Commission Delegated Regulation (EU) 2016/127 (supplementing Regulation (EU) No 609/2013): guidance
Updated 3 April 2024
Applies to England
Nutrition law
The UK left the European Union (EU) in January 2020 and entered a time-limited transition period until 31 December 2020. Now the transition period has ended, regulation becomes an autonomous matter for both Great Britain (GB) and EU as 2 separate legal and regulatory systems. The Protocol on Ireland/Northern Ireland (NIP) means that EU legislation relating to nutrition, as detailed in Annex 2 of the NIP, continues to be directly applicable in Northern Ireland (NI).
From 1 January 2021, EU Regulations and tertiary legislation relating to nutrition were retained under the powers contained within the European Union (Withdrawal) Act 2018 as UK law. Retained EU Regulations and tertiary legislation were subsequently amended by the Nutrition (Amendment etc.) (EU Exit) Regulations 2019 and the Nutrition (Amendment etc.) (EU Exit) Regulations 2020.
The Nutrition (Amendment etc.) (EU Exit) Regulations 2019 and the Nutrition (Amendment etc.) (EU Exit) Regulations 2020 transferred responsibilities from EU organisations involved in the risk assessment and risk management processes covered by nutrition legislation to bodies in GB.
NI continues to play a vital role in policy development for nutrition legislation in GB, as NI’s full participation in risk assessment and risk management processes ensure that any decisions taken in GB account for the potential impacts across the UK, as set out under the arrangements agreed in the UK-wide provisional common framework for Nutrition Related Labelling, Composition and Standards (NLCS). This is to ensure that any impacts on the UK internal market are minimised.
Intended audience
This guidance is aimed at companies that manufacture, process, distribute, use, sell or import infant formula and follow-on formula, and those local authorities who are responsible for enforcing the legislation in this area – but may also be of interest to relevant third sector organisations.
Legislation on infant formula and follow-on formula is a devolved matter for UK. While these guidance notes have been developed by the Department for Health and Social Care (DHSC) and specifically refer to England, the principles are similar throughout GB (Scotland and Wales).
The Protocol on Ireland and Northern Ireland (NIP) means that EU legislation relating to nutrition, as detailed in Annex 2 of the NIP, continues to be directly applicable in Northern Ireland (NI).
Equivalent guidance will be issued for Scotland, Wales and NI.
Executive summary
The guidance provides information, advice and sets out DHSC’s interpretation of the requirements of the legislation on infant formula and follow-on formula under Commission Delegated Regulation (EU) 2016/127 and is provided to facilitate adherence to and assessment of adherence to the legislation. Commission Delegated Regulation (EU) 2016/127 comes under the overarching Regulation on Food for Specific Groups (EU) No 609/2013 on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control, which was adopted on 12 June 2013 and applied from 20 July 2016. This is enforced in England by The Food for Specific Groups (Information and Compositional Requirements) (England) Regulations 2016. Similar enforcement legislation applies in Scotland, Wales and NI.
Commission Delegated Regulation (EU) 2016/127 was adopted in 2016 and applied from 22 February 2020 except in respect of infant formula and follow-on formula manufactured from protein hydrolysates, which was initially due to apply from 22 February 2021, but applied from 22 February 2022 (in both GB and the EU).
Commission Delegated Regulation (EU) 2016/127 specifies rules on the composition, labelling and advertising of infant and follow-on formula. It is enforced in England by The Food for Specific Groups (Food for Special Medical Purposes for Infants, Infant Formula and Follow-on Formula) (Information and Compositional Requirements) (Amendment etc.) (England) Regulations 2020.
From its initial date of application (22 February 2020) Commission Delegated Regulation (EU) 2016/127 replaced Commission Directive 2006/141/EC, which was implemented by The Infant Formula and Follow-on Formula (England) Regulations 2007. For infant and follow-on formula made from protein hydrolysates the requirements of Commission Directive 2006/141/EC continued until 22 February 2022.
These guidance notes do not apply to infant and follow-on formula made from protein hydrolysates until 22 February 2022, and until this date the guidance on The Infant Formula and Follow-on Formula (England) Regulations 2007 continues to be available and includes guidance for infant and follow-on formula made from protein hydrolysates. Further explanation on the requirements for infant and follow-on formula made from protein hydrolysates is available in appendix 1.
These guidance notes do not apply to foods for special medical purposes intended for young children which are legislated under Commission Delegated Regulation (EU) 2016/128 on the specific compositional and information requirements for food for special medical purposes.
Introduction
The content of these guidance notes has been prepared by DHSC. The content reflects DHSC’s interpretation of Commission Delegated Regulation (EU) 2016/127 (referred to throughout this document as ‘the Commission Delegated Regulation’).
These guidance notes aim to help industry, local authority enforcement officers and other interested parties interpret the provisions of the Commission Delegated Regulation and are provided to facilitate adherence to and assessment of adherence to the legislation.
In these guidance notes, references to ‘annexes’ refer to the annexes of the Commission Delegated Regulation and references to ‘appendices’ refer to the appendices of these guidance notes.
The guidance notes:
- focus on the provisions of the Commission Delegated Regulation which include labelling, notification, avoidance of risk of confusion between infant formula and follow-on formula, advertising, promotion, and the provision of information and education relating to infant and child feeding
- replace previous guidance notes on The Infant Formula and Follow on Formula (England) Regulations 2007 (which are referred to throughout this document as ‘the 2007 Regulations’)
- provide guidance on interpreting the requirements set out in the Commission Delegated Regulation and should be read in conjunction with the legislation itself
The text should not be taken as an authoritative statement on how the law should be interpreted, as this would be for a court to determine. Every effort has been made to ensure that these guidance notes are as helpful as possible. However, it is ultimately the responsibility of individual businesses to ensure their compliance with the law. Businesses with specific queries may wish to seek the advice of their local enforcement agency, which will usually be the Trading Standards or environmental health department of the local authority or Port Health Authority
These guidance notes have been developed by DHSC and refer to the implementation and enforcement legislation for England. The corresponding legislation for each of the 4 UK countries is as follows:
England
The Food for Specific Groups (Information and Compositional Requirements) (England) Regulations 2016
Scotland
The Foods for Specific Groups (Scotland) Regulations 2016
The Foods for Specific Groups (Infant Formula and Follow-on Formula) (Scotland) Regulations 2020
Wales
The Food for Specific Groups (Information and Compositional Requirements) (Wales) Regulations 2016
Northern Ireland
The Food Safety (Information and Compositional Requirements) Regulations (Northern Ireland) 2016
Background
Food for specific groups
Commission Delegated Regulation (EU) 2016/127 comes under the overarching Regulation on Food for Specific Groups (EU) No 609/2013 (referred to throughout this document as ‘the FSG Regulation’) on food intended for infants and young children, food for special medical purposes, and total diet replacement for weight control.
The FSG Regulation was adopted on 12 June 2013 and applied from 20 July 2016. From 1 January 2021, the FSG Regulation was retained under the powers contained within the European Union (Withdrawal) Act 2018 as UK law. This retained EU legislation was subsequently amended by the Nutrition (Amendment etc.) (EU Exit) Regulations 2019 and the Nutrition (Amendment etc.) (EU Exit) Regulations 2020. It is enforced in England by the Food for Specific Groups (Information and Compositional Requirements) (England) Regulations 2016.
The FSG Regulation covers:
- infant and follow-on formula
- processed cereal-based food and baby food
- food for specific medical purposes
- total diet replacement for use in energy restricted diets for weight control
Further information on the FSG Regulation can be found in the Nutrition Legislation Information Sheet.
The FSG Regulation sets out provisions to empower the appropriate authority (the competent authority in this respect for England means Secretary of State for Health and Social Care) to adopt delegated acts on general compositional, advertisement and labelling rules for the categories of foods covered by the regulation (as above).
The FSG Regulation defines the categories listed above, sets out information requirements and a ‘GB list’ of substances that may be added to these categories of food.
The following terms defined in the FSG Regulation apply for the purposes of the Commission Delegated Regulation:
- ‘infant’ means a child under the age of 12 months
- ‘young child’ means a child aged between one and 3 years
- ‘infant formula’ means food intended for use by infants during the first months of life and satisfying by itself the nutritional requirements of such infants until the introduction of appropriate complementary feeding
- ‘follow-on formula’ means food intended for use by infants when appropriate complementary feeding is introduced, and which constitutes the principal liquid element in a progressively diversified diet of such infants
Appropriate complementary feeding
In relation to complementary feeding the World Health Organisation (WHO) notes that “Around the age of 6 months, an infant’s need for energy and nutrients starts to exceed what is provided by breast milk, and complementary foods are necessary to meet those needs. An infant of this age is also developmentally ready for other foods. This transition is referred to as complementary feeding.”
The Scientific Advisory Committee on Nutrition (SACN), who provides advice to the UK government on nutrition and related health matters includes the WHO definition of complementary feeding in its 2018 report on feeding in the first year of life.
In that report, complementary feeding refers to the period when solid foods are given in addition to either breast milk or infant formula to complement the nutrients provided by breast milk (and/or infant formula) when breast milk (and/or infant formula) alone is not sufficient to meet the nutritional requirements of the growing infant. Complementary feeding replaces the term ‘weaning’ which can be misinterpreted to mean the cessation of breastfeeding rather than the introduction of solid foods. Complementary feeding includes all liquids, semi-solid and solid foods, other than breast milk and infant formula.
Commission Delegated Regulation (EU) 2016/127
The Commission Delegated Regulation provides the detailed labelling and compositional rules for infant and follow-on formula and replaced the 2007 Regulations, except for infant and follow-on formula made from protein hydrolysates where the requirements of the 2007 Regulations will continue until 22 February 2022.
The Commission Delegated Regulation updates and sets additional provisions to those of the 2007 Regulations. Differences in the requirements of the 2 regulations include:
- prohibiting the use of nutrition and health claims
- strengthening the requirement for infant and follow-on formula labels to be clearly distinct from each other
- the mandatory addition of docosahexaenoic acid (DHA)
- the inclusion of statements on DHA and lactose
- the requirement to notify the authority when placing infant formula on the market
- the pre-authorisation and notification required when placing follow-on formula on the market when it is manufactured from protein hydrolysates or substances other than those listed in Annex II of the Commission Delegated Regulation
Unless otherwise specified in the Commission Delegated Regulation, infant and follow-on formula shall also comply with labelling information requirements set out in Regulation (EU) No 1169/2011 on the provision of food information to consumers. Guidance on Regulation (EU) No 1169/2011 is available.
The Commission Delegated Regulation is enforced in England by the Food for Specific Groups (Food for Special Medical Purposes for Infants, Infant Formula and Follow-on Formula) (Information and Compositional Requirements) (Amendment etc.) (England) Regulations 2020.
The Commission Delegated Regulation consolidates legislation on the composition, labelling and marketing of infant formula and follow-on formula. The Commission Delegated Regulation reflects the latest scientific advice on the essential composition of infant formula and follow-on formula. The Commission Delegated Regulation and the overarching FSG Regulation together give effect to some but not all of the general principles and ambitions of the 1981 WHO Code on the Marketing of Breastmilk Substitutes covering marketing, information and responsibilities of health authorities in relation to infant formula and follow-on formula, as they set provisions which regulate labelling and restrict advertising and presentation of infant and follow-on formula so as not to discourage breastfeeding.
The provisions of the Commission Delegated Regulation, which supplements the FSG Regulation, relate to:
- placing on the market (Article 1)
The term ‘placing on the market’ is defined in Regulation (EC) 178/2002 which lays down the general principles and requirements of food law.
- the compositional requirements of infant formula and follow-on formula (Article 2)
- the suitability of ingredients for infant and follow-on formula (Article 3)
- the requirements on pesticides for infant and follow-on formula (Article 4)
For the purposes of this Article, ‘residue’ means the residue of an active substance as referred to in Article 2(2) of Regulation (EC) No 1107/2009 used in a plant protection product as referred to in Article 2(1) of that Regulation, including metabolites and products resulting from the degradation or reaction of that active substance. In April 2021 the EU laid Commission Delegated Regulation (EU) 2021/1041, which amends the requirements on pesticides in infant formula and follow-on formula.
- the name of the food (Article 5)
- the requirement for the packaging of infant and follow-on formula to differ from each other (Article 6)
- the requirements under Regulation (EU) No 1169/2011 on the provision of food information to consumers including the specific requirements on food information for infant and follow-on formula (Articles 6 and 7)
- the provisions of advertising infant formula and follow-on formula (Articles 6 and 10) – further information on what is considered advertising can be found in appendix 2 of this guidance
- the specific requirements on the nutrition declaration for infant and follow-on formula (Article 7)
- the prohibition of nutrition and health claims on infant formula (Article 8)
- statements relating to lactose and docosahexaenoic acid (DHA) (Article 9)
- presentation of the products relating to the way in which infant formula and follow-on formula are arranged for sale and the setting in which they are displayed (Article 10)
- provision of informational and educational material dealing with the feeding of infants (Article 11)
- the notification to the authority required when placing infant formula on the market and from 22 February 2022, the notification required when placing follow-on formula on the market when it is manufactured from protein hydrolysates or substances other than those listed in Annex II of the Commission Delegated Regulation (Article 12)
Specific requirements on food information
Article 6 of the Commission Delegated Regulation:
Article 6(2)(a) requires the following statement to be included on infant formula: ‘suitable for infants from birth when they are not breast fed’.
Articles 6(2)(b) and (3)(b) requires that instructions are provided for appropriate preparation, storage and disposal of infant formula and follow-on formula.
Information on the process for safe and appropriate preparation and the storage of formula can be found on the NHS website.
Articles 6(2)(b) and 3(b) require, in addition to instructions for appropriate preparation, storage and disposal of the infant formula or follow-on formula, a ‘warning about the health hazards of inappropriate preparation and storage’. This warning statement should stress the importance of the correct preparation of infant formula or follow-on formula without which there is an increased risk to the baby’s health including the baby suffering from serious stomach upsets, diarrhoea and vomiting, constipation and dehydration, and so on. This statement should appear on the label in a conspicuous place and be clearly visible and easily understandable. The statement should include wording such as ‘Failure to follow instructions may make your baby ill’.
In order to be clearly visible, DHSC suggests that the warning statement should have contrasting font in respect of both size and colour to ensure it stands out from the surrounding text.
Article 6(2)(c) relates to the ‘Important Notice’ requirement. The Important Notice is required only on infant formula and must state the superiority of breast feeding and make a statement recommending that the product is to be used only on the advice of independent persons having qualifications in medicine, nutrition or pharmacy, or other professionals responsible for maternal and child care. The Important Notice should be clearly visible and understandable and should be afforded a high degree of prominence on the label. Please refer to appendix 3 of this guidance for advice on the presentation of ‘Important Notice’ information on websites.
Article 6(3)(a) provides further labelling requirements for follow-on formula. Follow-on formula must state that the product is suitable only for infants over the age of 6 months, that it should form only part of a diversified diet, that it is not to be used as a substitute for breast milk during the first 6 months of life and that the decision to begin complementary feeding, including any exception to 6 months of age, should be made only on the advice of independent persons having qualifications in medicine, nutrition or pharmacy, or other professionals responsible for maternal and child care, based on the individual infant’s specific growth and development needs.
The NHS provides guidance on types of formula.
The guidance states:
“Follow-on formula is suitable from 6 months of age (but ask a health visitor for advice first) and that follow-on formula should never be fed to babies under 6 months old.
“Research shows that switching to follow-on formula at 6 months has no benefits for your baby. Your baby can continue to have first infant formula as their main drink until they are 1 year old.”
Article 6(6) seeks to ensure that the labelling of infant formula and follow-on formula provides the necessary information about the appropriate use of the products so as not to discourage breast feeding and does not contain the terms ‘humanised’, ‘maternalised’ and ‘adapted’ or any similar terms.
Article 6(6) also requires infant and follow-on formula to be clearly distinct from each other in order to ensure appropriate product use and prevent confusion. This is to ensure parents and caregivers provide the most suitable product for their child. They must differ in relation to text, images and colours used on the packaging. All 3 of these elements must be different to enable a clear distinction to be made between different formulas. DHSC does not consider using different shades of the same colour to be an appropriate difference. This requirement also serves to prevent cross promotion and the indirect marketing of infant formula by advertising a product that looks almost identical. Further information on the requirements for distinct packaging can be found in appendix 4.
Article 10 of the FSG Regulation
Article 10(2) of the FSG Regulation states that the labelling of infant formula and follow-on formula shall not include a picture of an infant or any other picture or text which may idealise the use of the product. The labelling may include graphic representations for easy identification of the product or for illustrating methods of preparation.
Examples of representations which may be considered to ‘idealise’ the use of infant or follow-on formula should they feature on infant or follow-on formula labelling (for example, representations which may imply that formula is equivalent or superior to breast feeding) include (this list is not exhaustive):
- pictures of infants, young children or carers (for example, mothers or fathers)
- graphics that represent nursing mothers and pregnant women
- pictures or text which imply that infant health, happiness or wellbeing, or the health, happiness and wellbeing of carers, is associated with infant or follow-on formula
- references to an infant’s or carer’s emotions
- baby or child-related subjects and anthropomorphic characters, pictures and logos
- non-mandatory pictures or text which refers, directly or indirectly, to ‘the best’ or ‘the ideal method’ of infant feeding
- references to non-mandatory text or pictures on infant formula and follow-on formula labelling which refers to ‘human milk’, ‘human milk oligosaccharides (HMO)’, ‘breastmilk’, ‘breastfeeding’, ‘moving on from breastfeeding’ or ‘closer to or inspired by breastmilk’.
In light of these requirements, DHSC recommend that manufacturers should ensure the following when producing infant formula and follow-on formula labelling:
The specific terms ‘infant formula’ and ‘follow-on formula’ should be clearly featured on the packaging.
Non-mandatory references to breastmilk or breastfeeding should not be made on follow-on formula packaging as consumers may associate these terms with feeding infants from birth, whereas follow-on formula should be used only from 6 months.
Specific requirements on the nutrition declaration
Unless otherwise provided for in the Commission Delegated Regulation including derogations, the nutrition declaration of infant and follow-on formula shall comply with labelling information requirements set out in Regulation (EU) No 1169/2011.
Article 7(1) of Regulation (EU) No 1169/2011 states food information should not be misleading, particularly by the information suggesting that the food possesses special characteristics – for example, by specifically emphasising the presence or absence of certain ingredients and/or nutrients when in fact all similar foods possess such characteristics.
In light of these requirements, DHSC recommends the highlighting or repeating of mandatory nutrients should not be included on the labelling of infant and follow-on formula as it could be considered that it implies that the product possesses special characteristics when all similar foods possess such characteristics.
Article 7 of the Commission Delegated Regulation:
Dictates the mandatory nutrition information that must be included on the packaging of infant formula and follow-on formula irrespective of the size of the packaging or container. The article also refers to the supplementary nutrition information that can be included on the packaging.
Under Article 7(1), in addition to the mandatory particulars in Article 30(1) of Regulation (EU) No 1169/2011, the nutrition declaration of infant and follow-on formula must include the amount of the minerals and vitamins listed in Annex I and Annex II of the Commission Delegated Regulation.
Under Article 7(3), the information included in the mandatory nutrition declaration for infant and follow-on formula shall not be repeated on the labelling.
An exception to Article 30(1) of Regulation (EU) No 1169/2011 is the inclusion of salt on the mandatory nutrition declaration of infant and follow-on formula. Under Article 7(1), infant and follow-on formula must not have the amount of salt on the label.
Under Article 7(1), the mandatory nutrition declaration for infant formula must include the amount of choline, inositol and carnitine.
The nutrition declaration for infant and follow-on formula may be supplemented with the amounts of nutrients as described in Article 7(2).
Article 7(6) notes that energy value and the amounts of nutrients of infant formula and follow-on formula shall be expressed per 100 ml of the food ready for use after preparation in accordance with the manufacturer’s instructions rather than as sold.
The use of daily reference intake is not permitted on infant formula.
Nutrition and health claims for infant formula
Article 8 of the Commission Delegated Regulation states:
Nutrition and health claims are prohibited on infant formula.
The following definitions, which are set out in Regulation (EC) No 1924/2006 on nutrition and health claims made on foods, apply for the purposes of the Commission Delegated Regulation:
‘Claim’ means any message or representation, which is not mandatory under any enactment, including pictorial, graphic or symbolic representation, in any form, which states, suggests or implies that a food has particular characteristics.
‘Nutrition claim’ means any claim which states, suggests or implies that a food has particular beneficial nutritional properties due to:
(a) the energy (calorific value) it:
- provides
- provides at a reduced or increased rate, or
- does not provide
And/or:
(b) the nutrients or other substances it:
- contains
- contains in reduced or increased proportions, or
- does not contain
‘Health claim’ means any claim that states, suggests or implies that a relationship exists between a food category, a food or one of its constituents and health.
‘Reduction of disease risk claim’ means any health claim that states, suggests or implies that the consumption of a food category, a food or one of its constituents significantly reduces a risk factor in the development of a human disease.
Examples of ‘claims’ on infant formula that could be considered as ‘non-permitted’ claims include:
- ‘contains all the nutrients your baby needs to grow strong and healthy’
- ‘easy to digest’
- ‘gentle’
- highlighting the addition or exclusion of any ingredients such as:
- taurine
- fructo-oligosaccharides and galacto-oligosaccharides (GOS/FOS)
- nucleotides
- DHA without accompaniment of either of the statements in Article 9
Statements related to lactose and docosahexaenoic acid (DHA)
Article 9 of the Commission Delegated Regulation:
Article 9(1) dictates when the statement ‘lactose only’ is permitted on the packaging of infant and follow-on formula.
Article 9(2) dictates when the statement ‘lactose free’ is permitted on the packaging of infant and follow-on formula. Article 9(2) also specifies the circumstances in which the wording ‘not suitable for infants with galactosaemia’ must be included on the packaging. Where the statement ‘not suitable for infants with galactosaemia’ is used, it should be in close proximity to the area of packaging highlighting the statement of ‘lactose free’.
Article 9(3) refers to the mandatory addition of DHA in infant formula. Due to this addition being mandatory, packaging that wishes to highlight the presence of DHA in the formula must include either of the following statements:
- ‘contains Docosahexaenoic acid (as required by the legislation for all infant formula)’
- ‘contains DHA (as required by the legislation for all infant formula)’
DHSC suggests the statement should be in close proximity to the area of the packaging highlighting the presence of DHA.
The statements may only be used for infant formula that has been placed on the market before 22 February 2025. After this date, highlighting of the presence of DHA will be prohibited.
Requirements for promotional and commercial practices for infant formula
Article 10 of the Commission Delegated Regulation:
When providing information to the public or healthcare professionals, infant formula manufacturers must comply with the requirements of Article 10. Further information on what may constitute as advertising can be found in appendix 2.
The aims of Article 10 are to ensure that:
- advertising of infant formula shall be restricted to publications specialising in baby care and scientific publications (Article 10(1)) and shall contain only information of a scientific and factual nature – such information shall not imply or create a belief that bottle-feeding is equivalent or superior to breast feeding – see further information on scientific publications in appendix 5
- point-of-sale advertising, the giving of samples and any other promotional devices to induce sales of infant formula directly to the consumer at the retail level, are prohibited – for example, companies should not use special displays such as prominent shop window displays, free-standing displays or ‘shelf-talkers’ (that is, attachments that add a company’s logo or sales message to the edge of a shelf), discount coupons or special sales which relate to infant formula products (Article 10(2))
- manufacturers and distributors of infant formula shall not provide free or subsidised products, samples or any other promotional gifts to members of the general public including pregnant women, mothers or members of their families – this could include multi packs (bulk buys), loyalty or reward card schemes, free formula, price reductions, discounts or mark downs and ‘buy one get one free’ and gifts provided via baby clubs or similar activities are also prohibited (Article 10(3))
- when advertising follow-on formula, it must be clear that the advertisements for follow-on formula relate exclusively to products for older babies and not infant formula – these advertisements should not promote, either directly or indirectly, infant formula, or formula milks/bottle-feeding in general
Article 10 of the FSG Regulation
Article 10(1) of the FSG Regulation states that labelling, presentation and advertising of infant formula and follow-on formula shall be designed so as not to discourage breastfeeding.
The term ‘presentation’, including the manner in which products are arranged and the setting in which they are displayed, is defined in Regulation (EC) 178/2002 which lays down the general principles and requirements of food law.
It is advised that when advertising follow-on formula, companies do not:
- promote a range of formula products by making the brand the focus of the advert, rather than specific products (for example, where specific products are mentioned only in a footnote or in a picture of a tin of formula within the advertisement)
- include text or images which relate to pregnancy (for example, pregnancy test kits) or the feeding or care of infants under 6 months
- include pictures or text which directly or indirectly relate or compare products to breastmilk
- focus on carers’ emotions in relation to the feeding or care of infants under 6 months
- feature babies which consumers may perceive as being under 6 months (even if they are over 6 months)
- focus primarily on the promotion of ingredients, or the effect of ingredients, which are common to both follow-on formula and infant formula
Requirements on information relating to infant and young child feeding
Article 11 of the Commission Delegated Regulation:
Article 11 requires information and educational material (whether this is written or audio-visual) that deals with feeding of infants and is intended to reach pregnant women and mothers of infants and young children, to include certain information. This information must not use any images or text which idealise the use of infant formula.
Article 11(2) states that the following information must be included:
- the benefits and superiority of breastfeeding
- maternal nutrition and the preparation for and the maintenance of breastfeeding
- the possible negative effect on breastfeeding of introducing partial bottle-feeding
- the difficulty of reversing the decision not to breastfeed
- where needed, the proper use of infant formula
Where materials contain information about the use of an infant formula Article 11(2) states that the following information is included:
- the social and financial implications of its use
- the health hazards of inappropriate foods or feeding methods
- the health hazards of improper use of infant formula
Article 11(3) regulates the donation of informational or educational materials and explains the requirement to seek approval from the appropriate authority to approve the donation of informational or educational materials to third parties. The appropriate authority in England is DHSC.
The following is a non-exclusive list of some of the types of materials which, if they provide information or are educational, would be controlled by Article 11(3):
- CDs and DVDs
- wallcharts, posters
- booklets or leaflets which are designed for reference purposes
- electronic files that can be downloaded directly from a website
- non-broadcast electronic media such as YouTube videos, Instagram or other social media (refer to appendix 3 for further guidance with regard to the internet)
To gain approval from the appropriate authority, such informational or educational materials:
- should contain information which is consistent with current government policies on breastfeeding and the promotion and advertising of infant formula and follow-on formula
- must not be marked or labelled with the name of a proprietary brand of infant formula
- must only be distributed through the healthcare system, such as via GPs, nurses midwives and other healthcare professionals. Donations may only be made on request and with the written approval of the appropriate authority
Companies who wish to seek approval from DHSC for donating such materials should write to (preferably via email as there may be a significant delay in receiving and responding to postal enquiries):
Nutrition Legislation Team
Healthy Weight and Nutrition Division
Office for Health Improvement and Disparities
Department of Health and Social Care
39 Victoria Street
London SW1H 0EU
Tel: 020 7972 4340 E-mail: nutritionlegislation@dhsc.gov.uk
Notification
Article 12 of the Commission Delegated Regulation:
Article 12 provides that no food business operator may place an infant formula on the market unless they have given prior notice to the competent authority where the product is being marketed. The competent authority in this respect for England means the Secretary of State for Health and Social Care (DHSC).
DHSC is centrally coordinating notification forms for all 3 GB nations (England, Scotland and Wales) for the purposes of notifying each of the applicable competent GB authorities. Please see the notification forms.
The ‘food business operator’ must forward a model of the label used for the product and any other information the competent authority may reasonably request to establish compliance with this Commission Delegated Regulation.
Regulation (EC) 178/2002, which lays down the general principles and requirements of food law, provides the following definitions:
A ‘food business operator’ means the natural or legal persons responsible for ensuring that the requirements of food law are met within the food business under their control.
A ‘food business’ means any undertaking, whether for profit or not and whether public or private, carrying out any of the activities related to any stage of production, processing and distribution of food.
See further guidance on how and when to notify the relevant competent authority. Additionally, the link provides guidance on placing an infant formula on the market in the European Union and Northern Ireland.
When follow-on formula is manufactured from protein hydrolysates or contains substances other than those listed in Annex II of the Commission Delegated Regulation, the food business operator must notify the relevant competent authority. Further information is available in appendix 1.
Appendix 1: infant and follow-on formula made from protein hydrolysates
Paragraph 21 of the introductory text of the Commission Delegated Regulation explains that manufacturers of infant formula and follow-on formula which are made from protein hydrolysates must demonstrate the safety and suitability of each specific formula containing protein hydrolysates has been established by clinical evaluation. This requirement is also covered in Article 3. Additionally, Article 12(2) of the Commission Delegated Regulation requires follow-on formula made from protein hydrolysates to be notified by the food business operator to the competent authority where the product is being marketed. For this Article, the competent authority in England is the Secretary of State for Health and Social Care (DHSC).
Providing it has been demonstrated that a specific formula manufactured from protein hydrolysates reduces the risk of developing allergy to milk proteins, further consideration will be given to how to adequately inform parents and caregivers about that property of the product.
The Nutrition (Amendment) and Food for Specific Groups (Food for Special Medical Purposes for Infants, Infant Formula and Follow-on Formula) (Information and Compositional Requirements) (Amendment) Regulations 2021 came into force on 21 February 2021. This statutory instrument (SI) amended the Nutrition (Amendment etc.) (EU Exit) Regulations 2020 in order to make an amendment to the Commission Delegated Regulation (EU) 2016/127. This resulted in a change to the date of application for the provision related to infant and follow-on formula made from protein hydrolysates in England, Scotland and Wales from 22 February 2021 to 22 February 2022. It also amended the date of enforcement of the same provisions in England. Scotland (see The Foods for Specific Groups (Infant Formula and Follow-on Formula) (Scotland) Amendment Regulations 2021) and Wales have similarly amended their enforcing SIs to reflect this new date and Northern Ireland are progressing similar changes.
The EU introduced Regulation (EU) 2021/572 that extended the date of application from 22 February 2021 to 22 February 2022, relating to infant and follow-on formula made from protein hydrolysates. The Protocol on Ireland/Northern Ireland (NIP) means that the EU provisions apply in Northern Ireland.
Therefore, until 22 February 2022 the requirements of Directive 2006/141/EC continued to apply to infant and follow-on formula made from protein hydrolysates.
To apply for the authorisation of the safety and suitability of protein hydrolysates used to manufacture infant and follow-on formula, please see the appropriate application form and guidance. The guidance and application form sets out the steps which applicants need to follow for the authorisation of protein hydrolysates used in infant and follow-on formula. The process to submit an application for the safety and suitability of protein hydrolysates used to manufacture infant and follow-on formula has been agreed and will be reflected as part of the NLCS common framework.
Appendix 2: advertising
All infant and follow-on formula must comply with the requirements of the Commission Delegated Regulation.
Article 6 and Article 10 of the Commission Delegated Regulation restrict the advertising, including promotional and commercial practices, of infant formula.
Article 11 of the Commission Delegated Regulation provides information on the requirements for producing and publishing information and educational material relating to infant and young feeding when it is permitted. Informational and educational materials which fulfil the requirements of Article 11 would not be considered to be advertising however the provisions for advertising also apply to advertising in scientific journals aimed at health professionals. Whether a representation of information is considered informational or educational material or as advertising will depend on its content and context, regardless of whether it is claimed to be informational or educational material.
The following list includes some examples of the means by which a ‘representation’ of information could be considered to be within the context of advertising:
- newspapers, magazines, brochures, leaflets, circulars, direct mailings, e-mails, text transmissions, catalogues, follow-up literature and other electronic and printed material (including advertorials)
- publications for healthcare professionals which are not scientific publications
- posters and other promotional media in public places, including moving pictures
- cinema and video commercials
- non-broadcast electronic media such as YouTube videos, Instagram or other social media, (refer to appendix 3 for further guidance with regard to the internet)
- television and radio broadcast commercials
- correspondence between a trade, business or company and their customers, in writing, orally (including telephone calls and company carelines), electronically or by other means
- press releases and other public relations material and activities that can be accessed by consumers
- tickets, timetables and price lists.
- celebrity endorsements in connection with a trade, business, or company
- product placement in websites
The list is not definitive due to the fact that the nature of advertising is always changing and is intended as guidance only.
Appendix 3: guidance on website information relating to infant formula, follow-on formula and infant feeding
All website content, including editorial text, pictures or videos, that may constitute an advertisement, is subject to the provisions of the Commission Delegated Regulation, supplementing the provisions of the FSG Regulation.
All website content must comply with the requirements of the Commission Delegated Regulation, including Article 6 and Article 10.
Online sale of infant formula
In the case of websites where infant formula can be purchased, only the information that is permitted on the label should be provided, with no particular emphasis given to any of that information. The information provided must comply with the labelling requirements of infant formula set out in Article 6 and Article 10 of the Commission Delegated Regulation.
Informational and educational material
DHSC advises that any informational and educational material about infant formula, or infant feeding, which is included on the website should be accessed only by means of an intermediate page which includes statements which inform the user that:
- by proceeding, they will be able to view information about ‘Brand X’ infant formula and other formula products and that if they choose to proceed, they are accepting that ‘Brand X’ is supplying this information at their individual request
Those responsible for preparing the website are legally obliged to ensure that the content relating to infant formula may not constitute advertising and may only constitute information.
Where informational and educational material about infant formula is provided by means of a website, such information should comply with the requirements of Articles 10 and 11 of the Commission Delegated Regulation.
The information described in the above paragraphs should have the same readability as the main text of the website. Any button which must be clicked to progress beyond the information described above should be positioned at the end of the text to ensure the reader has been presented with all the required information.
The Advertising Standards Authority (ASA) regulates advertising across all media in the UK. Since September 2010, this also includes advertisers’ own marketing communications on their own websites and in other non-paid-for space under their control, such as social networking sites like Facebook and Twitter.
For more information on the ASA and rulings relating to infant formula, visit the ASA website.
Appendix 4: differentiating infant formula and follow-on formula
The lack of differentiation between the following examples of product labels (labels A and B) (same colour, graphics and placement and style of text, logos and graphics) may prevent consumers being able to easily distinguish between infant formula and follow-on formula.
Figure 1: labels A and B
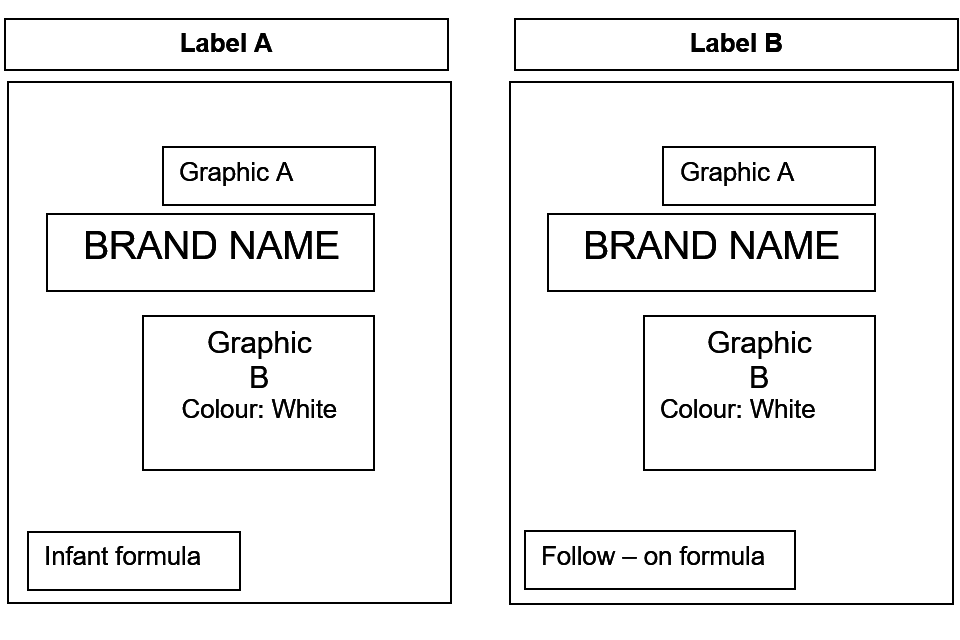
Text alternative to figure 1
Label A depicts a label with 2 sets of graphics: graphic A is placed at the top and graphic B is placed in the middle of the label. The brand name is placed centrally between the 2 graphics and the type of product (infant formula) is placed in the bottom left hand corner of the label.
Label B also depicts a label with the same 2 graphics as label A: graphic A is also located at the top of the label and graphic B is also located in the middle of the label. The same colour graphics (white) are used on both labels.
Label B also has the brand name placed centrally between the 2 graphics and the type of product (follow-on formula) is located at the bottom left hand corner of the label. The same font style and colour for the type of product and brand name is used for label A and label B.
The following amended product labels (labels C and D) provide an example of where the packaging style differs in text (both placement and style), graphics (including placement) and colours, and therefore should allow consumers to distinguish between infant and follow-on formula. DHSC does not consider using different shades of the same colour to be an appropriate difference.
Figure 2: labels C and D
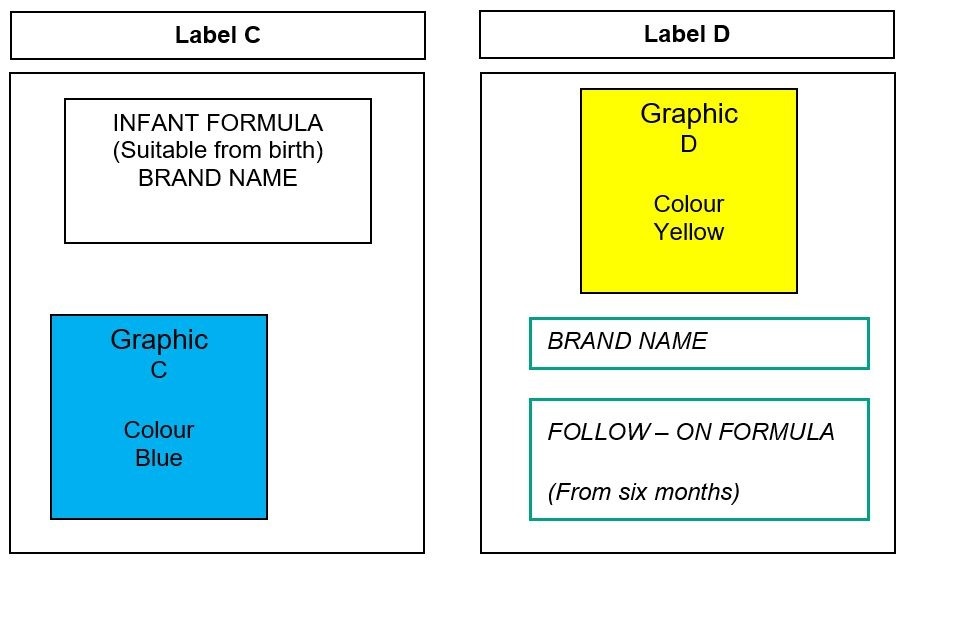
Text alternative to figure 2
Label C depicts a label with one graphic. Graphic C is at the bottom of the label, is positioned to the left of the label and is a blue colour. The type of product (infant formula) is located above graphic C at the top of the label and is centrally aligned, with the brand name located underneath the type of product.
Label D depicts a label which includes graphic D. Graphic D is placed at the top of the label and is a yellow colour. Graphic D is not used on label C.
Label D has the brand name placed underneath graphic D and the type of product (follow-on formula) is located underneath the brand name. The type of product and brand name is placed within a box with a teal colour outline. A different font style is used for the type of product and brand name for label C and label D.
Appendix 5: guidance on scientific publications and information of a scientific or factual nature
Article 10 of the Commission Delegated Regulation only allows infant formula to be advertised in scientific publications and publications specialising in baby care and puts in place controls on the content of such advertisements. Below is guidance on what constitutes a scientific publication and also guidance on the nature of the information that can be included in advertisements for infant formula.
Scientific publication
Scientific publications are usually published periodically and aimed at academic and/or professionals in a scientific field, such as GPs, nurses and midwives. They consist of an aggregation of original articles by different authors published under an umbrella title. Articles included in scientific publications are those that report new scientific research or review existing scientific research. In addition, scientific publications may also include editorials, opinion pieces and book or other reviews dealing with a scientific theme.
In addition, they:
- are static, rather than dynamic (that is, the core content is fixed at the time of publication)
- may have been assigned an International Standard Serial Number (ISSN)
Content of infant formula advertisements
Where advertisements for infant formula are permitted, the advertisements can only include information that is of a scientific and factual nature (Article 10(1)). In DHSC’s view, to comply with this requirement, manufacturers must be capable of supporting any further information provided with research from peer-reviewed scientific journals.
All nutrition and health claims are prohibited on infant formula. DHSC considers this to include the advertising of infant formula. The nutrition and health claims section of this guidance (above) provides information on what constitutes a nutrition or health claim.
