Ebola: overview, history, origins and transmission
Updated 4 September 2025
Background
Ebola disease (EBOD) is a severe disease caused by orthoebolaviruses, members of the filoviridae family, which occurs in humans and other primates. The disease was identified in 1976, in almost simultaneous outbreaks in Zaire (now the Democratic Republic of the Congo (DRC)) and Sudan (now South Sudan).
Between 1979 and 1994, no human cases or outbreaks were detected. However, since 1994, outbreaks have been recognised with increasing frequency.
Until 2014, outbreaks of EBOD were primarily reported from remote villages close to tropical rainforests in Central and West Africa. Most confirmed cases were reported from the DRC, Gabon, the Republic of the Congo, Sudan and Uganda. In 2014, an EBOD outbreak was reported for the first time in West Africa, involving Guinea, Liberia and Sierra Leone. During this outbreak, which was ongoing between 2014 and 2016, there was intense transmission in urban areas, resulting in over 28,000 reported cases. Multiple countries including Italy, Mali, Nigeria, Senegal, Spain, the UK and the US reported imported EBOD cases associated with this outbreak.
4 species of orthoebolavirus are known to cause disease in humans:
- Ebola virus disease (EVD): caused by Ebola (Zaire) virus (orthoebolavirus zairense, EBOV)
- Sudan virus disease (SVD): caused by Sudan virus (orthoebolavirus sudanense, SUDV)
- Bundibugyo virus disease (BVD): caused by Bundibugyo virus (orthoebolavirus bundibugyoense, BDBV)
- Tai Forest (formerly Ivory Coast) virus (orthoebolavirus taiense, TAFV)
Reston virus (orthoebolavirus restonense, RESTV), was first detected in 1989 in Reston, Virginia (US), in a colony of monkeys imported from the Philippines. It had caused severe illness outbreaks in non-human primates linked to imported animals. Several research workers became infected with the virus during these outbreaks but did not become ill. In 2008, RESTV was isolated from sick pigs in the Philippines. Several animal facility workers developed antibodies, but none reported any symptoms.
A sixth species of Ebola virus was discovered in bats in Sierra Leone in 2018 and named Bombali virus (orthoebolavirus bombaliense, BOMV). It is not yet known if this species is pathogenic to humans.
See information on current EVD outbreaks.
Figure 1: Map of Ebola disease (EBOD) in Africa. Please see the HCID: country specific risk webpage for details of travel-associated EVD cases reported outside of Africa.

Natural reservoir
The natural reservoir for Orthoebolaviruses is believed to be fruit bats from the Pteropodidae family. Non-human primates are known to have been a source of human infection in a number of previous EBOD outbreaks. However, they are considered incidental rather than reservoir hosts. This is because they typically develop severe, fatal illness when infected and viral circulation is not believed to persist within their populations. In addition to bats, EBOV RNA has also been detected chimpanzees, gorillas and forest antelopes (see Judson and others, 2016).
Transmission
Orthoebolaviruses are introduced into the human population through contact with blood, organs, or other bodily fluids of an infected animal. The first human EBOD case in the West Africa outbreak (2014 to 2016) was likely infected via exposure to bats. In addition to bats, EBOD has also been documented in people who handled infected chimpanzees, gorillas and forest antelopes, both dead and alive, in Cote d’Ivoire, the Republic of the Congo and Gabon.
Orthoebolaviruses can be transmitted from person to person through direct contact with the blood, organs, or other bodily fluids of an infected person. People can also become infected with orthoebolaviruses through contact with objects, such as needles or soiled clothing, that have been contaminated with infectious secretions.
Burial practices that involve direct contact with the body or body fluids of an infected person may also contribute to transmission.
Orthoebolaviruses can persist in some areas of the body even after acute illness and this persistence carries the risk of resurgent outbreaks. These areas include the testes, interior of the eyes, placenta, and central nervous system. Transmission via sexual contact with a convalescent case or survivor has been documented. The virus can be present in semen for over a year after recovery.
Where there are insufficient infection prevention and control measures, including the use of personal protective equipment (PPE), healthcare workers or those caring for infected individuals at home are at risk of infection through close contact with EBOD patients. Laboratory-acquired EBOD has been reported in England (in 1976) and Russia (in 1996 and 2004). These have predominantly been associated with handling infected animals or viral cultures.
There is no evidence of transmission of orthoebolaviruses through intact skin or infected individuals spreading the virus to others through coughing or sneezing.
Symptoms
The incubation period of EBOD ranges from 2 to 21 days, with an average of 8 to 10 days.
The onset of illness is usually sudden, with symptoms of fever, headache, fatigue, muscle pain and a sore throat. Gastrointestinal symptoms such as nausea, diarrhoea and vomiting usually follow after a few days. Some patients may develop a rash, cough, shortness of breath, red eyes, hiccups, impaired kidney and liver function and internal and external bleeding.
Between 25 to 90% of all clinically ill cases of EBOD are fatal, depending on the virus species, patients’ age and other factors.
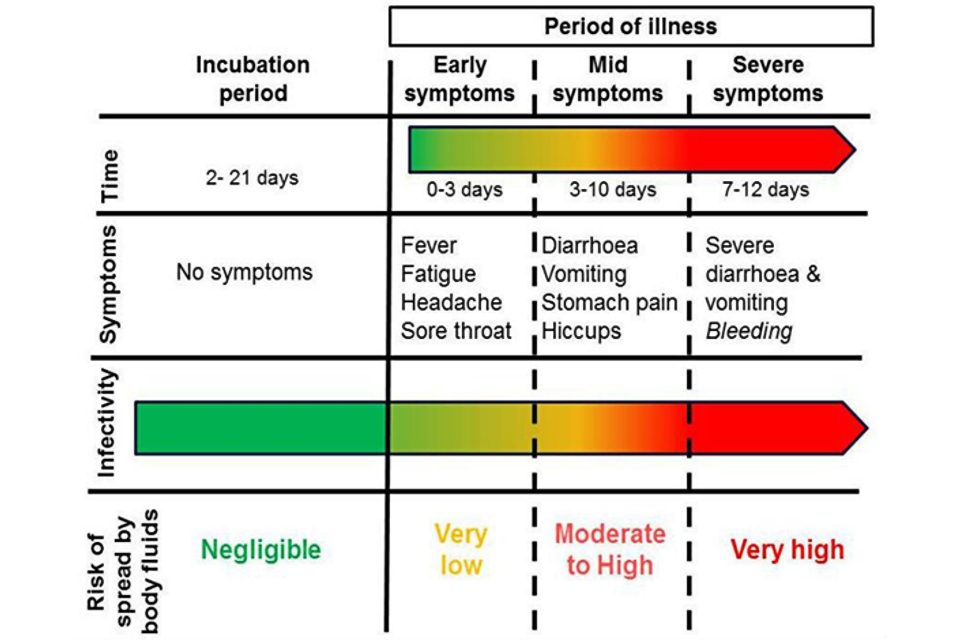
The diagram below outlines how a person’s infectiousness changes over time, following infection with orthoebolaviruses. When a person is displaying no symptoms, or early symptoms such as fever, the level of virus in the body is very low, and therefore poses a very low risk to others. Once an individual becomes unwell with symptoms such as diarrhoea and vomiting, then all body fluids are considered to be infectious, particularly blood, faeces and vomit which may contain high levels of the virus. When someone reaches the point at which they are most infectious, they are unlikely to be well enough to move or interact socially. Therefore, the greatest risk at this stage of infection is to people involved in their care. Skin is likely to be contaminated in the late stage of disease, because of the difficulty of maintaining good hygiene.
Diagnosis
Clinical diagnosis of EBOD in the early stages of infection is difficult, as early symptoms are non-specific and similar to those of other infections such as malaria, typhoid and meningitis.
Laboratory diagnosis must be carried out under high-level biological containment conditions. Diagnosis of acute infection is by reverse-transcriptase polymerase chain reaction (PCR) for viral RNA.
In the UK, laboratory diagnosis is performed by the Rare and Imported Pathogens Laboratory.
See Viral haemorrhagic fever: sample testing advice.
Treatment
EBOD patients require intensive supportive therapy, including intravenous fluids or oral rehydration with solutions including electrolytes as well as maintenance of oxygen status and blood pressure.
Two monoclonal antibodies, REGN-EB3 (Inmazeb™) and mAb114 (Ebanga™) are available for the treatment of Ebola virus disease (EVD) caused by caused by the Ebola (Zaire) virus (EBOV).
The WHO’s Therapeutics for Ebola virus disease guidance recommends the use of REGN-EB3 and mAb114 for patients with laboratory confirmed EVD infection and for neonates 7 days old or younger with unconfirmed EVD status, who are born to mothers with confirmed EVD.
There are currently no licensed therapeutics for the treatment of EBOD caused by orthoebolavirus sudanense (SUDV), or any of the other Ebolavirus species except EBOV.
Prevention
To avoid person-to-person transmission of orthoebolaviruses, great care needs to be taken when caring for patients to avoid contact with infected bodily fluids.
Patients should be isolated, and strict barrier nursing techniques should be used, including PPE as described in guidance for the management of viral haemorrhgic fevers. Invasive procedures such as the placing of intravenous lines, handling of blood, bodily secretions, catheters and suction devices are a particular risk and strict infection prevention and control is essential.
The bodies of those that have died of EBOD remain highly infectious and should be promptly and safely buried or cremated.
Two vaccines are licensed for the prevention of EVD and are specific to the stain of the virus. The rVSV-ZEBOV vaccine (ERVEBO®) protects against EVD caused by EBOV is used for adults over 18 years old. rVSV-ZEBOV is given as a single injection into muscle (intramuscular) around the shoulder or thigh. rVSV-ZEBOV can be used as part of the response to an EVD outbreak caused by EBOV as only a single dose is required to elicit an immune response. In the context of an outbreak, the Strategic Advisory Group of Experts (SAGE) advising the WHO, recommends that individuals who have already been vaccinated more than 6 months earlier, should be revaccinated if they are among the contacts, or contacts of contacts, of a confirmed case.
The second vaccine available to protect against EVD caused by EBOV is delivered as 2 doses. The first dose, Ad26.ZEBOV-GP (Zabdeno), is given followed by a second dose, MVA-BN-Filo (Mvabea), 8 weeks later. The 2 doses are administered as injections into the muscle (intramuscular) around the shoulder or thigh. Ad26.ZEBOV-GP and MVA-BN-Filo can be used in adults and children over one year old. The vaccine requires 2 doses and is therefore not suitable for use in outbreak response where immediate protection is necessary. People who have previously received Ad26.ZEBOV-GP and MVA-BN-Filo injections more than 4 months earlier and are at immediate risk of infection can receive a booster dose of Ad26.ZEBOV-GP.
There are currently no licensed vaccines for the prevention of SVD caused by SUDV. According to WHO, current evidence shows that the rVSV-ZEBOV vaccine (which is highly effective against EBOV), does not provide cross protection against SUDV. Vaccines against SUDV are in different stages of development, including vaccines with Phase 1 data (safety and immunogenicity data in humans).
UK guidelines
The UK has specialist guidance on the management (including infection control) of patients with EBOD and other viral haemorrhagic fevers.
This guidance provides advice on how to comprehensively assess, rapidly diagnose and safely manage patients suspected of being infected within the NHS, to ensure the protection of public health.
See the public health guidance in UK settings for EBOD contact tracing.
Healthcare workers who suspect a case of EBOD or other VHFs should refer to the VHF assessment algorithm and discuss with their local infection services (microbiology, virology, infectious diseases). Infection services can contact the Imported Fever Service (IFS) for advice on risk assessment, testing and clinical advice.
Cases in the UK
Imported EBOD cases are extremely rare in the UK. Since 1976, there have been 4 confirmed cases of EBOD reported in the UK. One was a laboratory-acquired case in 1976 and 3 cases were in healthcare workers associated with the West African epidemic 2014 to 2015. There have been no UK deaths from EBOD.
