HPR volume 13 issue 23: news (8 July)
Updated 20 December 2019
Malaria imported into the UK: 2018
A total of 1,683 cases of imported malaria were reported in the UK in 2018, according to recently published annual data, 6.1% lower than reported in 2017 [1].
A total of 6 UK deaths were associated with malaria importations in 2018, this has been steady since 2015. All were due to Plasmodium falciparum malaria acquired in Western Africa (3), Middle Africa (1), and an unspecified region of Africa (1) - the travel region was not stated for one case.
Cases were reported in England (1,597), Scotland (52), Wales (23) and Northern Ireland (11). Most cases in 2018 were caused by Plasmodium falciparum, which is consistent with previous years.
Of the cases that travelled abroad from the UK, where the reason for travel was documented, 85% were for those visiting friends and relatives. This group of travellers appear not to be receiving, understanding or acting on health messages emphasising the importance of appropriate chemoprophylaxis.
Advice for healthcare workers who advise UK-based travellers is available [2]. Country-specific advice for travellers is available on the National Travel Health Network and Centre (NaTHNaC) website [3].
References
- GOV.UK (5 July 2019). Malaria imported into the UK: 2018. Implications for those advising travellers.
- GOV.UK. Malaria prevention guidelines for travellers from the UK.
- NaTHNaC website. Countries A to Z.
EVD outbreak in eastern DRC: eleventh update
The outbreak of Ebola virus disease in eastern Democratic Republic of the Congo (DRC) continues.
As of 3 July 2019, 2,288 confirmed and 94 probable cases have been reported in North Kivu and Ituri provinces [1]. Of these, 356 were newly confirmed cases reported between 1 and 30 June, a lower monthly total than April and May (406 and 459 cases respectively). There have been 1,606 deaths, 252 since the last update.
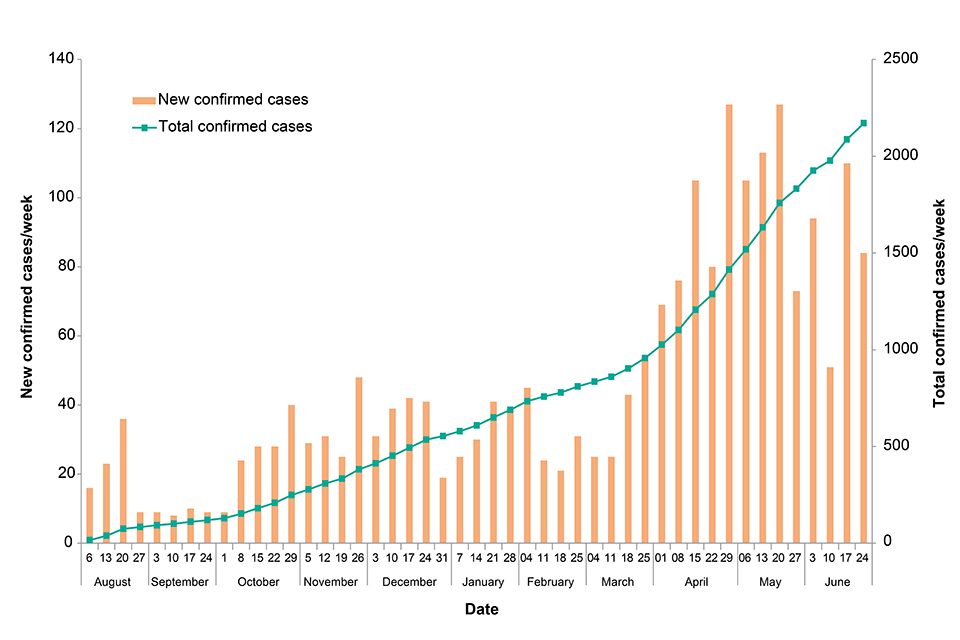
Figure: New and total confirmed cases by week. Data provided by DRC MoH [2]
A new health zone, Ariwara in northern Ituri province, reported its first confirmed case in late June [3], when a woman fled there from Beni after being identified as a contact (her five children were all confirmed cases). Ariwara is located the farthest north so far in this outbreak, some 500 km (289 miles from Beni, and about 70 km or 43 miles from the border with South Sudan.
There are now 23 affected health zones. Twenty of these reported confirmed cases in the last 21 days [4], representing the widest geographic spread of cases thus far seen in 2019. However, most cases came from just three health zones (Mabalako, Beni and Mandima), which together reported 63% of cases in this period.
A further 20 healthcare workers have been confirmed as cases, bringing the total to 130 and indicating ongoing issues with transmission in healthcare settings.
On 11 June 2019, the Ugandan Ministry of Health reported a confirmed Ebola case in Kasese district [5]. This was a child from the DRC who had travelled with his family after attending a funeral. Two other family members were also rapidly confirmed as cases, but in the period since there have been no further cases in Uganda. All identified contacts completed 21 days of follow-up without developing symptoms.
Outbreak response activities continue to be intermittently hampered by security incidents and there is ongoing uncertainty regarding the completeness of surveillance and detection of all new cases. The outbreak remains confined to the same two provinces in DRC, without spread within the country. [4]
Despite the critical situation in DRC and the limited occurrence of linked cases in Uganda, the risk to the UK public remains very low to negligible. The situation is being monitored closely and the risk assessment regularly reviewed.
Further information sources
- GOV.UK website collection Ebola virus disease: clinical management and guidance
- NaTHNaC website travel advice Travel Health Pro website
- WHO website EVD homepage Ebola virus disease
- FCO travel advice DRC advice
References
- DRC Ministry of Health update, 4 July 2019 (in French).
- DRC Ministry of Health daily updates (in French).
- DRC Ministry of Health update, 1 July 2019 (in French)
- WHO Disease Outbreak News: Update, 4 July 2019.
- WHO Disease Outbreak News: Uganda cases, 13 July 2019.
Guidance on PEP following changes to the supply of immunoglobulin products
As a result of changes in the availability of immunoglobulin products procured by PHE, including temporary shortages in some cases, updated information on dosages has been circulated to clinicians on the use of immunoglobulin products for post-exposure protection against a range of infections, in particular: measles, hepatitis A, hepatitis B, and varicella/shingles.
Varicella or shingles
In the case of prophylaxis following exposure to varicella or shingles, updated guidance has been published, taking account of the resumption of supply of varicella-zoster immunoglobulin (VZIG) following a shortage, in 2018, that necessitated both prioritising use of stock to protect the most vulnerable groups, and the use of antivirals as an alternative to VZIG in other cases [1].
On the suitability of antivirals for post-exposure prophylaxis (PEP), the guidance takes account of a series of evaluations of the efficacy of antiviral agents, concluding that, taking account of the likely continued precariousness of VZIG supply in future:
- VZIG should be issued to VZ antibody-negative pregnant contacts exposed in the first 20 weeks of pregnancy
- for susceptible women exposed more than 20 weeks before delivery - either VZIG or oral aciclovir may be issued
Change in vials and dosage of immunoglobulin products supplied by PHE
The vial size of human normal immunoglobulin and hepatitis B immunoglobulin supplied by BPL is changing, and updated guidance for the amount of immunoglobulin for post-exposure prophylaxis for measles, hepatitis A and hepatitis B has been issued [2,3,4].
References
- Updated guidelines on post-exposure prophylaxis (PEP) for varicella or shingles.
- Updated guidance on the use of HNIG following hepatitis A exposure.
- Updated post-exposure prophylaxis guidelines for measles.
- Hepatitis B immunoglobulin.
Infection reports in this issue
This issue includes:
