR test kit. As sample collection could be distressing for children under 5 years of age, it was recommended that the child’s parent or guardian collected the sample from the child, but only if they felt comfortable to do so.
The expansion enabled key workers with symptomatic family members under 5 years of age to be able to confirm or exclude infection, allowing workers with ‘negative households’ to return to work and only those that were contacts of confirmed cases to appropriately isolate.
Context of assessment
As part of NHS Test and Trace’s responsibility to ensure safe implementation, an initial safety assessment was conducted in June and July 2020 at 18 RTSs comprising observational data collection by questionnaire and enhanced monitoring of potential incidents in those under 5 years of age when using standard swabs for sample collection.
In parallel to the RTS observational data being collected in the initial assessment, the testing programme actively monitored national data for the testing of children under 5 years of age. From May to July 2020, a total of 125,142 tests were requested for those under 5 years of age, with 78,188 (62%) being completed or returned. Out of these completed and returned tests, 58,332 (74.6%) tests were conducted on testing sites, with 95% success rate and only a 4% void rate and 1% null rate (null meaning not yet processed or notification not sent to end user). Out of the monitored tests in children under 5 years of age, there were no reported incidents registered (summary of total incidents to date in addendum 1).
Subsequently during August and September 2020, a new mini-tip swab, with a smaller tip than standard swabs (see appendix B), became potentially available for use within testing sites. With this opportunity for innovation in testing, there was a need to evaluate if the mini-tip swabs could provide a better user sample collection experience for group with children under 5 years of age and make testing more accessible in a mass-testing context, where sample collection swabbing for children under 5 years of age was undertaken by parents and guardians, and not by clinically-trained individuals.
As there was no available user evidence on the benefits of mini-tip swabs over standard swabs in children under 5 years of age, the inclusion of mini-tip swabs among swabs within the testing programme provided the opportunity to assess this via a managed pilot. A rapid literature search at the time of the writing of this review also did not identify a large peer-reviewed literature comparing the tolerability of standard and mini-tip swabs for paediatric use.
Assessing the value of providing bespoke mini-tip swab kits for children under 5 years of age prior to wider implementation across all RTSs (requiring bespoke procurement, supply, logistics, digital development and opportunity cost to other aspects of the programme) was key to inform decision-making on how to improve the testing service for this age group. With that aim, a focussed roll out of mini-tip swabs for those under 5 years of age took place in October and November 2020 to assess the potential benefits.
The pilot consisted of a second period of enhanced observational data collection by questionnaire undertaken on swab usability and incidents on the use of this smaller mini-tip swab.
Questions being addressed
First assessment
Is the expansion of testing to children under 5 years of age in a mass-testing context being conducted in a safe manner?
Second assessment
Do mini-tip swabs provide substantial benefits in the sample-collection experience compared to the use of standard swabs, for children under 5 years of age, to justify implementing a bespoke system within the testing programme to ensure availability of mini-tip swabs?
Assessment parameters
The 2 assessments were designed as rapid observational service improvement exercises in the live service via enhanced surveillance of testing of children under 5 years of age, to ensure testing in that group was being conducted in a safe manner, as well as to evaluate benefits from the introduction of mini-tip swabs for children under 5 years of age. As such, the exercises were not formal evaluations of swab performance and not powered to collect a sample size for statistical comparisons required to assess swab performance.
The first assessment, conducted from 8 June to 27 July 2020, was set up to provide assurance that testing in children under 5 years of age was being delivered safely when using standard swabs for sample collection was implemented.
Observations using a standard questionnaire from 434 participants aged 4 years and under were recorded by a convenience sample of more than 18 staff members in 18 different RTSs (age distribution of participants in appendix A). Two standard swabs in use in the testing programme at the time were used for sample collection in the assessment, ‘standard swab A’ and ‘standard swab B’ (swab specification summaries in appendix B).
The second assessment, conducted between 16 October to 4 November 2020, was set up to assess potential benefits of using mini-tip swabs compared to standard swabs for sample collection in children under 5 years of age, to assess whether these justified implementing a bespoke testing service for that group.
Observations by standard questionnaire from 128 participants aged 4 years and under, were recorded by 4 staff members in Kettering RTS. The mini-tip swab was used for sample collection in the assessment and the results compared to the use of standard swabs in the original assessment.
Assessment parameters for standard swabs and mini-tip swabs
Standard swabs assessment (‘standard swab A’ and ‘standard swab B’)
Locations:
-
Ebbw Vale RTS
-
Guildford RTS
-
Gatwick RTS
-
Lincoln RTS
-
Midland Met RTS
-
Newcastle Great Park RTS
-
Nottingham RTS
-
Penrith RTS
-
Perth RTS
-
Portsmouth RTS
-
Preston RTS
-
Salisbury RTS
-
Slough RTS
-
Stafford RTS
-
Stansted RTS
-
Taunton RTS
-
Telford RTS
-
York RTS
Number of participants: 434 children aged under 5 years
Number of site staff recording observations: more than 18 staff across all sites
Time period over which data was collected: 8 June to 27 July 2020
Mini-tip swabs assessment (‘mini-tip swab’)
Location: Kettering RTS
Number of participants: 128 children aged under 5 years
Number of site staff recording observations: 4 staff
Time period over which data was collected: 16 October to 4 November 2020
Eligibility criteria
Participants for both assessments periods were required to be aged under 5 years and were attending the RTS with the intention of being tested (with their parent or guardian consent) for SARS-CoV-2. Their parent or guardian needed to be willing to provide additional user feedback on the experience.
Methodology
The parents or guardians of the child were given verbal and written instructions to collect the sample, as well as the necessary kit (swab, instructions booklet, and so on) to perform the PCR test sample collection. Standard written national instructions were provided.
In the standard swab assessment, a testing kit containing a standard swab was used for sample collection, whilst in the mini-tip swab assessment, a testing kit containing a mini-tip swab was used for sample collection.
After the swab sample was collected by the parent or guardian, additional observations related to swab usability were collected by members of site staff. The observations collected were based on a 7-question questionnaire that a member of staff would ask the parent or guardian after the swab sample was collected.
The majority of answers were registered as free text; data was retrospectively merged into analysis categories for the 2 swab types and assessment time periods. As the assessment was conducted in an enhanced incident reporting context, definitions of ‘distress’ and ‘harm’ were left open to interpretation by the parent or guardian.
Questionnaire used to collect observations after each participating child under 5 years of age is tested
Question 1a: Have there been any reports of distress from testing this child?
Possible answers:
- no distress
- distress
Question 1b: Have there been any reports of harm from testing this child?
Possible answers:
- distress or no incident
- incident
- free text description of incident
Question 2: How easy to follow were the instructions?*
Possible answers:
- easy
- neither easy nor hard
- hard
Question 3: What other information (if any) would be helpful to include in the instructions?*
Possible answers:
- free text
Question 4: Did the swab prove challenging for use?
Possble answers:
- not challenging
- challenging and completed
- challenging and abandoned
Question 5: Feedback from the user on which part of the test was particularly challenging and why (if applicable)*
Possible answers:
- free text
Question 6: Was the test taken on throat and nose, or nose only?
Possible answers:
- throat and nose
- nose only
Question 7: Additional comments
Possible answers:
- free text
*Feedback regarding the standard national sample-collection instructions were positive, with most participants on both assessments reporting instructions as being ‘easy to follow’ for the standard and mini-tip swab assessments. Results were fed into programme improvement activities regarding instructions for use and not reported here.
After the assessments were completed, the results were analysed to see what could be learned about the use of standard swabs and the possible benefits of using mini-tip swabs with respect to safety and tolerability.
Key findings
Testing tolerance
Image 1 is a bar chart showing testing tolerance of standard swabs and mini-tip swabs. The mini-tip swab had a higher incidence of distress reported (78%) when compared to the standard swab (25%). However, it should be noted that a greater proportion were successful with the mini-tips in completing a nose and throat swab rather than opting for a nose only swab (see section on swab location). The nature of distress was not described for the majority of the standard swab incidents. However, when using the mini-tip swab, 57% of the incidents of distress were described as involving ‘crying’, and 9.3% involving ‘discomfort’.
Image 1: Summary of results for ‘testing tolerance’
| Testing tolerance | ‘Standard swab’ | ‘Mini-tip swab’ |
|---|---|---|
| No distress or incident | 325 (74.8%) | 27 (21.2%) |
| Distress | 108 (28%) | 100 (78.0%) |
| Reported incident | 1 (0.2%) | 1 (0.8%) |
There were 2 instances of reported incidents, one while using the standard swab (0.2%) and one while using the mini-tip swab (0.8%), both involving nose bleeds. The standard swab incident reported that there was blood on the swab after swabbing the nose. The mini-tip swab incident reported was a nosebleed after swabbing the nose.
Swabbing technique
Regarding difficulty to use the swabs during the sample collection, results were similar for both swab types, with the majority of the participants finding the swabs ‘not challenging’ to use.
Image 2 is a bar chart showing the level of difficulty/ease in using the standard swabs and the mini-tip swabs. 75% of participants reported the standard swabs were ‘not challenging’ to use and 77% reported the same for the mini-tip swab. Of those that responded with ‘challenging but completed’, 22% reported this for the standard swab and 23% reported the same for the mini-tip swab. Responses of ‘challenging and abandoned’ accounted for 3% of standard swab responses and 0% for mini-tip swab responses.
Image 2: Summary of results for ‘difficulty to use’
| Difficulty to use | ‘Standard swab’ | ‘Mini-tip swab’ |
|---|---|---|
| Not challenging | 326 (75%) | 99 (77%) |
| Challenging but completed | 95 (22%) | 29 (23%) |
| Challenging and abandoned | 13 (3%) | 0 (0%) |
Swab location
The recommended swabbing technique for both swab types was ‘nose and throat’ (throat and single mid-turbinate nasal). However, an option for testing in those that found this challenging (for any age group) was to undertake nose only swabbing.
A portion of the participants could only swab the nose of children under 5 years of age. Image 3 is a bar chart of swabbing location for both standard swab and mini-tip swab. The success rate for ‘nose and throat’ swabbing was higher for the mini-tip swab (84%), compared to the standard swab (68%).
Image 3: Summary of results for ‘swabbing collection’
| Swab location | ‘Standard swab’ | ‘Mini-tip swab’ |
|---|---|---|
| Nose and throat | 295 (68%) | 107 (84%) |
| Nose only | 135 (31%) | 13 (10%) |
| Other or no response given | 4 (1%) | 8 (6%) |
A total of 31% of respondents reported swabbing in the nose only for the standard swab and 10% for the mini-tip swab. The most common reason for nose-only swabbing was the child becoming too uncomfortable or distressed while the parent or guardian was swabbing their throat, which represented an acceptable reason to utilise a ‘nose-only’ swabbing method; 1% of standard swab and 6% of mini-tip swab provided an ‘other’ response or no response at all.
In parallel to investigating better sample collection methodologies for children under 5 years of age, the testing programme also conducted wider improvement activities around ensuring access to service. This included examining whether a more convenient alternative to the ‘nose and throat’ sample collection swabbing methodology for PCR testing could be implemented.
Feedback suggested nose and throat swabbing could be particularly challenging in Adult Social Care settings and special educational needs settings. NHS Test and Trace conducted a service evaluation in September 2020 comparing the effectiveness of 2 sample collection swabbing methodologies, anterior nares (nose-only) and the recommended throat and mid-turbinate nasal (nose and throat). A total of 2,169 subjects aged 18 years and over participated and collected 2 samples each, using both of the sampling methods.
The results showed a statistically significant difference between the 2 techniques, with the nose-only swabs associated with lower viral load and fewer positive results compared to the nose and throat swabs. The results did not recommend the use of nose-only swabbing in all settings. However, it supported the use of nose-only swabbing for sample collection as an exception, where the technique could increase compliance in a population that finds nose and throat swabs to be unacceptable, such as in group of children under 5 years of age.
Challenges and limitations
Given the pace at which the data collection exercises were required to be conducted as part of live testing service rollout, there were challenges and limitations with potential implications for the assessment exercise delivery, data analysis, and results:
COVID-19 pandemic context in 2020
Due to the accelerated pace of the pandemic, high demand for COVID-19 PCR tests and urgency for evidence to facilitate the testing programme planning and policy, the time available for preparation, set up and delivery was limited.
Number of test sites
As there were multiple RTSs and staff involved in evaluating the standard swab assessment (18 sites and >18 staff) compared to the mini-tip swab assessment (1 site and 4 staff), the standard of data collected may have varied between assessment periods. Within the mini-tip assessment there was greater potential for local factors to influence the generalisability of results; in the initial assessment urgency to maximise throughput of individuals tested may have affected the completeness of data collection.
Time period variation
The data collected for the standard swabs was done relatively early in the COVID-19 pandemic, where a high proportion of the population were swabbing for the first time. With a time period of 3 months between assessments, a higher proportion of individuals on the mini-tip swab assessment were likely to be experienced in swab sample collection for a PCR test. This may have impacted results and limited comparison.
Nature of participants
Due to the nature of the assessment, it was not possible to conduct 2 sample collections on the same subject to compare different swabs, as the results related to distress and incidents at time of swabbing. Therefore, a like-for-like comparison of the swab types was not possible.
Subjective nature of data
Due to the enhanced-monitoring nature of the assessments, the key criteria of ‘distress’ was open to being influenced by, amongst other things, the observer’s (usually the parent’s) perception of distress in the child. In addition, as the assessment actively questioned the parent or guardian, a higher number of reports for distress were expected, compared to normal incident reporting procedures. This may have inflated reports of distress in the mini-tip assessment particularly.
Conclusion
The testing programme has a proactive approach to ensure the safety and improvement of services. As such, results for each assessment are carefully analysed and discussed by the leadership team, feeding into established clinical governance structures and supporting decision-making for key areas of the programme.
Following completion of the first assessment, a summary of observations was shared with the NHS Test and Trace Clinical Issues Group on 31 July 2020. The level of incidents being experienced as a result of the use of the standard swabs was such that enhanced monitoring was stood down and incidents for all age groups continued to be monitored via the programme’s incident and clinical governance systems (details in addendum 1). Data on distress specifically supported the need for nasal-only swabbing to be an option available to parents or guardians and that they needed to be comfortable with undertaking the swabbing process.
Following the completion of the second assessment, a summary of the results analysis containing data from both assessments was presented to the NHS Test and Trace Clinical Issues Group on 15 December 2020, where key findings and limitations were discussed.
Reports of incidents were acknowledged as not significant, and the nature of the 2 instances of harm between assessments were not of significant concern.
The subjective nature of the term ‘distress’ and the implication of the observer having different interpretations of ‘distress’ was acknowledged.
It was noted that the difference in the sample size and potential differences between the test sites may limit the conclusions that can be drawn from the data.
Benefits of the mini-tip swabs regarding completion of both nose and throat swabbing in children under 5 years of age were not thought to justify setting up a bespoke system to implement the use of mini-tip swabs across the testing programme particularly in the context of mitigations around options for nose-only swabbing.
Potential benefits of the mini-tip swabs were acknowledged as very limited. To draw more comprehensive conclusions regarding benefits, a larger study would be required, with variables such as subjectivity of distress addressed
A rapid literature review was undertaken in May 2022 to help determine if the position taken by the programme should be revisited. Searches were of the PubMed database covering the previous 5 year period (20 May 2017 to 19 May 2022) and one individual undertook screening activity of papers. Relatively little published data was identified specifically regarding children, with only 4 relevant papers in the 452 identified using the search terms (see references). While none provided sufficient evidence to suggest a change in the current testing programme approach, there was a general theme that nasal-only swabbing was identified as a preference.
Appendix A: Swab specifications
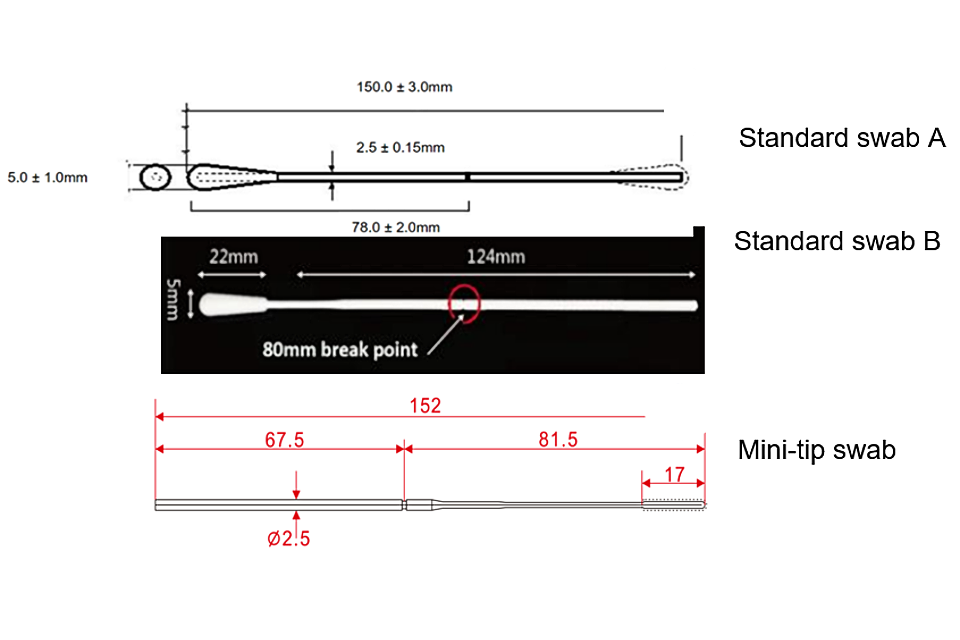
| Swab type | Breakpoint | Length | Bud width | Bud material |
|---|---|---|---|---|
| Standard swab A | 78mm | 150mm (+/- 3mm) | 5mm (+/-1mm) | Viscose (Rayon) |
| Standard swab B | 80mm | 150mm | 5mm | Flock (Nylon) |
| Mini-tip swab | 81.5mm | 152mm | 3mm | Flock (Nylon) |
Appendix B: Age distribution of participants
Appendix B is a bar chart showing the ages of the participants who used mini-tip swabs and who used standard swabs. The y-axis shows the age category of the participant, starting at under 6 months, 6 to 12 months, 12 to 18 months, 18 months to 2 years, 3 years and finally 4 years. The x-axis shows the percentage of participants who were each age.
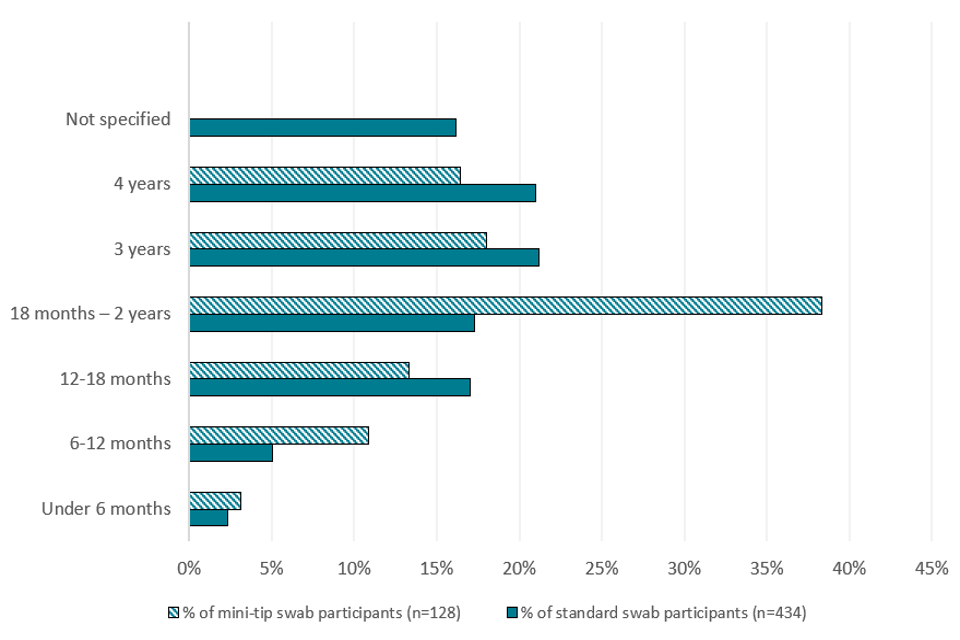
The most common age (over a third) for those who used mini-tip swabs was 18 months to 2 years. For standard swabs, the most common age was both 3 and 4 years (accounting for over 20% each). The least common age for both tests were participants under 6 months old.
Addendum 1: Continued monitoring of testing incidents for children under 5 years of age (November 2020 to February 2022)
After the assessments were concluded, the programme continued to monitor testing incidents for all age groups. From November 2020 to February 2022, there were over 5 million tests conducted for the group of children under 5 years of age. Out of all those tests, there were 45 incidents registered within the health and safety category for children under 5 years of age; the actual number may be slightly higher due to reporting artefacts. Not all incidents have recorded the age of the child involved. The incidents reported here include only those with an age reported as under 5 years.
Healthcare and safety incidents can refer to multiple issues, such as safeguarding issues and individual healthcare conditions needing medical attention within the testing site. Of the 45 incidents registered, 5 involved swabs breaking during use, and 6 involved reports of a nosebleed. The remainder were not related to swabbing.
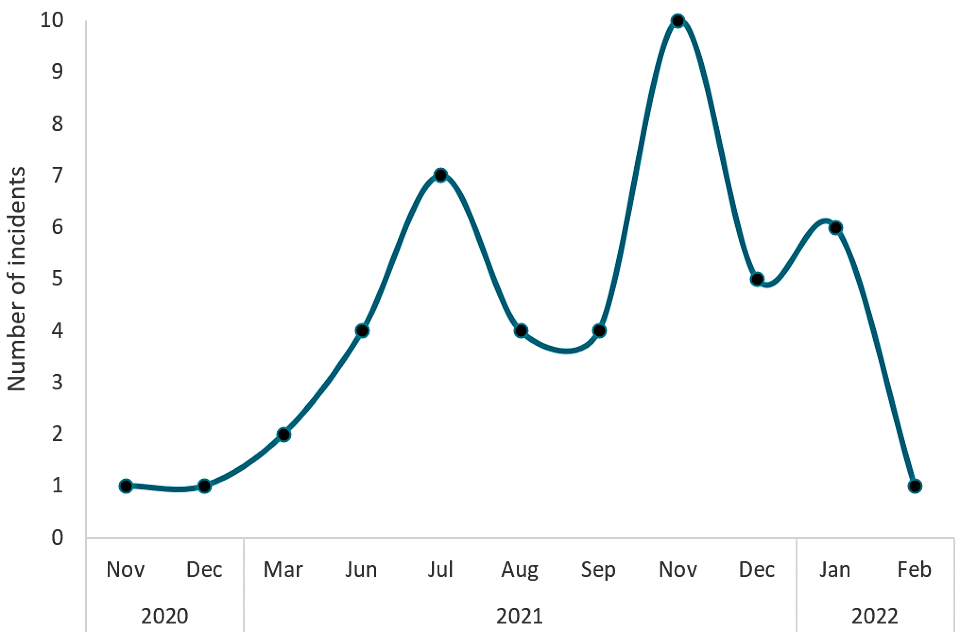
The above line graph shows the number of incidents reported each month from November 2020 to February 2022 for children under 5 years of age. The y-axis shows the number of incidents from 0 to 10. The x-axis shows the month. November and December 2020 and February 2022 show the fewest incidents (1). November 2021 had the highest number of incidents (10).
References
1. Harwood R, Rad L, Larru B, Kelly C, Kenny S and the LAVA Study Team. ‘Comparison of the pain experienced with anterior nasal swabs and nose and throat swabs in children’. Archives of Disease in Childhood 2022: volume 107, page 207
2. Bidkar, V, Selvaraj, K, Mishra, M, Shete, V, and Sajjanar, A. ‘A comparison of swab types on sample adequacy, suspects comfort and provider preference in COVID-19’. American Journal of Otolaryngology 2021: volume number 42, issue number 2, 102872
3. Giordano P, Moriondo M, Trapani S, Ricci S, Calistri E, Pisano L and others. ‘Nasal Swab as Preferred Clinical Specimen for COVID-19 Testing in Children’. The Pediatric Infectious Disease Journal 2020: volume, 39, issue 9, pages e267 to e270
4. Frazee BW, Rodríguez-Hoces de la Guardia A, Alter H, Chen C, Fuentes E, Holzer A and others. ‘Accuracy and Discomfort of Different Types of Intranasal Specimen Collection Methods for Molecular Influenza Testing in Emergency Department Patients’. Annals of Emergency Medicine 2018: volume 71, issue 4, pages 509 to 517.e1
