Quality assurance
Updated 15 May 2025
Applies to England
For naming conventions used throughout this guidance and other general information, please see the handbook overview.
The NHS Fetal Anomaly Screening Programme (FASP) has a defined set of screening standards providers must meet to make sure local services are safe and effective.
The aim of quality assurance (QA) is to maintain these standards and drive continuous improvement in the screening programme. It covers the entire screening pathway, from identifying who is eligible to be invited for screening, through to receipt of referral where required. The screening quality assurance service (SQAS) performs QA for the NHS screening programmes.
QA is essential to minimise harm and maximise the benefits of screening.
Antenatal and newborn (ANNB) QA makes sure all women and their babies have access to high quality screening wherever they live. ANNB QA has quality assurance procedures for checking standards are met.
To make sure services are set up correctly and meet standards, all commissioners and service providers must refer to the:
- Down’s syndrome, Edwards’ syndrome and Patau’s syndrome screening pathway requirements specification
- 20-week screening scan pathway requirements specification
In line with the pathway requirements specifications, providers should have the following posts in place:
- a screening midwife or coordinator and deputy to oversee the screening programme and act as a link between other members of the local and regional screening teams
- a screening support sonographer (SSS) (see section 3) and deputy with administrative support to specifically support the ultrasound part of the combined and quadruple test
Providers should make sure there is continual cover for all posts.
1. Quality assurance of laboratories undertaking T21, T18 and T13 screening
Laboratories undertaking trisomy 21 (T21), trisomy 18 (T18) trisomy 13 (T13) screening (see sections 4.1, 4.2 and 4.3 of the handbook overview) must:
- be accredited by the UK Accreditation Service (UKAS) according to International Organization for Standardization (ISO) Medical laboratories – Requirements for quality and competence (ISO 15189)
- participate in external quality assurance (EQA) schemes accredited to ISOs Conformity assessment – General requirements for proficiency testing schemes (ISO 17043)
- meet the screening programme QA requirements mapped to ISO 15189
- use ISO 15189 accredited reference laboratories
- submit required data (see the accompanying request form and data fields attachment) to the Down’s syndrome screening quality assurance support service (DQASS) and National Congenital and Rare Diseases Registration Service (NCARDRS)
- include an annual vertical audit of an antenatal screening sample in its laboratory audit schedule
The audit should randomly select a higher chance T21, T18 or T13 sample. In laboratories providing both combined and quadruple testing it is acceptable for the annual audit to relate to either test. Wherever possible this should alternate between the 2 tests.
The audit should include arrival and receipt of an antenatal screening sample at the laboratory, and acknowledgment of higher chance results by clinical services.
UKAS looks at both ISO 15189 and the screening requirements on behalf of NHS England (NHSE).
More information is available on the QA of laboratories.
2. Down’s syndrome screening quality assurance support service (DQASS)
DQASS is a service commissioned by NHSE to support the NHS FASP.
DQASS monitors and supports the quality and effectiveness of T21, T18 and T13 screening in England.
3. Quality assurance of ultrasound: the role of the screening support sonographer
The NHS FASP recommends the provider has a dedicated SSS, with a deputy to provide continual cover. These roles are to oversee the implementation, delivery and monitoring of the ultrasound part of the combined and quadruple screening pathways.
Details of the functions of the SSS are available.
3.1 Nuchal translucency and crown rump length diagnostic plot self-assessment tool
A diagnostic plot self-assessment tool is provided to assist monitoring of NT and CRL measurements. We recommend using weekly or monthly depending on the number of scans performed. The tool is not designed to be used at the time of the scan to compare measurements to the Fetal Medicine Foundation (FMF) reference curve.
4. Departmental review of ultrasound images
Reviewing ultrasound images and providing feedback to ultrasound practitioners can help to improve the quality of images. This is not designed to be used as a performance management tool.
The review of ultrasound images aims to:
- assist and support ultrasound practitioners in making continuous improvements in their nuchal translucency (NT) and crown rump length (CRL) measurement techniques (see section 4.3 in Screening for Down’s syndrome, Edward’s syndrome and Patau’s syndrome)
- provide regular feedback to ultrasound practitioners
- encourage shared learning
Reviewing ultrasound images is an essential part of the:
- practical training process for trainees (see section 2.2 of Education and training)
- induction process for new staff (see section 2.3 of Education and training)
- management of red flags (see guidance on the Down’s syndrome screening quality assurance support service)
- 3-monthly departmental image review
The SSS should make sure there is a:
- documented process for departmental review of ultrasound images
- log of image review dates and any agreed actions – SQAS requires evidence this is performed
4.1 3-monthly departmental image review
Regular departmental review of ultrasound images is equally as important as reviewing the ultrasound practitioner’s DQASS NT feedback plot.
The SSS should undertake a 3-monthly image review of paired NT and CRL images taken by all ultrasound practitioners, including agency staff. If an ultrasound practitioner works at more than one provider, image review must be performed at each provider.
To perform the 3-monthly image review the SSS:
- Reviews 3 randomly selected paired NT and CRL images for each ultrasound practitioner.
- Assesses each image using the image review tool.
- Provides timely feedback to the ultrasound practitioner.
- Keeps a record that these reviews are undertaken.
Providing feedback
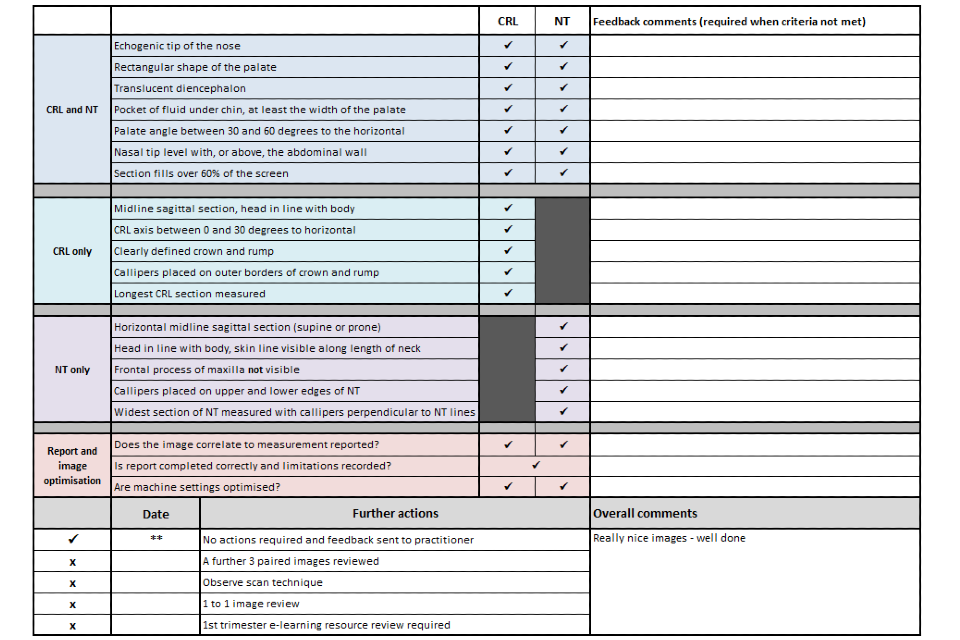
There are 12 criteria for both NT (see section 6.4 in Screening for Down’s syndrome, Edward’s syndrome and Patau’s syndrome) and CRL images (see section 6.5 in ‘Screening for Down’s syndrome, Edward’s syndrome and Patau’s syndrome’).
The image criteria assume the baby is supine but can be applied when the baby is prone, although it may not be possible to assess criteria 3 (echogenic tip of nose).
Further information on the NT and CRL criteria is available in the First trimester resource for ultrasound practitioners e-learning module.
The image review tool supports the SSS to:
- assess the paired NT and CRL images against the NHS FASP criteria
- provide feedback on images
- identify any need for further support
As a minimum the SSS should use this tool to provide feedback on any criteria that are not met.
The SSS will identify one of the following outcomes after performing the image review:
- no further support required
- further support required
If no further support is required, it means the SSS has no concerns following the image review. The ultrasound practitioner receives feedback on any minor improvements that could be made.
If further support is required, it means the SSS has concerns with the image review. It is recommended that a further 3 paired images are reviewed, and feedback provided.
If concerns continue after reviewing a further 3 paired images, the ultrasound practitioner should be offered further timely support. This support should be recorded and may include:
- observation of scan technique
- one to one image review
- review of completed e-learning resources
If concerns continue after providing further support, the SSS should escalate these concerns according to local policy.
4.2 Shared learning
Suggestions of local activities to encourage shared learning include:
- group review of a selection of images
- anonymised previously reviewed images shared within the department
- each practitioner measures selected NT and CRL images previously stored on an ultrasound machine; results are then compared and shared
- buddy scanning to improve image optimisation
- sharing key learning points from all image reviews with the department
These are suggestions and will not be externally audited.
4.3 Image review resources
Image review resources include the image review tool and a template for logging the 3-monthly image review. It also includes example sheets of how these could be completed.
4.4 Examples of image review and feedback
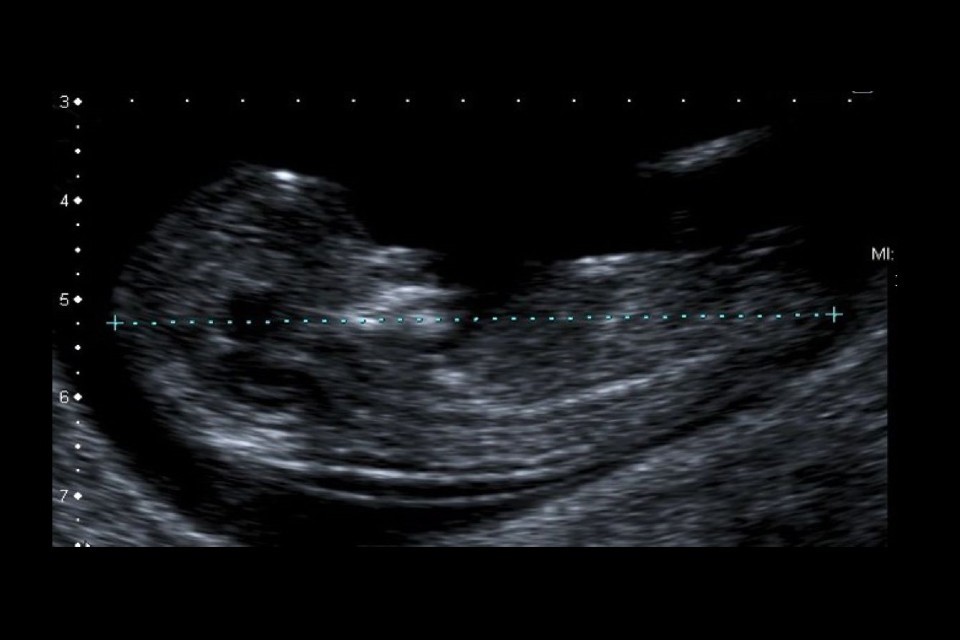
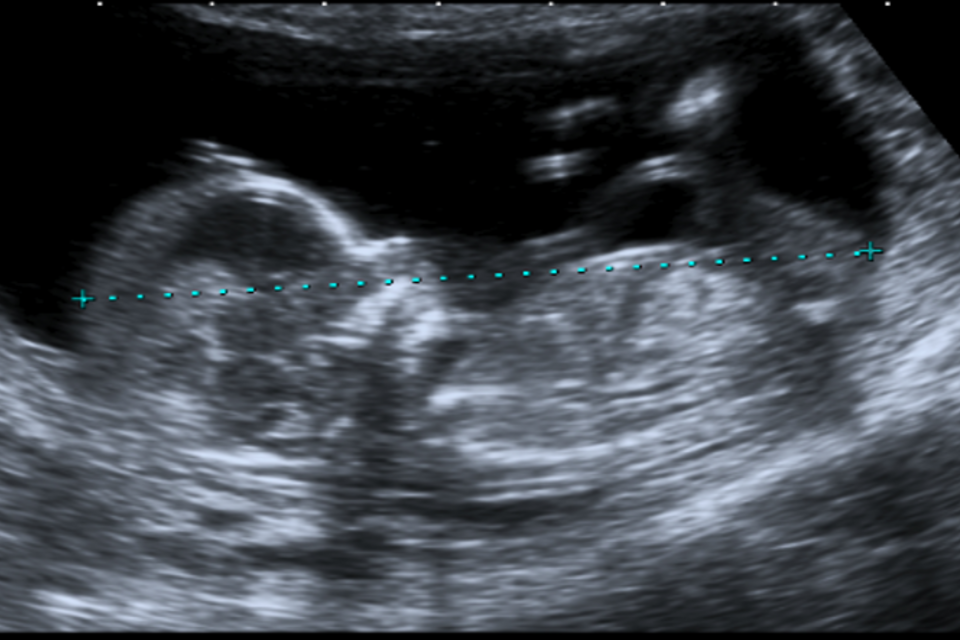
Example 1 shows 2 ultrasound images of a CRL and an NT that meet all the criteria. There is also a screenshot of what the accompanying image review tool would look like for both images.
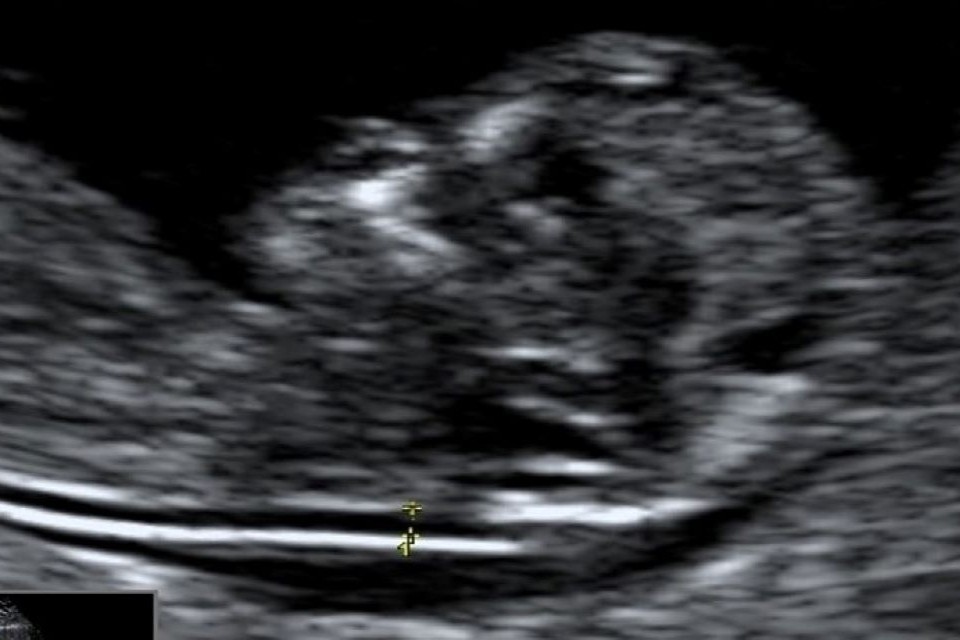
Example 2 shows 2 ultrasound images of a CRL and an NT that do not meet all the criteria. There is also a screenshot of what the accompanying image review tool would look like for both images.
Example 1

Example 2

5. Screening safety incidents
A screening safety incident is any unintended or unexpected incident(s) that could have or did lead to harm to one or more persons who are eligible for NHS screening. This also applies to staff working in the screening programmes.
A screening safety incident can affect populations as well as individuals. It is an actual or possible failure in the screening pathway.
Providers will comply with the national guidance for the management of safety concerns and incidents in screening programmes and NHS England’s guidance for managing safety incidents in NHS screening programmes.
Laboratory and ultrasound services are expected to inform the Medicines and Healthcare Products Regulatory Agency (MHRA) of any adverse incidents.
