MAVIS Newsletter edition 107
Published 26 July 2018
1. News
1.1 Non-Executive Directors (NEDs) re-appointments and recruitment
The terms of appointment for the current NEDs, David Corner and Julia Drown have been extended to 1 May 2020 and 1 May 2021 respectively.
Andrew Coulson has stepped down from his post with effect from 31 May 2018. Recruitment for a replacement NED will be carried out in the near future.
1.2 The VMD Annual Report and Accounts 2017/18
The VMD’s Annual Report and Accounts were presented to the House of Commons in accordance with Section 7(3)(c) of the Government Resources and Accounts Act 2000 and ordered by the House of Commons to be printed 5 July 2018.
1.3 The VMD achieves excellent ratings in the Benchmarking of European Medicines Assessment (BEMA) exercise
The recent BEMA assessment of the VMD by a team of assessors from other European medicines regulatory agencies scored the VMD an average of 4.5 out of 5 against a wide range of indicators covering our main responsibilities, functions and processes. We improved our score from an average of 4.3 in our BEMA assessment in 2014.
This is an excellent outcome from a comprehensive and rigorous independent assessment by peer agencies. Improving on the very high score of our last BEMA assessment shows the VMD’s continuing commitment to develop and enhance our systems and processes to meet the needs of our stakeholders in assuring the safety and effectiveness of veterinary medicines.
BEMA has been developed by the Heads of Medicines Agencies network to help create a world-class medicines regulatory system based on a network of agencies operating to best practice standards. It is based on the assessment of the systems and processes in individual agencies against a set of indicators covering: management systems; assessment of marketing authorisation applications; pharmacovigilance activities and inspection services.
1.4 Invitation to attend the Open Meetings of the Veterinary Medicines Directorate (VMD) and Veterinary Products Committee (VPC) on 28 September 2018
The VMD and VPC will hold Open Meetings on Friday 28 September 2018 at the Animal and Plant Health Agency, Addlestone, Surrey, KT15 3NB. The VMD Open Meeting will begin at 10.30am followed by the VPC Open Meeting and close by 1pm. Admission is free but by ticket only. The VMD will give presentations based on advance questions, followed by presentations by VPC members and then a ‘question and answer’ session. You should send questions and requests for tickets to openmeeting@vmd.defra.gsi.gov.uk by Friday 31 August 2018. Please include the names of all attendees.
1.5 Invitation to attend VMD Pharmaceutical Industry Information Event on 14 November 2018
The VMD will be holding an Information Event for the pharmaceutical industry on Wednesday 14th November 2018. We are currently developing the schedule for the day and are inviting your suggestions on areas you would like to see covered. Admission is free but by ticket only.
The event usually starts around 10am until 3pm. Timings will be confirmed once we have the final agenda.
2. Licensing
2.1 Suspension of Veterinary Medicines containing the excipient Diethanolamine (DEA)
The VMD has suspended products for food-producing animals that contain the excipient diethanolamine (DEA). A list of these products is below:
| Product | Vm Number | MAH |
|---|---|---|
| Allevinix 50 mg/ml Solution for Injection for Cattle, Pigs and Horses | 15052/4144 | Ceva Animal Health Ltd |
| Cronyxin Injection, 5% w/v Solution for Injection | 12597/4014 | Cross Vetpharm Group Ltd |
| Dugnixon 50 mg/ml Solution for Injection for Cattle, Pigs and Horses | 36167/4005 | Global Vet Health S.L. |
| Finadyne 50 mg/ml Solution for Injection | 01708/4582 | Intervet UK Ltd |
| Flunixin 50 mg/ml Solution for Injection for Cattle, Horses and Pigs | 02000/4170 | Norbrook Laboratories Limited |
| Meflosyl 5% Solution for Injection | 42058/4085 | Zoetis UK Limited |
| Norixin 5% Solution for Injection | 02000/4137 | Norbrook Laboratories Limited |
| Pyroflam 50 mg/ml Solution for Injection for Cattle, Horses and Pigs | 02000/4253 | Norbrook Laboratories Limited |
| Tribrissen 48% Suspension for Injection | 01708/4593 | Intervet UK Ltd |
The VMD has done this in the light of the scientific opinion of the Committee for Medicinal Products for Veterinary Use (the scientific advisory committee to the European Medicines Agency) that there may be a risk to humans from consuming food from animals treated with products containing DEA. Information on newly authorised and expired MAs, and Public Assessment Reports for homeopathic products are available in the Product Information Database.
2.2 Marketing Authorisations
Details of clinically significant variations are published in the Veterinary Record and the Veterinary Times.
You can see lists on GOV.UK of:
2.3 Quarterly reporting against VMD Published Standards for 2018/19 licensing work
This report is published on a monthly basis under Our Statistics on GOV.UK.
2.4 Summary of Company visit questionnaires from 1 April 2017 to 31 March 2018
Background
This is the ninth full year of the VMD seeking feedback from companies who request a meeting with us, on the effectiveness, accuracy and relevance of the advice provided. The outcomes for 2017/18 are similar to previous years, with consistently high levels of satisfaction being achieved.
These qualitative results complement the many quantitative measures we have in place and help to provide a more rounded summary of the performance and service that industry can expect to receive.
For the 2017/18 financial year, the VMD set a target that the overall median score from meeting questionnaires for individual VMD company meetings should be not less than 4 out of 5 for at least 90% of the meetings. In addition, any feedback received is used to enable the VMD to continue to provide a service that meets the industry need and helps to identify areas where improvements can be made.
Meetings
Between 1 April 2017 and 31 March 2018 a total of 48 meetings were held at the request of companies in order to discuss potential projects. A total of 16 completed questionnaires were received from companies reporting on the experiences that they had in arranging and attending meetings, which was a lower return rate than in previous years. In addition feedback was provided on the effectiveness, accuracy and relevance of the advice given.
The VMD would like to thank those who took the time to respond to this questionnaire. Your feedback is valued and we will be looking at the individual comments made to see where we can improve further. We are disappointed not to have received more completed questionnaires and we appreciate everyone is very busy and this is an additional task but we would like to encourage all companies on all occasions to provide us with feedback.
Results
The questionnaire relies on a simple scoring system from 1 to 5 with 1 being at the lower end of the scale and 5 at the top end.
- 15 out of the 16 respondents rated the overall usefulness of these meetings as 4 or above, with one giving a rating of 3. The average score was 4.6.
- Ease of arranging meetings – all but one company rated this as 4 or above with the other rating this at 3. The average score was being 4.5.
- Respondents thought that the VMD staff were well prepared for these meetings with the average score being 4.6.
- The VMD returned the draft set of minutes with our comments to the company within an average of 14.9 calendar days from receipt.
- On average respondents scored the advice given by each discipline as follows:
| Discipline | Score |
|---|---|
| Biologicals | 5.00 |
| Quality | 4.83 |
| Safety | 4.50 |
| Efficacy | 4.45 |
| Admin | 4.50 |
| Question | Score |
|---|---|
| Overall Usefulness of VMD Advice | 4.63 |
| Ease of Arranging Meetings | 4.5 |
| VMD Staff Prepared | 4.56 |
| Biologicals Advice | 5 |
| Quality Team Advice | 4.83 |
| Safety Team Advice | 4.5 |
| Efficacy Team Advice | 4.45 |
| Administration Team Advice | 4.5 |
| Welcome on Arrival | 4.69 |
| VMD Comments on Minutes | 4.53 |
| Follow up of Post Meeting Actions | 4.33 |
| Accuracy of Advice Provided | 4.3 |
As a balance to the company views, the VMD also completes a questionnaire after each company meeting. This questionnaire seeks views on the quality of the agenda provided; whether all the agenda points were covered or any additional ones added at the meeting; on the engagement of the company during the meeting; and also on the quality of the minutes provided.
| Question | Score |
|---|---|
| Was the Agenda Sufficiently Detailed | 4.08 |
| Did the Company Take a Full Part in the Discussions | 4.85 |
| Was the Company Well Prepared for the Meeting | 4.21 |
| Did the Company Listen to Advice | 4.84 |
| Overall Positivity of the Meeting | 4.94 |
Conclusions
As with previous years the clear message is that industry welcomes the VMD’s open approach to hosting meetings. These are easy to arrange, usually within the timescale requested by the company. Appropriately qualified people attend, which enables constructive debate around the issues that are of importance to the company. The feedback is that the advice offered by VMD staff across all disciplines is valued, relevant and of good quality; and that companies come well prepared and willing to discuss and exchange views.
The VMD welcomes the early provision of agendas with key points or questions for discussion clearly indicated. The VMD is grateful that companies, in the majority of cases, provide agendas at least one week prior to the meeting which provides sufficient time for preparation, enabling more focused discussions to take place. Furthermore, the VMD encourages companies to provide draft minutes so that these can be reviewed, ratified and consequently retained as a record of the discussions and of any agreements which may have been reached. It is important that when completing the minutes that sufficient detail and key points / agreements are recorded. Often there can be a gap between the meeting itself and the project being progressed to the point of submission or in compiling the dossier. The minutes provide a valuable reference point for both parties, especially when personnel may have changed during the intervening period.
2.5 Top ten imported veterinary medicines - Quarterly report 1 April to 30 June 2018
We provide a list on a quarterly basis of the ten products for which most Special Import and Special Treatment Certificates (SIC and STC) have been issued. This list contains details of the product, the active substance and the number of certificates.
We hope the pharmaceutical industry find this list helpful in considering where there might be a need for a UK authorised product.
Product | Active Substance | No. of Certificates Issued -|-|- # Artuvetrin® Therapy, suspension for subcutaneous injection in dogs | Allergens | 2,588 # Vet-Goid | Allergens | 249 # Spectrum Hyposensitisation Vaccine – Injectable Solution | Allergens | 238 # Smartshot B12 Prime Lamb | Allergens | 175 # Aquamid | Polyacrylamide | 154 # Botulism Vaccine | Clostridium botulinum type C toxoid Clostridium botulinum type D toxoid | 130 # European Viper Venom Antiserum (Antytoksyna Jadu Zmij) | European Viper Venom Antiserum | 121 # Greer Allergenic Extract Patient Prescription | Allergens | 119 # Oncept (Canine Melanoma Vaccine) | Canine Melanoma DNA | 102 # Ekyflogyl 125mls (Solution of prednisolone 2mg/ml, Lidocaine 0.01g/ml, Dimethyl sulphoxide 0.8ml/ml) Prednisolone Acetate Lidocaine Hydrochloride Dimethyl Sulphoxide | 87
3. Inspections and investigations
Breaches of the Veterinary Medicines Regulations are investigated in accordance with our published Enforcement Strategy.
3.1 Good Manufacturing Practice
Manufacturing sites must comply with Good Manufacturing Practice (GMP) standards. Eudralex Volume 4 provides guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use.
3.2 GMP inspection deficiency findings 1 April 2018 to 30 June 2018
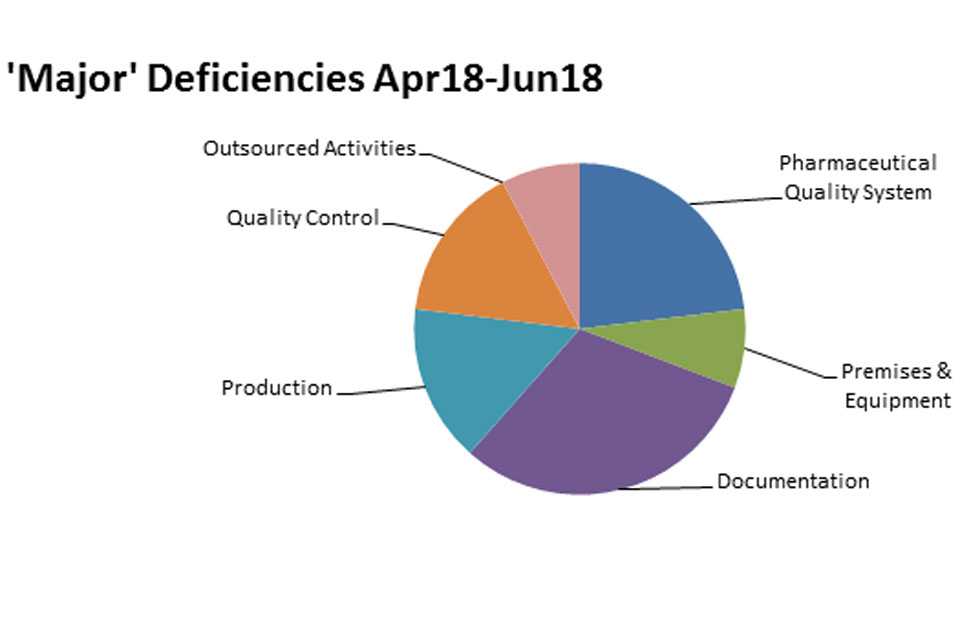
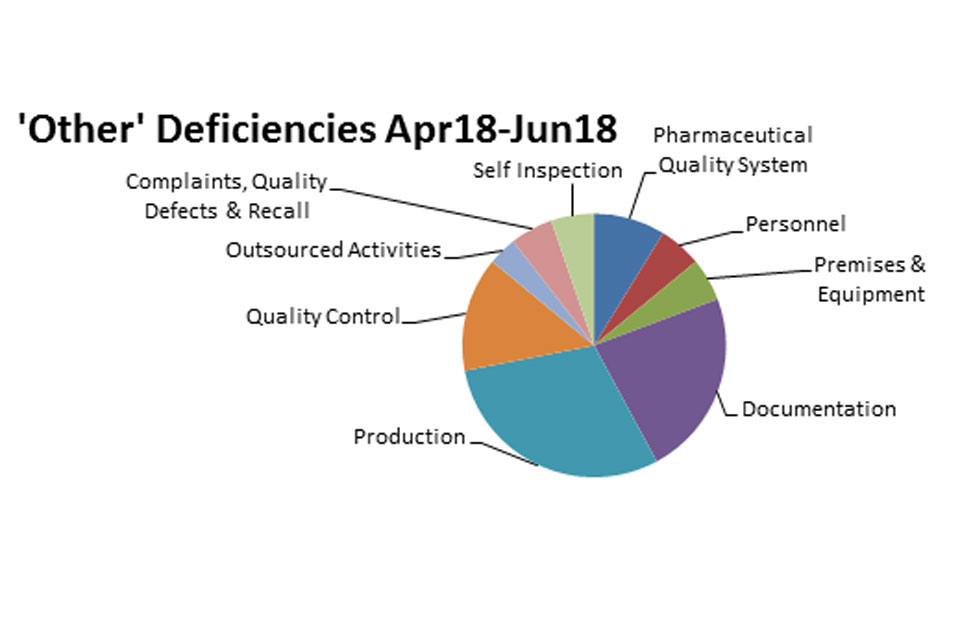
The distribution of inspection deficiencies for each of the nine GMP chapters is shown below. No critical deficiencies were cited in this period:
‘Other’ Deficiencies Apr 18 - Jun 18
GMP Inspection Major Deficiencies April 2018 to June 2018
‘Major’ Deficiencies Apr 18 - jun 18
GMP Inspection Other Deficiencies April 2018 to June 2018
4. Pharmacovigilance reports
4.1 Quarterly report
During the period 1 December 2017 to 28 February 2018, the VMD received 1,559 animal suspected adverse event reports. Of the 2,381[footnote 1] products involved in these reports, 2,252 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 2,225 | Prescription Only Medicine Veterinarian (POM V) |
| 106 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 63 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 36 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
The remaining 108 products were:
| Amount | Type |
|---|---|
| 43 | Authorised human medicines |
| 1 | Medicine used in a trial under an Animal Test Certificate |
| 11 | Veterinary products without any medicinal claim |
| 5 | Authorised medicines imported from other countries |
| 8 | Medicines sold under the exemption for small pet animals |
| 1 | Pesticide products |
| 10 | Specially formulated veterinary medicines |
| 29 | Incompletely identified products |
During this period 48 reports of human suspected adverse reactions were received. Of the 52[footnote 1] products involved in these reports, 51 were authorised veterinary medicines which belong to the following distribution categories:
| Amount | Legal Category |
|---|---|
| 35 | Prescription Only Medicine Veterinarian (POM V) |
| 13 | Prescription Only Medicine Veterinarian, Pharmacist, SQP (POM VPS) |
| 1 | Non Food Animal Veterinarian, Pharmacist, SQP (NFA VPS) |
| 2 | Authorised Veterinary Medicine General Sales List (AVM GSL) |
One Environmental Incident report was received during this period which involved an incompletely identified product.
5. Enforcement
We publish a list of prosecutions and notices involving illegal activity with veterinary medicines in the last year.
You can report information about suspected illegal medicines or breaches of the Veterinary Medicines Regulations to enforcement@vmd.defra.gsi.gov.uk. Details of how to report and how we will deal with the reports can be found in the guidance Report illegal animal medicines.
If you have concerns about a non medicinal product such as a product making an unauthorised claim, you can submit them using the Unauthorised Product Complaint Reporting Form.
All information will be treated confidentially.
6. Antimicrobial Resistance
6.1 Defra Antimicrobial Resistance Co-ordination (DARC) group
The DARC group met on 20 June 2018 to discuss the recent trends in antibiotic resistance (AMR) in bacteria of importance to human and animal health. The group received an update on antibiotic consumption data projects under development, as well as recent international collaborations. Topical presentations were given discussing antimicrobial usage data systems and how perceptions in industry are changing towards increased responsible use. Minutes of the meeting will be published on GOV.UK in due course. Minutes of the meeting will be published on GOV.UK in due course. Minutes from previous meetings are available.
6.2 UK AMR strategy High Level Steering Group meeting
The most recent meeting of the High Level Steering Group (HLSG) for the AMR Strategy took place on 25 June 2018. HLSG members discussed progress on the drafting of the next UK AMR Strategy. The meeting is chaired by the Chief Medical Officer and is represented by cross-government department leaders, including devolved administrations.
6.3 Communications
The summer catch-up meeting in preparation for European Antibiotic Awareness Day and World Antibiotic Awareness Week 2018 activities was held on 5 June 2018. One Health colleagues from across government and animal health experts from the private sector gathered to discuss plans and identify areas for collaboration.
Members of the AMR team were involved in judging nominations for the agriculture and food category of the Antibiotic Guardian Awards. The awards were held on 27 June 2018.
7. Veterinary Products Committee
7.1 Meetings of the Veterinary Products Committee (VPC)
The VPC met in June 2018. Summary minutes of the meetings held from October 2014 are available on GOV.UK.
Minutes of meetings held between 2009 and May 2014 are available on the National Archives.
8. Residues controls and monitoring
8.1 Results of Statutory Surveillance
Sampling commenced in January 2018 and full details of UK surveillance results, together with information on any action taken, can be found on GOV.UK.
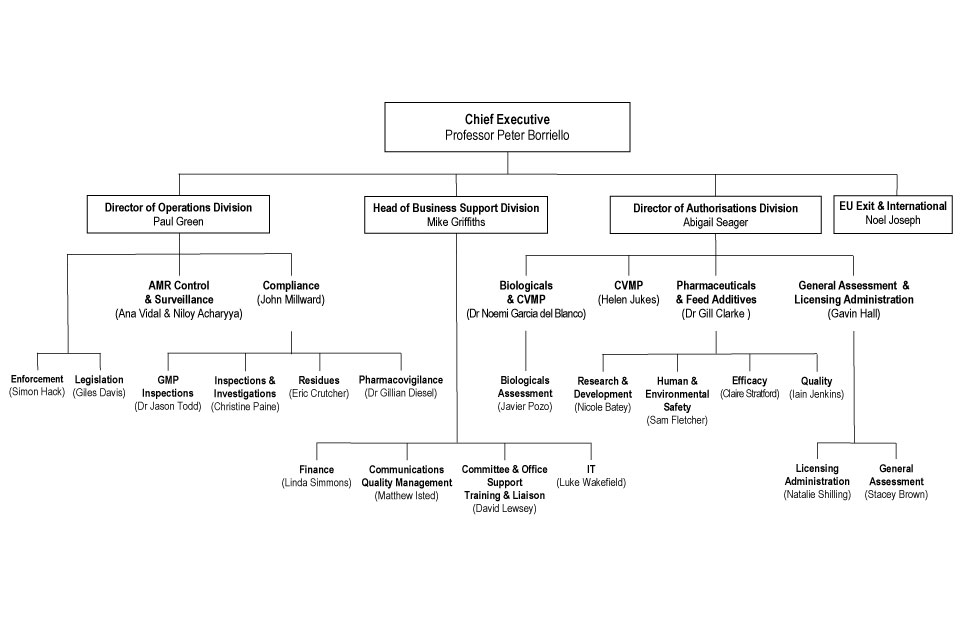
9. Organogram as at 31 July 2018
VMD Organogram as at July 2018
