MAVIS Hub 110
Published 30 April 2019
1. Product News
Veterinary Medicine Supply Problem: Isoflurane
Continuity of supply of veterinary medicines
Veterinary medicine: Reminder how a MAH can report a supply problem
Update on veterinary medicine supply in a no deal EU Exit
Reclassification of pregabalin and gabapentin to Schedule 3 drugs from 1 April 2019
Anaestamine 100mg/ml injection - Product defect recall alert
Known supply problems with animal medicines
Report a product defect: veterinary medicine
Top ten imported veterinary medicines - Quarterly report 01 January to 31 March 2019
2. Enforcement
Animal medicines improvement notice: UK Belgica Ltd Colchester Essex
Animal medicines improvement notice: Taggart Jack, Omagh, County Tyrone
Animal medicines improvement notice: Fruugo.com Ltd
Animal medicines improvement notice: Aurivo (NI) Ltd , Omagh, County Tyrone
Animal medicines improvement notice: APG Supplies and Services, County Fermanagh, Northern Ireland
Animal medicines improvement notice: Pilgrims Veterinary Practice
Police caution issued 18 February 2019
Police caution issued 29 November 2018
Animal medicines improvement notice: Meditech UK, Trade Stand Doncaster National Spring Pigeon show
Animal medicine seizure notice: Emiliano Espinar Veterinary Surgeon
2.1 Collection page
3. AMR (Antimicrobial Resistance)
3.1 New this quarter
The DARC group met on 20 February 2019 to discuss the recent trends in antibiotic resistance (AMR) in bacteria of importance to human and animal health. The group received an update on antibiotic consumption data projects under development, as well as recent international collaborations. Topical presentations were given that outlined latest research and development in AMR with contributions from the Veterinary Medicines Directorate and Food Standard Agency. Summary minutes of the meeting are available GOV.UK.
3.2 Sales Data and Antibiotic Resistance Surveillance Report
Data for the 2018 UK Veterinary Antibiotic Resistance and Sales Surveillance (UK-VARSS) Report are currently being collated for publication later in the year. The report collates UK data on antibiotic sales from Marketing Authorisation Holders and antibiotic resistance data from the VMD’s surveillance programmes.
UK-VARSS 2017 and previous reports.
The second AMR One Health Report (joint report on antibiotic use in animals and humans and antibiotic resistance in the UK between 2013 and 2017) was published on GOV.UK on 31 January
3.3 UK AMR strategy High Level Steering Group meeting
The new UK 5-year AMR National Action Plan (NAP) and 20-Year Vision have been published on 24 January 2019. The new Governance arrangements to replace the “High Level Steering Group” are being discussed amongst government departments involved.
3.4 Communications
During February, the VMD supported the Responsible Use of Medicines in Agriculture Alliance (RUMA) in their #ColostrumIsGold campaign. Using social media platforms, facts were broadcast to raise awareness on how quality colostrum delivered at the right time and of the right quantity can reduce the need for antibiotics during animals’ lifetimes, especially during the early stages.
3.5 Collection page
4. Guidance Updates
Authorisations to manufacture veterinary medicines
Apply for a Manufacturing authorisation for veterinary medicines
Report a supply problem with a veterinary medicine
Export drugs and medicines: special rules
Apply for an Autogenous Vaccine, Non-Food Animal Blood Bank, Equine Stem Cell Centre Authorisation
Using the Veterinary Medicines Digital Service
Timetables for national applications for MAs, ATCs and VHRs
Apply to change a veterinary marketing authorisation or homeopathic remedy
Apply for a Marketing Authorisation for a veterinary medicine or expiry
Apply to renew a marketing authorisation for a veterinary medicine
Marketing Authorisations for Parallel Import of veterinary medicines
Validation of applications for veterinary medicines
Approval of premises for retail supply of veterinary medicines by suitably qualified persons (SQP)
5. Surveillance
Residues of veterinary medicines in food: 2019
Residues of veterinary medicines in food: 2018
5.1 Collection page
6. Stakeholder Engagement
Veterinary Medicines Directorate’s EU Exit Information Hub
VMD EU Exit Information Hub: Accessible Format
Veterinary medicines: National authorisation application timetables from 1 April
7. Corporate News
Veterinary Medicines Directorate: privacy notices
Veterinary Medicines Directorate – Our governance
VMD Published Standards 2018 to 2019: Monitoring Performance
7.1 VMD Organogram as at 01 April 2019
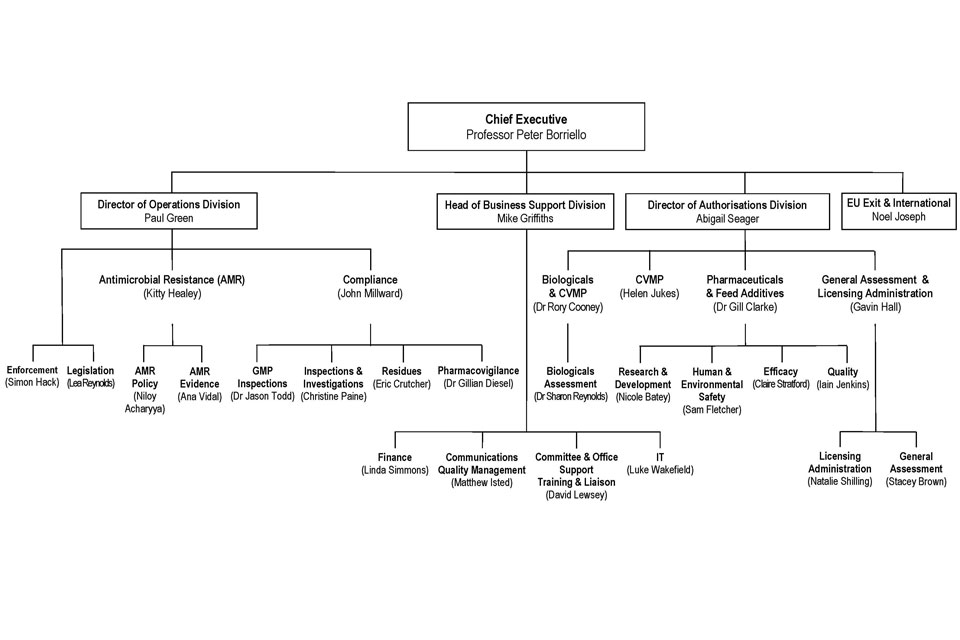
VMD Organogram as at 1 April 2019
