JCVI statement on vaccination of children aged 5 to 11 years old
Published 16 February 2022
On 22 December 2021, the Joint Committee on Vaccination and Immunisation (JCVI) advised that children aged 5 to 11 years in a clinical risk group, or who are a household contact of someone who is immunosuppressed should be offered primary course vaccination with two 10 microgram (mcg) doses of the Pfizer-BioNTech COVID-19 vaccine (Comirnaty®). JCVI has since reviewed evidence on the potential impact of extending COVID-19 vaccination to other children aged 5 to 11. Consideration had been given to the health benefits of vaccination in this age group, the potential educational benefits, and the impact on NHS services of delivering a 2-dose vaccination programme to around 5 million young children.
Advice
JCVI advises a non-urgent offer of two 10 mcg doses of the Pfizer-BioNTech COVID-19 vaccine (Comirnaty®) to children aged 5 to 11 years of age who are not in a clinical risk group. The 2 doses should be offered with an interval of at least 12 weeks between doses.
The intention of this offer is to increase the immunity of vaccinated individuals against severe COVID-19 in advance of a potential future wave of COVID-19.
For deployment teams, JCVI advises that:
- the offer of COVID-19 vaccination to 5 to 11 year olds who are not in a clinical risk group should not displace the delivery of other paediatric non-COVID-19 or COVID-19 immunisation programmes
- delivery of paediatric non-COVID-19 immunisation programmes across all ages should receive due attention, particularly where vaccine coverage has fallen behind due to the COVID-19 pandemic and where there is evidence of health inequalities
- use of the Pfizer-BioNTech 10 mcg paediatric formulation vaccine should be encouraged for all pupils in the relevant academic year for children aged 11 to 12 to reduce complexity in programme delivery and expected reactogenic events for individuals
This advice on the offer of vaccination to 5 to 11-year olds who are not in a clinical risk group is considered by JCVI as a one-off pandemic response programme[footnote 1]. As the COVID-19 pandemic moves further towards endemicity in the UK, JCVI will review whether, in the longer term, an offer of vaccination to this, and other paediatric age groups, continues to be advised.
Informed consent
In all instances, the offer of vaccination must be accompanied by appropriate information to enable children, and those with parental responsibility, to provide informed consent prior to vaccination. Teams responsible for the implementation and deployment of COVID-19 vaccination for persons aged 5 to 11 should be appropriately trained and confident regarding the information relevant to the vaccination of these persons.
Key considerations
Most children aged 5 to 11 have asymptomatic or mild disease following infection with SARS-CoV-2. Some may experience post-COVID-19 symptoms lasting longer than a few days. Children aged 5 to 11 years who are not in a COVID-19 clinical risk group are at extremely low risk of developing severe COVID-19 disease (references 1-3). Of those admitted to hospital over the last few weeks comprising the Omicron wave, the average length of hospital stay was 1 to 2 days (reference 4). A proportion of these admissions are for precautionary reasons.
It is estimated that over 85% of all children aged 5 to 11 will have had prior SARS-CoV-2 infection by the end of January 2022 (reference 5), with roughly half of these infections due to the Omicron variant. Natural immunity arising from prior infection will contribute towards protection against future infection and severe disease.
Vaccination of children aged 5 to 11 who are not in a clinical risk group is anticipated to prevent a small number of hospitalisations and intensive care admissions in this population and would provide short-term protection against non-severe infection (asymptomatic and symptomatic infection that does not require hospital-based care). The extent of these impacts is highly uncertain. They are closely related to future levels of infection in the population in the period following vaccination; these in turn are influenced by the timing, size and severity of any future waves of infection, and the characteristics of any new variants that may dominate future waves of infection. Vaccination is commonly associated with systemic and local reactions (such as headache, fatigue and local arm pain) which typically resolve within 1 to 3 days.
Overall, the committee agreed that the potential health benefits of vaccination are greater than the potential health risks when not including the opportunity costs of a programme to vaccinate all children aged 5 to 11 due to this being part of a pandemic response. The impact of vaccination on school absences was indeterminate; the balance between school absences due to reactions following vaccination versus school absences avoided due to prevention of infection is highly influenced by the uncertain timing of any future wave of infection and of the vaccination programme. In particular, school absences are affected by whether an infection wave falls within the period of good protection against non-severe infection provided by the vaccine, and whether vaccination occurs during school term time or holiday periods.
Vaccination of children aged 5 to 11 who are not in a clinical risk group is not expected to have an impact on the current wave of Omicron infection. The potential benefits from vaccination will apply mainly to a future wave of infection; the more severe a future wave, the greater the likely benefits from vaccination. Conversely, the less severe a future wave, the smaller the likely benefits from vaccination.
Detailed considerations
In formulating the current advice for 5 to 11-year olds, JCVI considered evidence on
- potential direct health benefits and harms
- indirect educational impacts of vaccination
- wider anticipated opportunity costs
As vaccination against COVID-19 currently forms part of the UK pandemic response, a formal cost-benefit analysis incorporating these factors was not considered feasible and was not conducted. Policymakers may wish to take a narrower or wider set of factors into account.
1. Potential health benefits and harms from vaccination
In comparison to the rest of the population / older age groups, evidence indicates that children aged 5 to 11 are at the very lowest risk from COVID-19. Rates of hospitalisation, paediatric intensive care admission and death are lower in this age group than in all older age groups. In addition, the high level of prior infection in this age group of children can be expected to contribute towards their natural immunity against reinfection. There are some data to suggest that natural immunity may last longer than vaccine-induced immunity against non-severe infection.
Vaccine safety and effectiveness
There is good evidence that the Pfizer-BioNTech COVID-19 paediatric vaccine (10mcg, Comirnaty®) induces a strong immune response to vaccination. The currently available vaccine was developed based on the original wild-type SARS-CoV2 virus and is less well-matched to the Omicron variant. Therefore, whilst the current vaccine provides good protection against non-severe infection due to the wild type, Alpha and Delta variants, protection against non-severe infection due to the Omicron variant is less good and is anticipated to be of relatively short-duration (weeks). Data from adults indicate that vaccine effectiveness against symptomatic infection due to Omicron (Pfizer-BioNTech vaccine) wanes over time from around 70% shortly after 2 vaccine doses to around 25% after 10 weeks and 10% after 20 weeks.
In contrast to the protection provided against non-severe infection, vaccine-induced protection against severe disease (hospitalisation and deaths) is expected to be good and maintained for much longer, as evidenced in the UK data for adults.
The Pfizer-BioNTech paediatric COVID-19 vaccine is typically well tolerated, although local and systemic reactions following vaccination, such as fatigue, headache and injection site pain, are common in this age group. In the United States where over 8 million children in this age group have been vaccinated, 8 to 10% of persons reported at least one day absent from school following vaccination. Serious adverse events following vaccination are extremely rare in this age group. In the United States, less than 2 cases of vaccine-related myocarditis have been reported per million doses. The majority of cases of myocarditis were reported after the second dose. International data from adult programmes suggest that a longer interval between doses (greater than the 3 to 4-week schedule widely used in the United States) is associated with a lower reporting rate of myocarditis following vaccination. This association is expected to also apply to the paediatric dose and formulation when used in children.
The available data indicate that the clinical manifestations of myocarditis following vaccination are typically self-limiting and resolve within a short time. The medium to long-term (months to years) prognosis remains less certain.
Potential direct health benefits from vaccination
Vaccination of children aged 5 to 11 years who are not in a clinical risk group would prevent a relatively small number of hospitalisations, paediatric intensive care admissions and paediatric multisystem inflammatory syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] (PIMS-TS) cases. The size of these benefits will depend on the timing and severity of any future wave of infection. Two possible scenarios are considered below (fuller details in Annex A below). There is a high level of uncertainty over a range of assumptions in relation to future estimates of disease in children aged 5 to 11, particularly as regards the level of protection provided by prior infection against future reinfection, severe COVID-19 and PIMS-TS, and how they might vary by variant on both first exposure, and subsequent SARS-CoV2 infection. Should prior infection provide a high level of immunity against future severe COVID-19, the benefits from vaccination would be smaller, and vice versa.
Table 1 - Prevented cases in 5 to 11 year olds, per million vaccine courses and number needed to vaccinate to prevent one case
| Scenario | Measure | PIMS-TS (hospitalisations/ ICU admissions) | Hospitalisations due to acute COVID-19 | ICU admissions due to acute COVID-19 |
|---|---|---|---|---|
| More severe future wave* | Prevented per million courses of 2 doses | 58 | 98 | 3.0 |
| More severe future wave* | Number needed to vaccinate to prevent 1 case | 17,000 | 10,300 | 340,000 |
| Less severe future wave** | Prevented per million courses of 2 doses | 10 | 17 | 0.5 |
| Less severe future wave** | Number needed to vaccinate to prevent 1 case | 95,000 | 58,000 | 1,900,000 |
*More severe: may be a wave due to a variant with disease severity similar to a pre-Omicron variant; in a population with a lower level of natural immunity provided by previous infection.
**Less severe: may be a wave due to a variant with disease severity similar to Omicron; in a population with a higher level of natural immunity provided by previous infection.
2. Vaccine preventable impacts on education
JCVI has considered evidence on the educational impact of COVID-19 on children aged 5 to 11 years, and how vaccination of children might mitigate these impacts. A major reason contributing towards a loss in education during the Omicron wave is absence from school due to self-isolation in the event of SARS-CoV2 infection amongst pupils. Because most infections are mild, the duration of absence from school is likely to be mostly determined by existing self-isolation policies (such as the 5-day self-isolation ‘rule’), rather than the duration of symptoms severe enough to require time away from school for illness recovery; however firm data to confirm this observation are lacking.
For vaccination to have a substantial impact on school absences, persistent high levels of vaccine-induced protection against non-severe infection are required. However, with respect to highly transmissible variants, such as Delta and Omicron, currently available COVID-19 vaccines provide good protection against non-severe infections for only a limited period of time. Estimates of the balance between absences from school due to vaccine-related reactions compared to absences prevented through reductions in infection vary depending on the likely future incidence of infection. These estimates encompass a high degree of uncertainty, reflecting the uncertainties in predicting the size, timing and severity of any future wave(s) of infection in the coming year. Benefits in preventing school absences would be higher should a future wave happen to occur shortly after vaccination when levels of protection against infection are higher.
Overall JCVI considered that the benefits of vaccination in preventing school absences were indeterminate (figure 1). These estimates are sensitive to:
- timing of vaccination - vaccination over weekends or in school holiday periods would reduce the amount of school absences due to adverse reactions following vaccination
- prevailing isolation policies - a set duration of isolation regardless of symptoms (for example, the ‘5-day isolation rule’) likely increases the amount of school absences due to infection. Conversely, a relaxation of this policy might reduce the amount of school absences due to infection as many children aged 5 to 11 years experience very mild, or no symptoms
Figure 1 – Cumulative school days lost due to vaccination and infection – high incidence scenario*
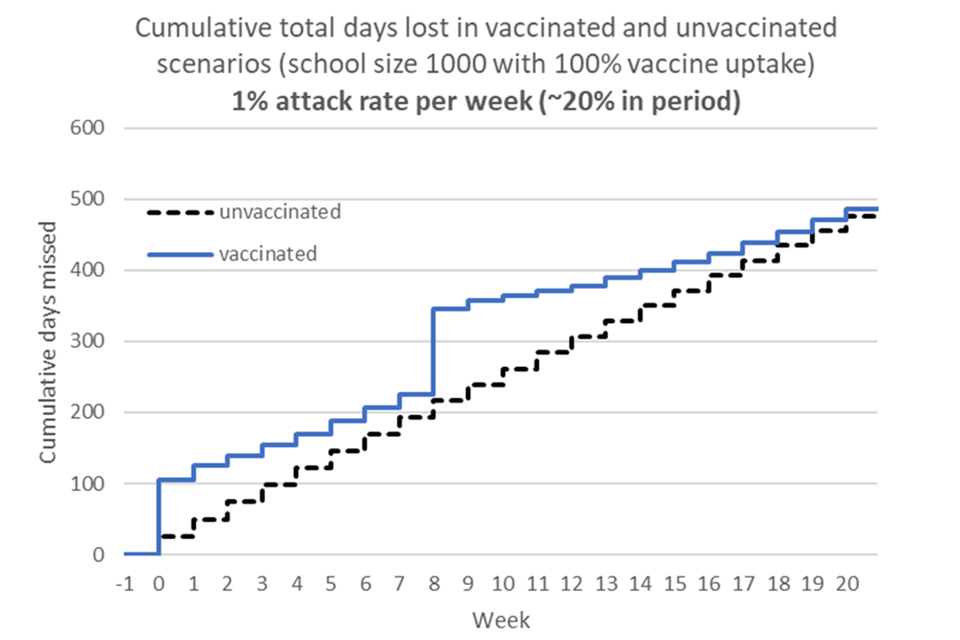
*assumptions: 1% attack rate per week with ‘5-day isolation rule’ in place and vaccinations occur during school term-time
3. Vaccine deployment and wider opportunities for health and education
Delivery of a vaccination programme that involves an injection to younger children is complex. It will typically require longer appointment times than for older children, child friendly settings and appropriately trained staff. Substantial time, care and resources will be necessary to deliver a positive programme. A poor experience of vaccination could adversely affect vaccine confidence towards other immunisation programmes available at older ages, such as against meningitis and human papilloma virus (HPV).
From UK experience with previous paediatric immunisation programmes, vaccine deployment via school-based approaches is associated with higher levels of vaccine coverage with less inequality as measured by ethnicity and indices of deprivation. However, school-based deployment will draw on many of the same resources required for the delivery of other routine immunisation programmes, including for HPV, MenACWY (meningitis) and MMR (measles, mumps and rubella), coverage of which was affected over the last 2 years because of the pandemic. As the UK moves towards ‘living with COVID-19’, these considerations of the opportunity costs related to COVID-19 vaccinations assume increasing importance, particularly where the direct health benefits from vaccination are more marginal. Opportunities to reduce health and educational inequalities through greater engagement in existing non-COVID-19 immunisation programmes that are widely held to be valuable deserve emphasis.
References
A list of all data considered over time is available in the previous statement.
- Ward and others, Risk factors for intensive care admission and death amongst children and young people admitted to hospital with COVID-19 and PIMS-TS in England during the first pandemic year
- Smith and others, Deaths in children and young people in England following SARS-CoV-2 infection during the first pandemic year: a national study using linked mandatory child death reporting data
- Harwood and others, Which children and young people are at higher risk of severe disease and death after SARS-CoV-2 infection: a systematic review and individual patient meta-analysis
- CO-CIN update January 2022
- Unpublished data, University of Warwick
Annex A
Estimating vaccine benefits in 5-11 year-old children in England
Introduction
The benefits of vaccination of 5 to 11 year olds who are not in a risk group in terms of serious COVID-19 disease prevented depend on a large number of factors. These include the:
- infection rate in the period following vaccination in children not previously infected
- rate of severe COVID-19 in those infected who were not previously infected (per 100,000 infections) and how this may differ by strain
- proportion of children already infected at the time of vaccination
- protective effect of a previous infection on the COVID-19 outcomes
- vaccine effectiveness of each dose in the period following vaccination against each outcome and whether this differs by past infection.
Two scenarios that show a range of vaccine benefits are considered based on future waves that may be more and less severe. For example, less severe might be a future wave where the population already has a high level of immunity which itself is protective and severity is similar to early data for Omicron severity. More severe may be a variant with severity similar to pre-Omicron severity and with a lower proportion already infected and with lower protection from that infection. In both scenarios it is assumed vaccine protection is expected to be similar to that initially estimated for Omicron.
Methods
The outcomes considered were ICU cases, hospitalisations and PIMS-TS. The benefits are expressed as cases prevented per million vaccinations. The table below gives the values of the parameters used and the source information or assumption. Two scenarios are considered reflecting a more severe future wave in which the vaccine has more benefit and a less severe future wave in which the vaccine has less benefit as shown in Table 2. Using these parameters the benefits can be calculated. This is done by separately calculating benefits by previous infection status and then combining.
Table 2: Parameters assumed for calculation of benefits
| Parameter | Value (more severe) | Source | Value (less severe) | Source |
|---|---|---|---|---|
| Infection rate in fully susceptible in a future wave | 33% | Fixed assumption (a) - this gives overall infection rate of 20% across those with and without previous infection | 27% | Fixed assumption (a) - this gives overall infection rate of 10% across those with and without previous infection |
| Hospitalisations per 100,000 infections | 64 | National child mortality dataset by risk status and modelled wave 2 infection rate (b) | 23 | (b) and assuming a 64% reduction in risk (c ) |
| ICU per 100,000 infections | 1.7 | National child mortality dataset by risk status and modelled wave 2 infection rate (b) | 0.6 | (b) and Assuming a 64% reduction in risk (c ) |
| PIMS-TS per 100,00 infections | 53 | From US publication (d) | 19 | (d) and assuming a 64% reduction in risk (c ) |
| Proportion already infected | 80% | Mathematical models (e) | 90% | Mathematical models (e) |
| Protection against disease outcomes if already infected | 50% | From test negative case-control analysis using the Omicron outcome (f). This may reflect a new variant. | 70% | From test negative case-control analysis averaging previous infection effect for Delta (90%) and Omicron (50%) (f). This may reflect a future Omicron wave. |
| Second dose vaccine effectiveness | ICU: 85%, Hospital: 70%, PIMS-TS: 55% | VE against Omicron 5-9 weeks post dose 2 (g). VE assumed to be the same irrespective of past infection | ICU: 85%, Hospital: 70%, PIMS-TS: 55% | VE against Omicron 5-9 weeks post dose 2 (g). VE assumed to be the same irrespective of past infection |
Table notes
a) 33% and 27% within the not previously infected give rates overall of 20% and 10% once combined with the other assumptions and cover a relatively large and medium future wave.
b) Based on child mortality dataset from wave 2 of the pandemic 1, stratified by children being in a risk group and using estimates of wave 2 infection of 21% (end of February 2021) from modelling (University of Warwick model).
c) Based on all age relative severity of Omicron compared to Delta and assuming Delta and Alpha/Wild type severity was similar to extrapolate from wave 2 data. 2
d) Uses US data where rates are 444 per million in age 0-5, 613 per million in age 6 to 10 and 224 per million in age 11-15. 3 To obtained 5-11 these are weighted as 1/7)444+(5/7)613+(1/7)*224 = 533 per million or 53 per 100,000. Similar rates were estimated for the original strain and the alpha variant waves in the UK (manuscript in preparation).
e) Range of modelled estimates for end of January 2021 from modelling groups (Warwick, LSHTM, Cambridge)
f) In the supplementary appendix of paper accepted by NEJM, using known previous positive test. 4
g) Vaccine effectiveness data are in UKHSA COVID-19 vaccine surveillance report 5 using adult data on 5-9 weeks post dose 2 and assuming for ICU the risk once hospitalised is reduced by a half by vaccination (85 = 100-((100-70)/2)). For PIMS-TS the VE is assumed to be the same as that for infection. Note that that paper by Zambrano LD et al. 6 shows VE of 90% for the Delta wave for PIMS-TS – similar to VE against symptomatic infection seen for children for Delta of 2 doses in England. (7)
References to tables notes
- Ward JL et al. Risk factors for PICU admission and death among children and young people hospitalized with COVID-19 and PIMS-TS in England during the first pandemic year. Nat Med. 2022 Jan;28(1):193-200
- Nyberg T et al. Risk of hospitalisation and death with SARS-CoV-2 Omicron variant (B.1.1.529) compared with Delta variant (B.1.617.2) in England. Manuscript submitted for publication by UKHSA/MRC Biostatistics Unit / Imperial College London
- Payne AB, et al. Incidence of Multisystem Inflammatory Syndrome in Children Among US Persons Infected With SARS-CoV-2.JAMA Netw Open. 2021 Jun 1;4(6):e2116420.
- Andrews N, et al. Effectiveness of COVID-19 Vaccines against the Omicron (B.1.1.529) Variant of Concern. NEJM – paper accepted
- UKHSA COVID-19 vaccine surveillance report – week 4
- Zambrano LD et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12-18 Years - United States, July-December 2021. MMWR Morb Mortal Wkly Rep. 2022 Jan 14;71(2):52-58
- Ladhani et al. Adolescent vaccination with BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine and effectiveness of the first dose against COVID-19: national test-negative case-control study, England. Submitted to The Lancet Infectious Diseases
Results
Table 3 shows the results for the two scenarios. Vaccine benefits are about 6 times greater for the more severe future wave compared to less severe. Note that most of these benefits can all be scaled to other scenarios of the parameters. For example, if the future wave was half the size, then the benefits would halve. If vaccine effectiveness was 90% instead of 70%, benefits improve by a ratio of 9/7. If the protection from past infection was 90% rather than 70%, then the benefits within those previously infected will be less (1/3 of the benefit since 1/3 = (1-0.9)/(1-0.7)).
Table 3: prevented cases per million doses and number needed to vaccinate to prevent one case
| Scenario | Measure | PIMS-TS | Hospitalisations | ICU |
|---|---|---|---|---|
| More severe future wave | Prevented per million courses of 2 doses | 58 | 98 | 3.0 |
| More severe future wave | Number need to vaccinate to prevent 1 case | 17,000 | 10,300 | 340,000 |
| Less severe future wave | Prevented per million courses of 2 doses | 10 | 17 | 0.5 |
| Less severe future wave | Number needed to vaccinate to prevent 1 case | 95,000 | 58,000 | 1,900,000 |
-
For deployment: this one-off programme applies to those currently aged 5 to 11, including those who will turn 5 years of age by the end August 2022. Based on current evidence, on-going eligibility for vaccination is expected to be for children aged 11 years and offered during the early part of the relevant academic year (year 7 in England and Wales, year S1 in Scotland, and year 8 in Northern Ireland). Children in these academic years in 2022/23, even those who are already aged 12, may be vaccinated using the paediatric formulation to support operational simplicity and to reduce the expected risk of reactogenicity which may interrupt education. ↩
