HPR volume 12 issue 37: news (19 October)
Updated 21 December 2018
Gonococcal resistance to antimicrobials surveillance programme (GRASP) annual report in summary
PHE has published its annual Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP) report, presenting latest data from surveillance of antimicrobial resistance in Neisseria gonorrhoeae [1].
Current first-line treatment for gonorrhoea involves dual therapy with ceftriaxone and azithromycin, but treatment effectiveness is threatened by antimicrobial resistance.
Between 2016 and 2017, gonococcal isolates collected through PHE’s sentinel surveillance system showed:
- no resistance to ceftriaxone
- an increase in azithromycin resistance from 4.7% to 9.2%
- an increase in resistance to ciprofloxacin from 33.7% to 36.4%
- an increase in the cefixime modal MIC from 0.015 mg/L to 0.03 mg/L
- a decline in penicillin resistance from 13.9% to 10.8%.
However, in 2018, one case of ceftriaxone resistance was confirmed by the national reference laboratory. This case was extensively-drug-resistant N. gonorrhoeae (XDR-Ng) and details have been published separately [2][3]. The strain was also resistant to cefixime, ciprofloxacin and tetracycline, but was susceptible to spectinomycin. The case reported one regular female partner in the UK and a female sexual contact in south east Asia in the month prior to symptom onset. The investigation co-ordinated by PHE concluded that there had been no spread of the strain within the UK.
Practitioners should ensure all patients with gonorrhoea are treated and managed according to national guidelines and be alert to changes in antimicrobials recommended for front-line use [4]. Sexual health services should report possible cases of treatment failure to PHE via the online HIV and STI web-portal (contact HIVSTI@phe.gov.uk for details).
References
- PHE (2108). Surveillance of antimicrobial resistance in Neisseria Gonorrhoeae. Key findings from the Gonococcal Resistance to Antimicrobials Surveillance Programme (GRASP): data up to May 2018.
- Update on investigation of UK case of Neisseria gonorrhoeae with high-level resistance to azithromycin and resistance to ceftriaxone acquired abroad. HPR 12(14).
- Eyre et al (2018). Gonorrhoea treatment failure caused by a Neisseria gonorrhoeae strain with combined ceftriaxone and high-level azithromycin resistance, England Euro Surveill. 23(27).
- British Association for Sexual Health and HIV (BASHH). BASHH Guidelines.
Third case of monkeypox diagnosed in England
Monkeypox is a rare viral infection which can be transmitted from animals to humans. Infection in humans is usually a mild, self-limiting illness and most people recover within a few weeks.
In early September 2018 2 imported cases of monkeypox were diagnosed in England and these have been described in recent publications [1,2]. Both cases had recently been in Nigeria where they are believed to have contracted the infection. Nigeria has been experiencing the largest documented outbreak of monkeypox, at least 262 suspected cases having been reported across 26 states in 2017 to 2018 [3]; the latest information reports a total of 115 confirmed cases in the country [4].
There is no UK epidemiological link between the 2 above-mentioned cases. However, in late September a third case was diagnosed in England [5,6], representing the first documented case of human-to-human onward transmission outside Africa. The third case is a UK-resident healthcare worker at Blackpool Victoria Hospital, where the second England case presented. This third case is believed to have contracted the infection whilst undertaking activities associated with the care of the second case, before monkeypox was diagnosed.
Individuals who had potential contact with any of the 3 monkeypox cases in England while they were infectious have been contacted and followed-up. A highly precautionary approach was taken with the third case: anyone who had contact with this individual 24-hours before they noticed a rash was traced, their degree of exposure risk-assessed and appropriate advice, information and follow-up provided. Contacts were monitored for 21 days after exposure to identify anyone developing symptoms consistent with monkeypox infection.
UK risk assessment
The risk of catching monkeypox in the UK is very low. Person-to-person transmission is very uncommon, but may occur through:
- contact with clothing or linens (such as bedding and towels) used by an infected person
- direct contact with monkeypox skin lesions or scabs, or
- through droplet spread (coughing or sneezing of an individual with monkeypox).
Monkeypox in travellers
Cases of monkeypox in travellers are very unusual [7]. However, monkeypox infection should be considered in those presenting with compatible symptoms and a history of travel to areas where the virus is endemic [8]. Specialist guidance from Public Health England should be followed [9]. All cases involving pyrexia of unknown origin in a returning traveller should be discussed with the local microbiology, virology or infectious disease consultant, who will discuss with the imported fever service if required.
Interestingly, on 12 October, 2018, the State of Israel Ministry of Health also reported a case of monkeypox imported from Nigeria [10]. The patient is an Israeli resident who had been working in Port Harcourt, Southern Nigeria.
References
- Vaughan A, Aarons E, Astbury J, Balasegaram S, Beadsworth M, Beck CR, Chand M, et al (2018). Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 23(38).
- Imported cases of monkeypox diagnosed in England. HPR 12(33), 14 September 2018.
- Nigeria Centre for Disease Control (12 September 2018). Monkeypox cases in the United Kingdom.
- Nigeria Centre for Disease Control (15 September 2018). An update of Monkeypos Outbreak in Nigeria for week 37 (15 Sept 2018).
- PHE website. Cases of monkeypox confirmed in England. News story (11 September 2018)
- NHS website. Monkeypox.
- NaTHNaC website. Monkeypox cases in UK (ex Nigeria), 13 September 2018.
- WHO website. African countries reporting human monkeypox cases, 1970-2017.
- State of Israel Ministry of Health (2018). Monkeypox patient diagnosed, 12 October.
Additional information
- PHE guidance on epidemiology, symptoms, diagnosis and management of monkeypox virus infections
- PHE monkeypox: guidance for primary care
- PHE monkeypox: guidance for environmental cleaning and decontamination
- World Health Organization (WHO) monkeypox factsheet
EVD outbreak in eastern DRC: second update
The outbreak of Ebola virus disease declared on 1 August in eastern Democratic Republic of the Congo continues. To date, 188 confirmed cases and 35 probable cases have been reported across 10 health zones in 2 provinces, North Kivu and Ituri (see table). In the past month, there have been 82 new confirmed cases and 4 new probable cases recorded, with an additional 2 health zones (Komanda and Tchomia) affected. The Béni Health Zone has reported more than 70% of all cases recorded since September 2018 and is the current active focus of transmission [2].
Case table for EVD outbreak in North Kivu and Ituri provinces, as of 17 October 2018. Data provided by DRC MoH [1].
| Health zone | Confirmed | Probable | Fatalities |
|---|---|---|---|
| Béni – North Kivu | 83 | 8 | 60 |
| Butembo – North Kivu | 14 | 2 | 8 |
| Kalunguta – North Kivu | 2 | 0 | 1 |
| Oicha – North Kivu | 2 | 1 | 1 |
| Mabalako – North Kivu | 71 | 21 | 67 |
| Masereka – North Kivu | 4 | 1 | 1 |
| Musienene – North Kivu | 0 | 1 | 1 |
| Komanda – Ituri | 1 | 0 | 0 |
| Mandima – Ituri | 9 | 2 | 3 |
| Tchomia – Ituri | 2 | 0 | 2 |
| TOTAL | 188 | 36 | 144 |
In the past 2 weeks a significant increase in case incidence has been reported (see figure). This increase is likely to be associated with (a) the recent period of mourning (also known as a ‘ville morte’) in response to community deaths at the hands of a militia group on 22 September and (b) ongoing small pockets of resistance, including violence towards responders, which have hampered control measures (contact tracing, vaccination, removal of suspected cases from the community and safe burials). An improvement in major response parameters, including contact tracing, has been noted in recent days but these activities are highly susceptible to disruption caused by the complex security situation in the area.
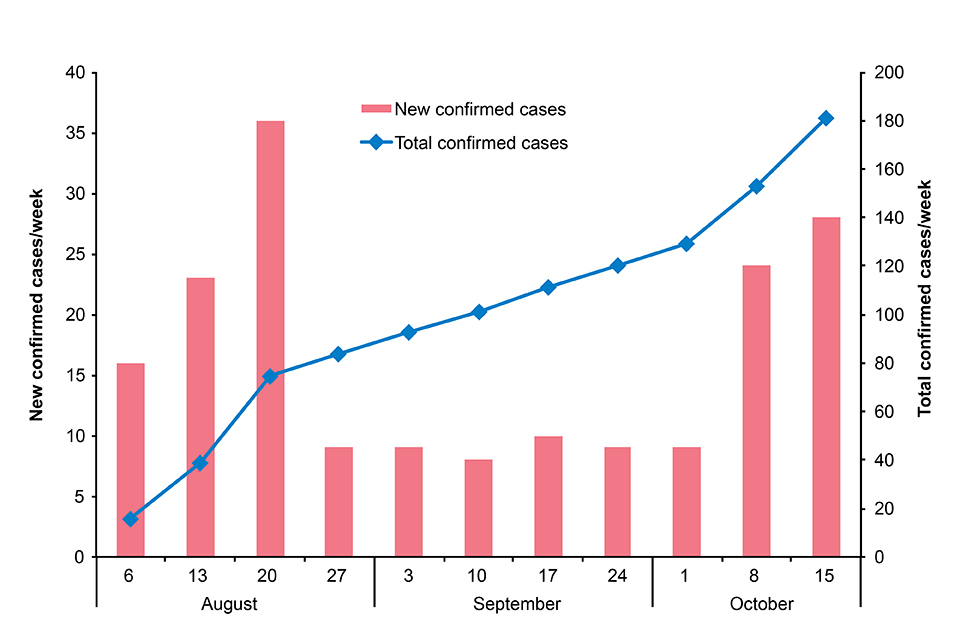
New and total confirmed cases by week. Data provided by DRC MoH [1].
On 17 October, the WHO Emergency Committee agreed that this current outbreak does not meet the requirements of a Public Health Emergency of International Concern (PHEIC) at this time [3]. The committee emphasised that response activities need to be intensified and ongoing vigilance is critical to the management of this outbreak.
The risk to the UK public remains very low to negligible. The situation is being monitored closely and the risk assessment is regularly reviewed.
Further information sources
- PHE website Ebola collection Ebola virus disease: clinical management and guidance
- NaTHNaC website for travel advice Travel Health Pro website
- WHO website EVD homepage Ebola virus disease
- Foreign and Commonwealth Office (FCO) DRC advice
References
- DRC Ministry of Health (in French).
- WHO AFRO Situation Report No. 11.
- WHO Emergency Committee statement, 17 October.
English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) fifth annual report
The fifth annual report of the English surveillance programme for antimicrobial utilisation and resistance will be published on Tuesday 23 October [1]. ESPAUR was established in 2013 in response to the cross-government UK 5-year antimicrobial resistance (AMR) strategy [2]. The latest report summarises antibiotic consumption, stewardship and resistance over the past 5 years.
The publication coincides with the launch of the Keep Antibiotics Working campaign. The campaign will run for 8 weeks supported by advertising on TV, radio, video on demand, social media, digital, and search; partnerships with local pharmacies, GP surgeries and local authority community hubs, such as children’s centres and libraries; and PR activity. Materials, including posters, leaflets and a social media toolkit, to support the national ‘Keep Antibiotics Working’ campaign, are available from the campaign resource centre.
Queries on any of the above, should be directed to: partnerships@phe.gov.uk.
References
- PHE website. The ESPAUR report 2018 will be available, from 23 October 2018, on the webpage: English surveillance programme for antimicrobial utilisation and resistance (ESPAUR) report.
- PHE (December 2015). UK 5-year antimicrobial resistance strategy 2013 to 2018.
Infection reports in this issue of HPR
This issue includes:
