Investigation into monkeypox outbreak in England: technical briefing 5
Updated 19 December 2024
Applies to England
The UK Health Security Agency (UKHSA) is working with the NHS and the public health agencies of the 4 nations to investigate the monkeypox outbreak. This briefing is produced to share data useful to other public health investigators and academic partners undertaking related work. It includes early evidence and preliminary analyses which may be subject to change.
Data reported in the technical briefing is as of 1 August 2022 (or as specified in the text) to allow time for analysis.
Potential levels of the outbreak in England
The outbreak can be considered to fall into one of 4 potential levels of transmission:
Level 1
Incursions from rest of the world – small numbers of imported cases with limited onward transmission.
Level 2
Transmission within a defined sub-population.
Level 3
Transmission within multiple sub-populations or larger sub-population.
Level 4
Wider significant community transmission – with potential for endemic and local epi-zoonotic disease.
These may be refined with better understanding of modes of transmission.
At present, England is judged to be in level 2.
Part 1. Risk assessment as of 1 August 2022
UK trajectory
Nowcast and growth
Over the past couple of weeks there has been approximately no change in the daily number of confirmed cases. Case ascertainment continues to challenge our understanding of the outbreak.
Assessment (confidence): zero (or low) daily case growth (high).
Geographic spread
The proportion of cases resident in London was more than 75% from the start of the incident up to 20 June 2022. This proportion stayed at around 66% in July 2022.
Assessment (confidence): remaining concentrated in London (moderate) but with evidence of increasing geographic spread (low).
Ascertainment
Whilst testing is freely available and a mixed population group is being tested, it is likely that there is under ascertainment. Atypical symptoms, including single or scarce lesions, are reported and there are international reports of subclinical infection.
Assessment (confidence): insufficient information.
UK transmission
Outbreak level
Level 2 is defined as transmission within a defined sub-population, currently gay, bisexual and men who have sex with men (GBMSM) connected by sexual networks. Enhanced surveillance data does not suggest a change in case mix, although it is available on only 26% (703 out of 2,638) of cases and may not be representative of the whole cohort. There are 22 women with confirmed or highly probable monkeypox in England (out of 2,615 with known gender). There is no robust evidence of sustained transmission outside some sexual networks of GBMSM, although the increase in female cases requires close surveillance.
Assessment (confidence): level 2 (moderate).
Route of transmission
Whilst the primary reported route is through close or sexual contact, monkeypox virus has been detected in air and environmental samples in the hospital room of infected patients. There are no confirmed instances of airborne transmission. Limited household transmission has been described in the UK. Detailed investigations of some cases have found small numbers with no known route of acquisition, due to reporting no sex or other potential exposures during their incubation period.
Assessment (confidence): transmitting primarily through close or sexual contact (moderate).
Severity
Observed clinical severity
There are no reported deaths in the UK. There is significant morbidity in the majority of people who are admitted to hospital for clinical care reasons, including severe pain and complications due to secondary bacterial infection.
Assessment (confidence): in current population group, low mortality but significant morbidity (moderate). In wider population, insufficient data.
Virological characterisation
UK genomic data is now available. The majority of outbreak cases are currently in a distinct lineage (B.1) which has mutations of unknown significance when compared with the closest previously characterised monkeypox virus genomes. A small number of cases, including 2 UK cases and some international cases, are in lineage A.2. Phylogeny suggest there may be under sampled circulation of this lineage alongside B.1, and this virus and outbreak requires separate characterisation. There is evidence suggestive of sustained human transmission prior to the 2022 outbreak but not clear-cut evidence of adaptation for improved human transmission. There is no phenotypic data available for this clade to date.
Assessment (confidence): insufficient data.
International trajectory
Global growth
There is now indication of varying trajectories in different countries, with several countries probably having a plateau in daily cases, and some continuing to have increasing number of daily cases. Given the varying reporting practices globally, further time is needed to confirm changes in trajectory.
Assessment (confidence): the outbreak continues to grow in some countries, with indications of plateauing or decline in others (moderate).
International transmission
Routes of transmission reported by other countries
Cases in previously non-endemic countries are reported as primarily in GBMSM. Small number of cases are reported in women and children. In countries with a longer history of monkeypox, apparent wider population transmission is reported with unclear routes. Phylogenetic data suggests that human transmission has been occurring for a number of years in some areas prior to the appearance of the outbreak clade and there is at present no reason to assume that the epidemic as a whole will naturally reduce without interventions globally.
Assessment (confidence): transmitting primarily by close or sexual contact in non-endemic countries (low), unknown transmission routes in endemic countries.
Part 2. Research and evidence gaps prioritisation and activity
2.1 Identification of research and evidence questions
UKHSA has carried out a research and evidence gaps analysis relating to the monkeypox outbreak in the UK. We are working collaboratively with academic partners, including National Institute for Health and Care Research Health Protection Research Units (NIHR HPRU) and national research funders, to develop and implement rapid studies to address these. The priority evidence gaps are shown in Table 1 and a full list of research questions developed with partners and shared with funding bodies in Table 2.
Table 1. Priority research and evidence gaps
| Research topic | Priority evidence gaps |
|---|---|
| Surveillance | Levels of undiagnosed disease Determining the start of the outbreak Trends Level of asymptomatic infection Wastewater surveillance |
| Transmission dynamics | Transmission risk to contacts Modes of transmission |
| Biological characterisation and virology | Genome sequencing and in-host variation Viral dynamics Virus characterisation, including biological significance of mutations |
| Clinical characterisation | Clinical presentation and outcomes. Groups at risk of worse outcomes |
| Vaccine response and immunology | Immune response to infection and vaccines Immunological correlates of protection Post-implementation effectiveness studies |
| Therapeutics | Is post exposure prophylaxis tecovirimat better or worse than vaccine? Does tecovirimat help with reducing isolation period? Does early treatment reduce risk of transmission? Impact on disease progression – severe disease |
| Diagnostics and evaluation | Best site to test Home sampling and testing Evaluation of Lateral Flow Devices (LFDs) Development of serology test |
| Evaluation of other interventions | Effectiveness of contact tracing |
| Behavioural and other social sciences | Public perception of risk Public understanding of disease Help seeking behaviour Vaccine acceptability Adherence to self-isolation Media coverage, behaviour and stigmatisation |
| Longer term consequences of infection | Are there longer-term consequences of infection? |
| Other | Reverse zoonosis risk |
Table 2. Monkeypox research questions
| Theme | Questions |
|---|---|
| Surveillance | How much undiagnosed disease is there? Prevalence How many cases could have been missed due to a presumption of a different cause, for example syphilis, herpes, chickenpox? Risk factors for infection When was virus introduced? |
| Surveillance | Understanding the extent of spread and assessing potential approaches to surveillance Genomic Analysis of Wastewater from sites under investigation – direct deep sequencing Can the virus be detected in wastewater? Can wastewater be used to monitor epidemic? |
| Transmission dynamics | What proportion of cases are infected in UK? What is the infectious period? What is the incubation period? Analysis of routine case and contact tracing data (and co-creation of enhanced contact cluster intelligence with key community, venue and event organisers) |
| Transmission dynamics | Risk of transmission to different groups on the population and contact categories. Risk of transmission in flights |
| Transmission dynamics | Can phylogeny provide any information on transmission? What information can we gain about intrinsic transmissibility of West African clade? Sequence and analysis. Is there any evidence that this is a new clade or that there has been any biologically significant change compared to previously described West African monkeypox? – In host variation |
| Transmission dynamics | Is there pre-symptomatic or prodromal transmission? How much transmission is asymptomatic, presymptomatic or symptomatic? Viral presence in semen |
| Transmission dynamics | What are the likely modes of transmission, including is there evidence of airborne transmission? Fomites Role of large droplets, aerosols and steam rooms Decontamination and mitigations – what is effective and needed in different settings? |
| Transmission dynamics | Risk to UK domestic animals Understanding reverse zoonotic risk, possible animal reservoirs |
| Transmission dynamics | Inference of biological characteristics from epidemic trajectories and mitigation efforts through modelling. Can interrupted chains of transmission inform biological characteristics? |
| Biological characterisation and virology | Viral dynamics |
| Clinical characterisation | What are the symptoms and should the case definition be refined? |
| Clinical characterisation | Does the current syndrome differ from the classical description of West African monkeypox? |
| Clinical characterisation | Do the outcomes (including critical illness and fatality) differ from the existing descriptions of West African monkeypox? |
| Clinical characterisation | Is more severe disease experienced by any population subgroup? |
| Clinical characterisation | What are the risks in pregnancy? |
| Clinical characterisation | Do different ages or demographics differ on clinical presentation? Does that lead to under- ascertainment of cases? |
| Vaccine response and immunology | What is the duration of the serological response to Imvanex after 1 or 2 doses? What are the immune responses to infection and vaccination? |
| Vaccine response and immunology | Intrinsic and innate immune barriers to monkeypox |
| Vaccine response and immunology | What are the immunological correlates of protection including cell-mediated immunity after vaccination, protection from smallpox vaccine? |
| Vaccine response and immunology | What is post-implementation vaccine effectiveness for the currently circulating strain? How do we deploy vaccine to maximise benefit? Effectiveness of vaccine for pre-exposure prophylaxis |
| Therapeutics | Is post-exposure prophylaxis tecovirimat better or worse than vaccine? Does tecovirimat help with reducing isolation period? Does early treatment reduce risk of transmission? Impact on disease progression – severe disease (occurring or expected, for example, immunosuppressed) |
| Therapeutics | Evaluation of other antivirals |
| Therapeutics | Pharmacokinetics and pharmacodynamic studies; either as part of clinical trials above, or possibly separate earlier phase studies in specific vulnerable populations (where the dosing is unclear) |
| Diagnostics | Best site to test, which site goes positive first |
| Diagnostics | What would enable home or point-of-care sampling and testing, including self-testing? Evaluation of swabs with inactivating buffer to enable community testing |
| Diagnostics | Development of serology test. |
| Diagnostics | Rapid LFD antigen test development and validation |
| Evaluation of other interventions | What is the effectiveness of contact tracing and activation? How do interventions act synergistically to reduce onward transmission? |
| Behavioural and other social sciences and equalities considerations | How are the public and affected groups perceiving the risk from monkeypox? What is the public understanding of the disease and What actions they need to take? What proportion of symptomatic cases seek care? Vaccine acceptability and intended or actual uptake What are current levels of adherence to self-isolation? What factors are associated with poor adherence or successful adherence to self-isolation? Is length of self-isolation a deterrent to help seeking? What factors are associated with compliance with other control measures? What factors are associated with effective symptom recognition and help-seeking when symptoms develop? How has media coverage of this as a sexual health problem affecting gay, bisexual, and other men who have sex with men (GBMSM) in particular affected public responses, including health seeking behaviours? How does the gay community wish to self-organise monkeypox transmission prevention and control? What characterises monkeypox misinformation and what are the scalable countermeasures? What are the potential harms from stigmatising monkeypox communications attached to specific risk groups? Impact of vaccination strategies |
| Longer term consequences of infection | What are the longer-term consequences of infection? Cohort for long-term follow up? Including: what are the consequences of infection in pregnancy, infancy or childhood? |
| Other | Systems context – how can we optimise surveillance, understanding transmission, destigmatised testing, behavioural insights, targeted vaccination, community involvement and so on as a system of action-research? In particular, bringing more behavioural understanding into adaptive observation or sampling to understand transmission? |
| Other | What can we learn from evaluation of the response to monkeypox to enhance our responses to future outbreaks of concern? Include consideration of mechanisms for collaborative working with international partners |
| Other | Are there any reasons to believe that this outbreak may be in some way related to coronavirus (COVID-19) and/or factors related to GBMSM? |
2.2 Studies underway to address identified gaps
UKHSA has developed proposals to address specific research and evidence questions related to monkeypox, both as leaders and in collaboration with partners, including NIHR HPRUs. Any queries about research in UKHSA should be directed to the Monkeypox Research and Science cell.
Studies led by UKHSA include:
- monkeypox virus culture from longitudinal samples from 7 patients to determine risk of onwards transmission, funded by the Healthcare Infection Society
- assessment of monkeypox levels in wastewater to assess incidence in community
- prevalence of monkeypox infection in England
- measuring environmental contamination with monkeypox virus in healthcare and other settings
- estimating the risk of transmission to contacts of monkeypox cases (in collaboration with the Behavioural Science and Evaluation HPRU)
- monkeypox diagnosis via near-patient lateral flow device (LFD)
- assessment of the duration of protective immunity to currently circulation monkeypox
- development of serology tests to increase diagnostic capacity
Other studies underway and in planning with UKHSA involvement include those designed to answer the following questions:
- risk of transmission to different population groups (in collaboration with the Behavioural Science and Evaluation HPRU)
- understanding transmission dynamics including infectivity and incubation period
In addition, UKHSA and the Medicine and Healthcare products Regulatory Authority (MHRA) have also received funding from the Coalition for Epidemic Preparedness Innovations) (CEPI) for the development of a monkeypox antibody standard and assays:
The tools CEPI is supporting the development of are: assays, with UKHSA, to test for the presence of specific antibodies - indicative of an immune response to monkeypox - following either natural infection or vaccination. A reference antibody standard, with the UK MHRA, to harmonise how different laboratories assess the strength and duration of immune responses generated by current vaccines and those in development against monkeypox.
2.3 Published literature
UKHSA researchers have authored or contributed to peer-reviewed publications for the current incident response. These can be accessed via the UKHSA Research Portal.
Part 3. Epidemiology update
3.1 Current epidemiological situation
Cases of monkeypox infection were confirmed in England from 6 May 2022. As of 1 August 2022, there were 2,759 laboratory-detected cases of monkeypox in the UK. This includes 2,672 confirmed and 87 highly probable cases, collectively referred to as ‘cases’ in this briefing for simplicity and defined respectively as those who tested positive with a monkeypox and orthopox polymerase chain reaction (PCR) test. Positive results for orthopox, the genus of viruses that includes monkeypox, are considered highly probable due to the rarity of other orthopox viruses in the UK.
Of all cases, 65 were in Scotland, 24 were in Northern Ireland, 32 were in Wales and 2,638 were in England. The median age of cases in the UK was 37 years old (interquartile range 31 to 44).
Table 3. Number of confirmed and highly probable monkeypox cases by devolved administrations, May 2022 to 1 August 2022
| Devolved administrations | Total | Confirmed | Highly probable |
|---|---|---|---|
| England | 2,638 | 2,551 | 87 |
| Northern Ireland | 24 | 24 | 0 |
| Scotland | 65 | 65 | 0 |
| Wales | 32 | 32 | 0 |
| Total | 2,759 | 2,672 | 87 |
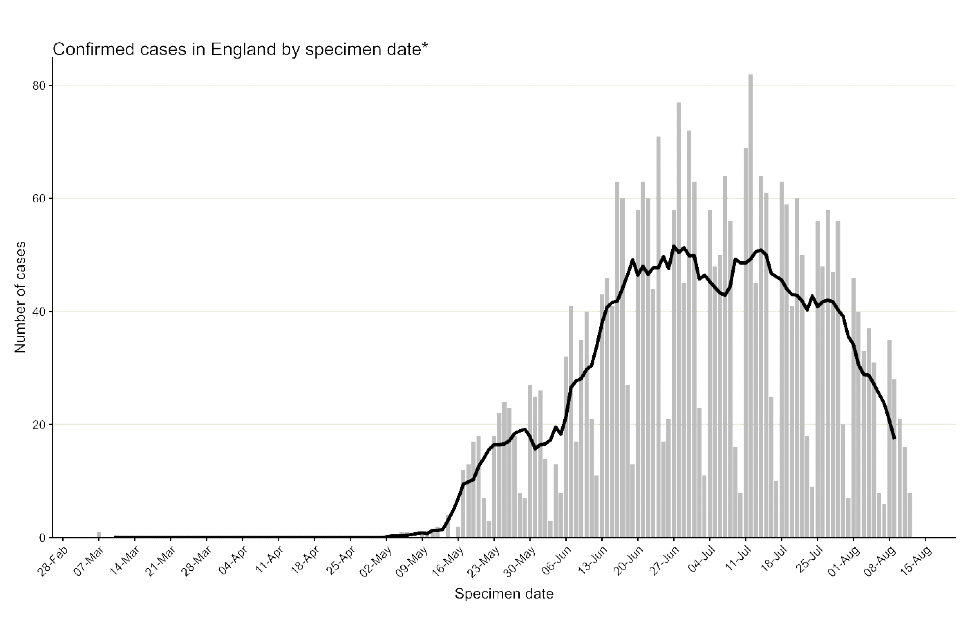
Figure 1 shows the specimen dates of monkeypox cases over time in England. While the first reported case in England was identified 6 May 2022 in a traveller from an endemic country for monkeypox, the earliest known case in England has a specimen date of 7 March 2022. This case was identified in late July through retrospective testing of a residual sample. An additional small number of cases have also been detected with specimen dates in May 2022 but with symptom onset dates in early April 2022. Some of these early cases had no travel history in their incubation period.
Figure 1. Incidence of confirmed and highly probable monkeypox cases by specimen date in England as of 4 August 2022
Where specimen date is missing, the date the laboratory received the specimen is used. The data used in this graph can be found in the accompanying spreadsheet]

In England, a high proportion of cases were known to be London residents (overall 73%, 1,906 out of 2,626 with reported home address), see Table 4. The number of cases in other regions has been steadily increasing, particularly in the South East and North West of England (Figure 2). The proportion of cases in London has stayed around 66% in July 2022 (Figure 3).
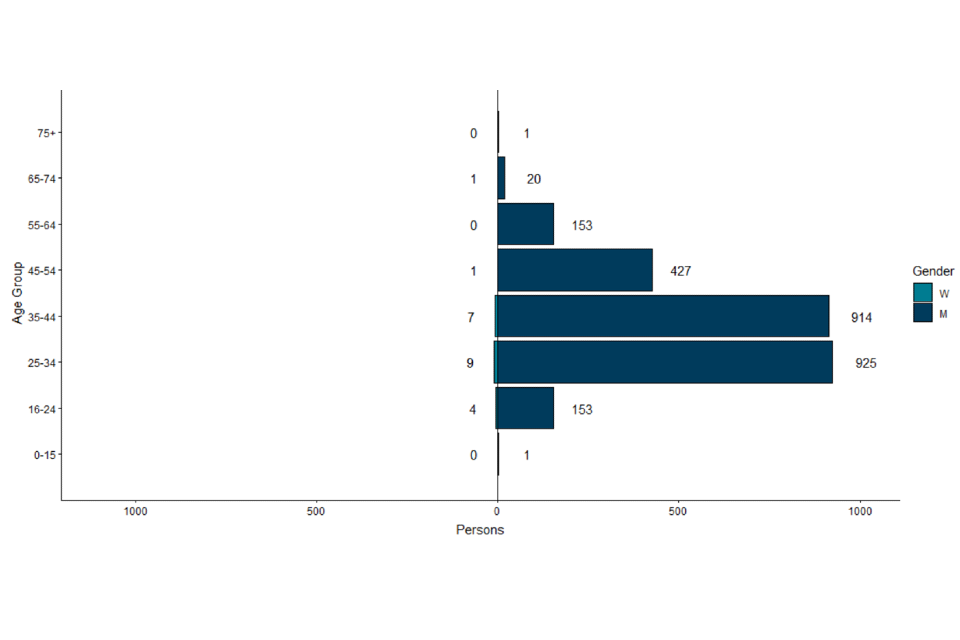
Where gender information was available, 2,593 out of 2,615 (99.1%) cases in England were men, and 22 were women (Figure 4).
Table 4. Number of laboratory confirmed and highly probable monkeypox cases by region of residence, England, May 2022 to 1 August 2022
| Region of residence | Total confirmed |
|---|---|
| East of England | 102 |
| East Midlands | 33 |
| London | 1,906 |
| North East | 29 |
| North West | 144 |
| South East | 233 |
| South West | 54 |
| West Midlands | 76 |
| Yorkshire and Humber | 49 |
| Unknown | 12 |
| Total | 2,638 |
Figure 2. Cumulative count of confirmed and highly probable monkeypox cases by region of residence in England as of 1 August 2022*
The data used in this graph can be found in the accompanying spreadsheet.
*If residential postcode is known, then region of residence is used, otherwise the region of the healthcare facility where testing occurred at is used
Figure 3. Seven-day rolling average of the percentage of confirmed and highly probable monkeypox cases in London, by specimen date
The data used in this graph can be found in the accompanying spreadsheet.
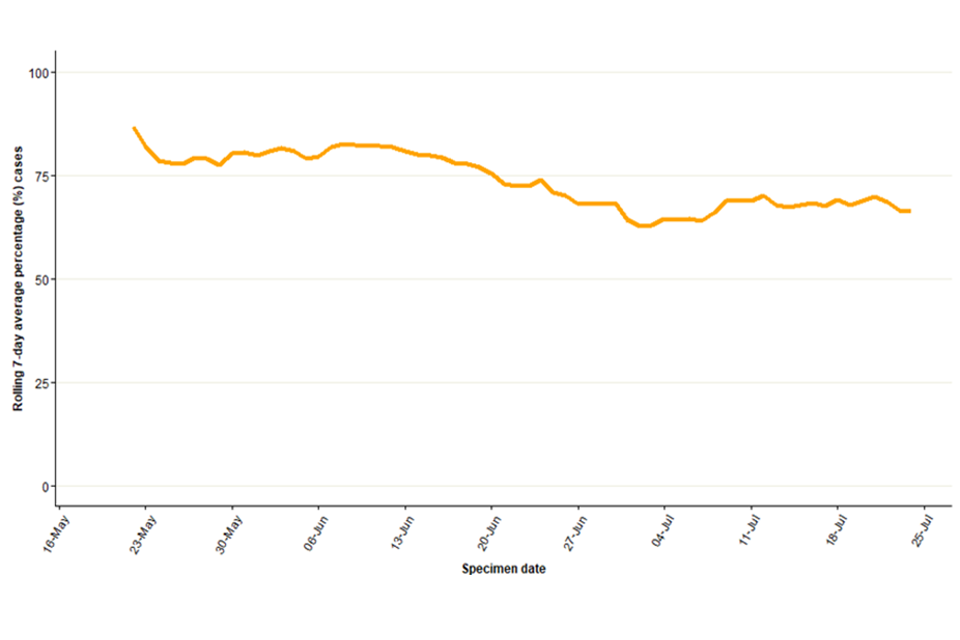
Figure 4. Age and gender distribution of confirmed and highly probable monkeypox cases in England as of 1 August 2022
The data used in this graph can be found in the accompanying spreadsheet.
3.2 Findings from enhanced surveillance questionnaires
Of the 2,638 cases identified in England up to 1 August 2022, a total of 703 (26%) had completed enhanced surveillance questionnaires. This includes data collected via rapid sexual health questionnaires administered during the initial weeks of the outbreak (used from 27 to 31 May 2022), questionnaires completed by health protection teams during telephone interviews (used from 1 to 24 June 2022), and self-completed questionnaires sent electronically to cases (used from 25 June 2022 onwards). Information from enhanced questionnaires should be interpreted with caution, due to the low uptake and because uptake has changed overtime. People completing questionnaires may not be representative of all those affected.
Most respondents were of white ethnicity (76%), followed by mixed (9%) and Asian (7%) ethnicities. Additionally, most cases were born in the UK or other European countries, although 10% reported being born in Latin American or Caribbean countries.
Table 5. Ethnicity and region of birth from enhanced surveillance questionnaires in confirmed monkeypox cases in England as of 1 August 2022
N=703
| Ethnicity | Count | Percentage (%) |
|---|---|---|
| White | 537 | 76.4 |
| Mixed | 64 | 9.1 |
| Asian | 50 | 7.1 |
| Black | 30 | 4.3 |
| Other | 1 | 0.1 |
| Unknown | 21 | 3.0 |
| Region of Birth | Count | Percentage (%) |
|---|---|---|
| UK | 364 | 51.8 |
| Europe | 148 | 21.1 |
| Latin America and the Caribbean | 72 | 10.2 |
| Asia | 42 | 6.0 |
| Africa | 22 | 3.1 |
| Oceania | 16 | 2.3 |
| Northern America | 12 | 1.7 |
| Unknown | 27 | 3.8 |
As shown in Table 6 and reported in previous technical briefings, monkeypox is predominantly being transmitted in interconnected sexual networks of GBMSM: the majority of cases report multiple sexual partners in the last 3 months, history of a sexually transmitted infection (STI) in the last year, and/or use of human immunodeficiency pre-exposure prophylaxis (HIV PrEP).
Table 6. Selected epidemiological metrics from enhanced surveillance questionnaires in confirmed monkeypox cases in England as of 1 August 2022
N=703, some metrics have smaller denominators due to missing values
| Metric | Count | Denominator (excluding cases with missing responses) | Percentage (%) |
|---|---|---|---|
| Gay, bisexual, or men who have sex with men | 648 | 680 | 95.3% |
| History of STI in the last year | 369 | 685 | 53.9% |
| One or no sexual partners in last 3 months | 96 | 690 | 13.9% |
| 10+ sexual partners in last 3 months | 210 | 690 | 30.4% |
| Living with HIV | 172 | 661 | 26% |
| Ever used PrEP (among HIV negative) | 373 | 474 | 78.7% |
Figure 5 shows trends of the selected epidemiological metrics over time, among cases with completed questionnaires and with symptom onset dates between epidemiological weeks 18 to 28 (from 1 May 2022 to 16 July 2022). Cases with onset dates before or after these dates were excluded from these analyses due to small numbers. These metrics were consistent over time, suggesting transmission of monkeypox virus in England has continued to occur in defined sexual networks of GBMSM.
More detailed analyses of questionnaire data, in combination with manual review of case management notes, was undertaken to describe the small number of cases that are known to not be GBMSM including possible routes of acquisition. Of 22 cases who were women, 9 (41%) were transgender women. From all 22 women, 8 had evidence of possible transmission during sexual contact, 5 appeared to have non-sexual routes of acquisition (either household or no identified exposure route), and 9 had no information on transmission. Additionally, there were 15 cisgender heterosexual men who had not reported having sex with another man in the past 21 days. Of these, a small number had evidence of possible transmission during sexual contact with cisgender women.
Figure 5. Trends of selected epidemiological metrics in monkeypox cases with completed questionnaires, by week of symptom onset in England, epidemiological weeks 18 to 26 (1 May 2022 to 16 July 2022)
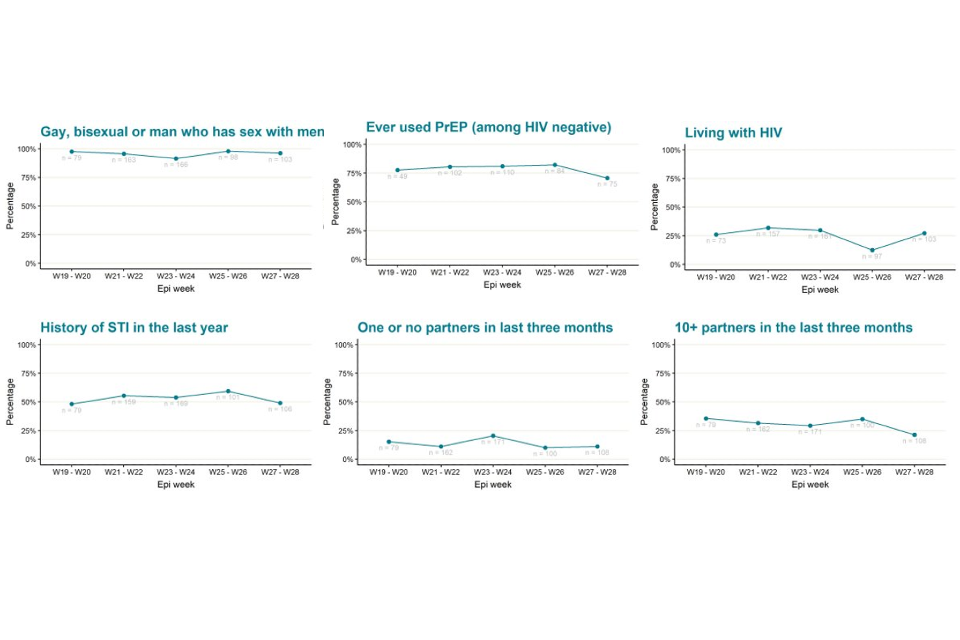
Supplementary data is not available for this figure. Due to delays between symptom onset and questionnaire completion, data for the most recent epidemiological weeks may change and should be interpreted with caution.
3.3 Testing data
Testing for monkeypox in the UK is conducted by the UKHSA Rare and Imported Pathogen Laboratory (RIPL) as well as several other NHS and private laboratories that have begun using monkeypox or orthopox PCR tests since early July 2022.
Testing analyses are available for RIPL, although these are currently not representative of testing across the UK and likely to under-represent testing in London.
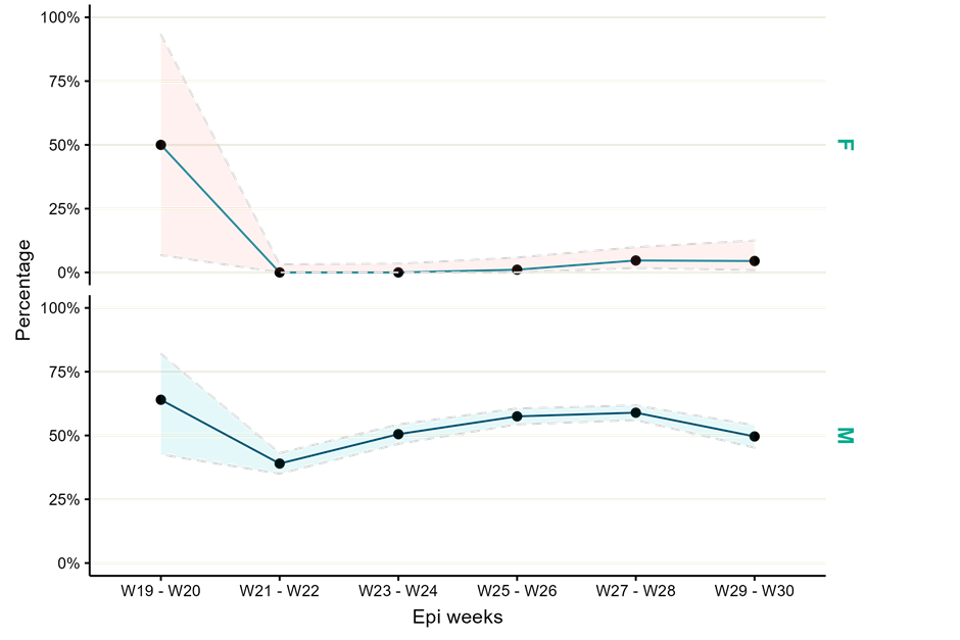
Up to 29 July 2022, 4,921 monkeypox tests were carried out in RIPL, of which 48% (2,204) were positive. Figure 6 shows positivity by sex. Positivity in females has increased slightly in recent weeks, whereas positivity in males was higher between weeks 25 and 28 but more recently has decreased to 50%.
Figure 6. Monkeypox test positivity over time among samples tested by UKHSA RIPL by sex, 1 May to 29 July 2022
Part 4. Transmission dynamics
There is additional evidence that transmission has slowed since technical briefing 3. Nowcasting by both specimen date and reporting date suggests the epidemic has plateaued.
4.1 Nowcasting by specimen date
As the epidemic has grown, the availability of data with recorded symptom onset date has declined. This biases the epidemic curve by symptom onset date, since the proportion of cases being recorded will decay over time, even when adjusting for reporting delays. Therefore, here we model the epidemic using specimen date, which is the date the sample is collected from a patient. This reflects an epidemic curve lagged by the delay between infection and seeking healthcare.
Visualising the epidemic curve by specimen date is affected by the delay from specimen date to the case being reported to UKHSA. To correct for this delay, nowcasting methods adjust the observed data by the distribution of reporting delays to create predictions of the actual frequency. We use a generalised additive model to nowcast current cases and apply this to non-travel associated cases in England.
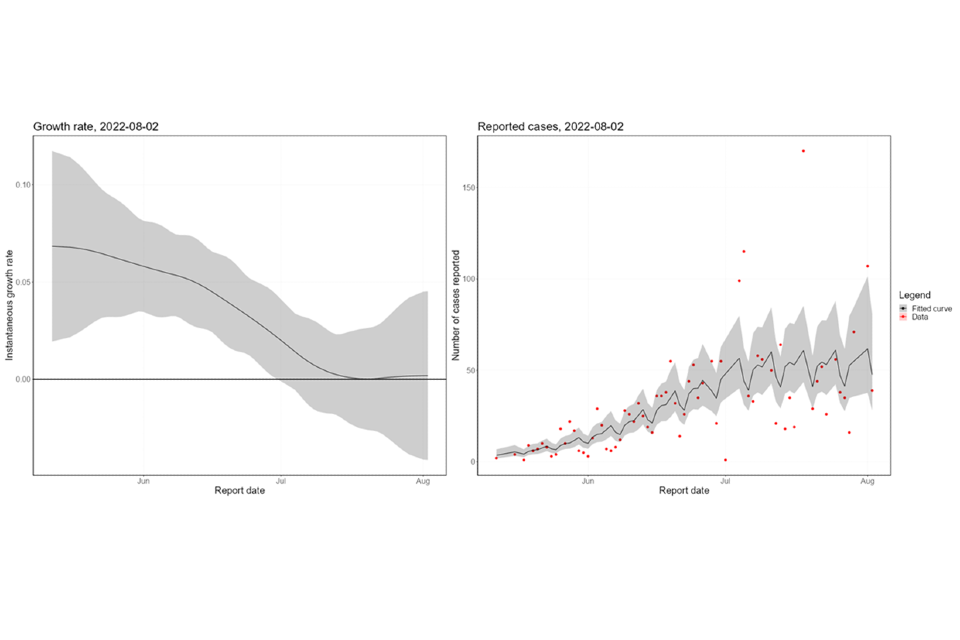
The nowcasting suggests that the growth in confirmed daily cases is zero, albeit with some uncertainty (Figure 7), with growth rates estimated between a 39-day doubling time and a 35-day halving time. Negative doubling times represent halving times. The nowcast estimate of cases is around 35 per day. The swathe that spans zero shows growth has slowed and is now consistently close to zero.
Figure 7. 7a shows estimates of nowcast growth rate and incidence by specimen date, 7b shows estimates of the growth rate and incidence by report date of monkeypox cases in England
The charts exclude cases associated with travel. The shaded area is the 90% Credible Interval (CrI). The dark shaded area is the 50% CrI and the lighter area is the 90% CrI. Note: y-axis denotes incidence of cases by specimen date. Supplementary data is not available for these figures.
Figure 7a
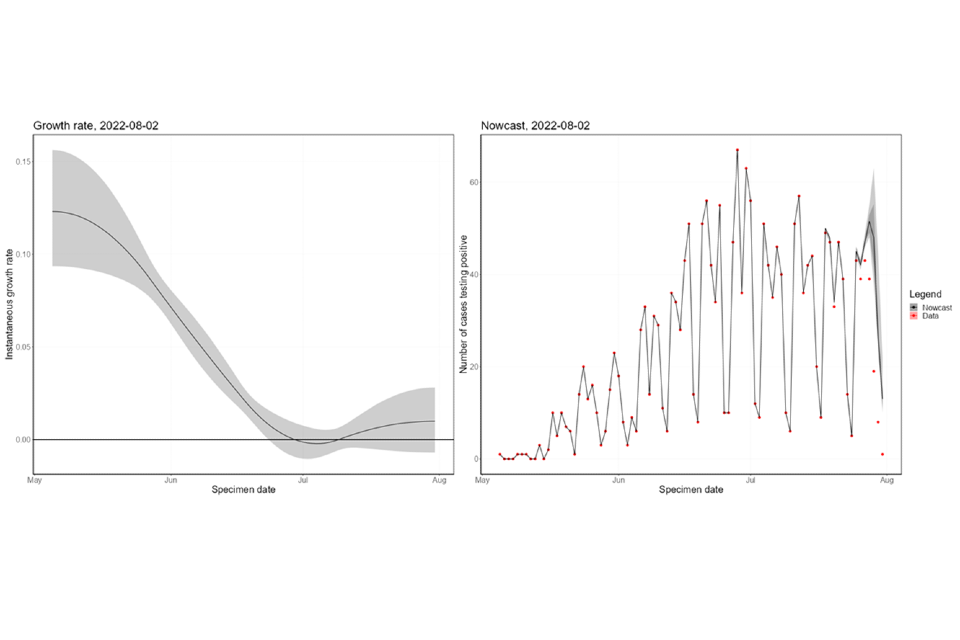
Figure 7b
4.2 Temporal trends in gender distribution
Thus far, the majority of cases have occurred in defined networks of GBMSM. To assess whether the outbreak is moving away from this initial subpopulation, we investigate temporal trends in the proportion of cases that do not identify as women. Assuming Monkeypox is being transmitted in sexual networks of GBMSM, the proportion of cases that identify as women should remain constant (allowing for random fluctuations in the observed proportion).
A small number of sporadic transmission events from GBMSM networks to women is expected. However, if the proportion of cases in women is increasing, this could suggest that there is sustained transmission outside GBMSM networks. If the proportion of cases in women with no known links to GBMSM networks is increasing, that could be even stronger evidence of sustained onward transmission.
To assess the evidence, we construct a null hypothesis of no change in the transmission network based on the gender-distribution of cases over the last 6 weeks. We then adjust this observed proportion to the number of cases in the current week, to create a 95% confidence region where we would expect the currently observed gender-distribution to lie if it follows the null hypothesis. By overlaying the currently observed gender-distribution, we can identify statistically significant perturbations. Note that although statistically significant, these perturbations may not reflect underlying transmission changes, since it could also be affected by changes in the relative ascertainment of cases in women relative to men, or unknown cases. We plot the statistical test as a rolling function of time for each week of the outbreak.
The proportion of cases in women is currently consistent with the null hypothesis, so there is insufficient evidence to support a change in the transmission dynamics (Figure 8). This is true whether cases in women which have a known link to a GBMSM primary case are included or excluded. However, over the last few weeks the proportion of cases in women has been increasing, so this trend needs to be monitored closely.
Figure 8. Binomial probability of male cases using a binomial exact test
The purple shaded area is the null hypothesis (which implies no change in the rate). The solid black line is the proportion observed in the latest week, plotted by the first day of the focal week.
Plot a) removes 5 cases (at time of analysis) which were known to have a link to a GBMSM primary case. Plot b) analysis including all women.
Figure 8a
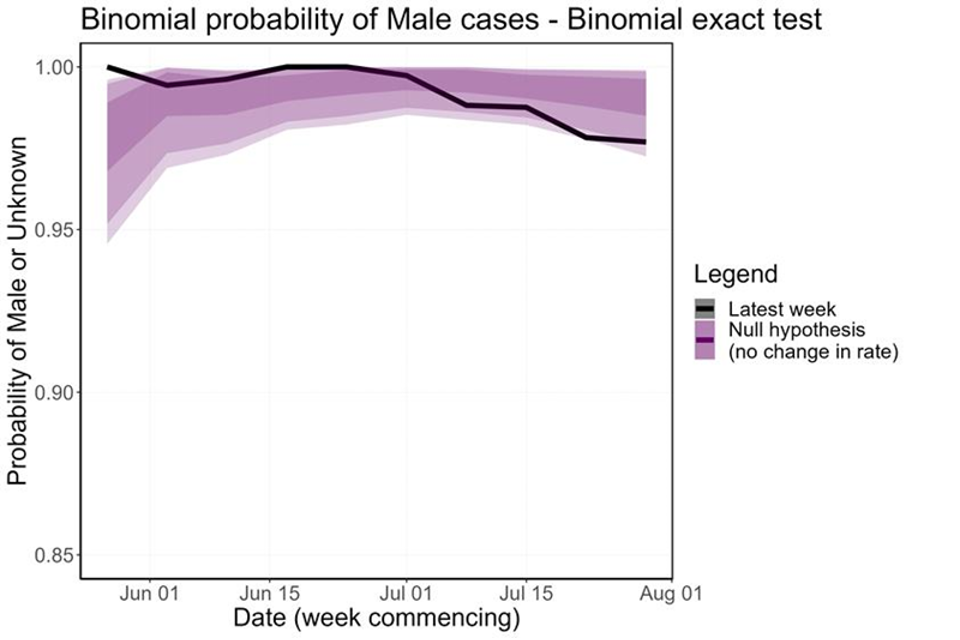
Figure 8b
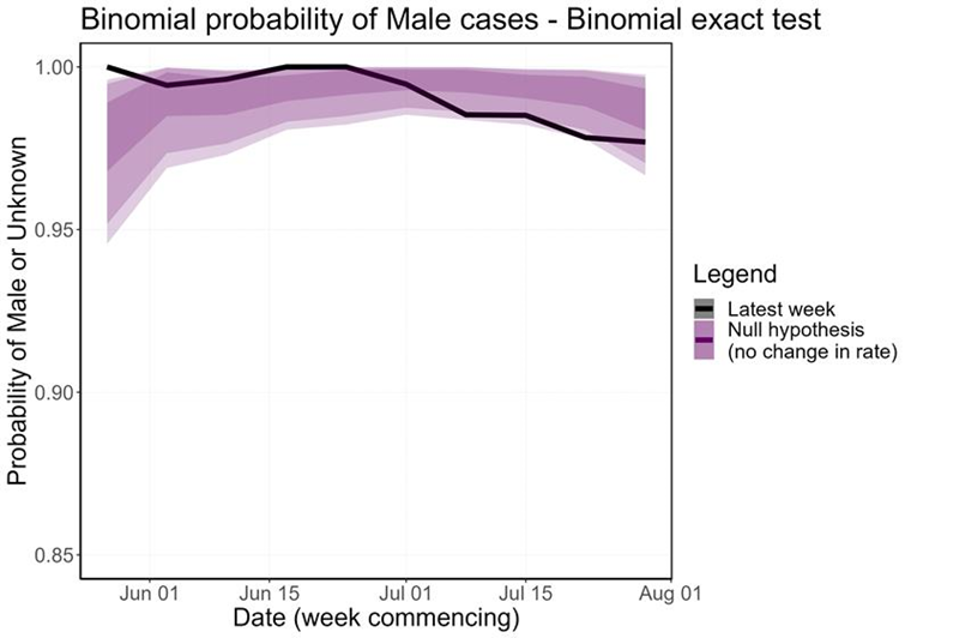
Supplementary data is not available for these figures.
Part 5. UK Genomics data
Summary
- The majority of UK genomes so far sequenced fall in the known outbreak lineage B.1, but 2 are designated as lineage A.2, which may be co-circulating internationally.
- There is evidence of genetic variation within individual lesion samples, which could be either acquired or transmitted diversity.
- This diversity may cause uncertainty in any attempts to undertake detailed molecular epidemiology at present.
- In host diversity requires further detailed exploration.
5.1 Samples sequenced
A total of 87 monkeypox genomes have been produced using Illumina metagenomics. Samples that have a monkeypox PCR cycle threshold (Ct) less than or equal to 20, are pre-treated to remove free nucleic acid before extraction and library preparation. This analysis includes information on the most recent 81 genomes that have been generated. This includes 2 pairs of sequences from the same samples in different runs. Both duplicates produced the same results.
5.2 Genomic diversity
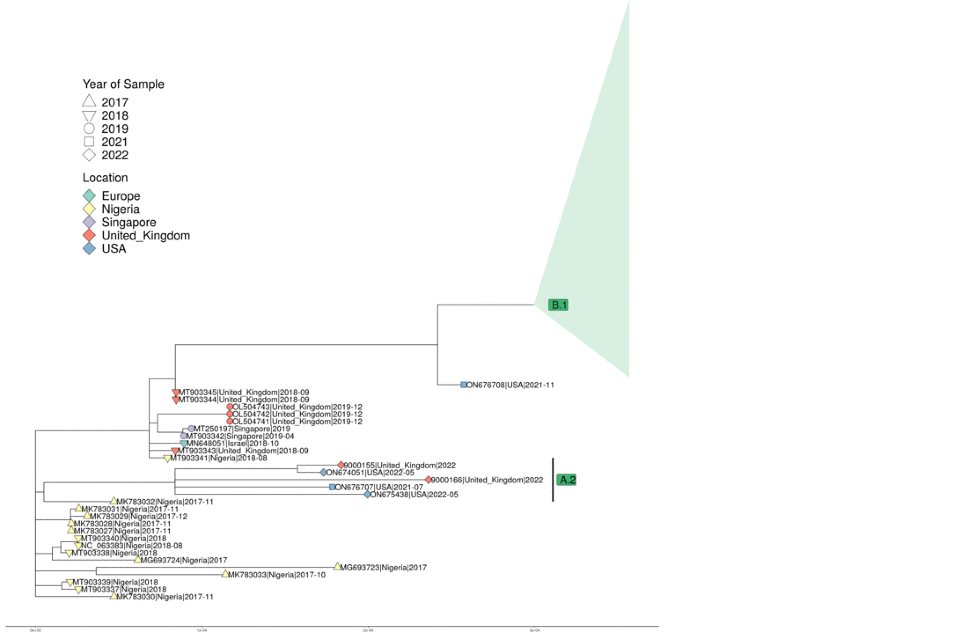
A phylogenetic tree is shown in Figure 9. A total of 79 of the sequences contain the 11 B.1 mutations described in technical briefing 3. This clade has been collapsed in the image due to the large number of sequences within the clade internationally. The remaining 2 sequences belong to clade A.2, which contains 16 mutations not found in other clades. The UK genomes that are within the B.1 clade contain 105 distinct point mutations when compared to ON563414.3. A total of 79 of the mutations (75.24%) are consistent with the action of host apolipoprotein B mRNA editing enzyme catalytic polypeptide 3 (APOBEC3) as described in the linked virological post. A further 15 mutations (14.29%) are either C>T or G>A changes but are not the canonical dinucleotides seen in APOBEC3 related mutations.
Figure 9. Phylogenetic tree of monkeypox genomes
The tree includes a sample of the international data available in Genbank and the 87 UK genomes from the 2022 outbreak, along with some historic samples for context. Clades B.1 and A.2 are labelled on the tree. B.1 (containing 85 UK genomes) has been collapsed due to the large number of sequences available internationally. Sequences are labelled by accession number for publicly available data, and by internal identifier for new UK genomes. Country and specimen collection date are included in the label and indicated by the colour and shape, respectively. Underlying data is not available for this figure.
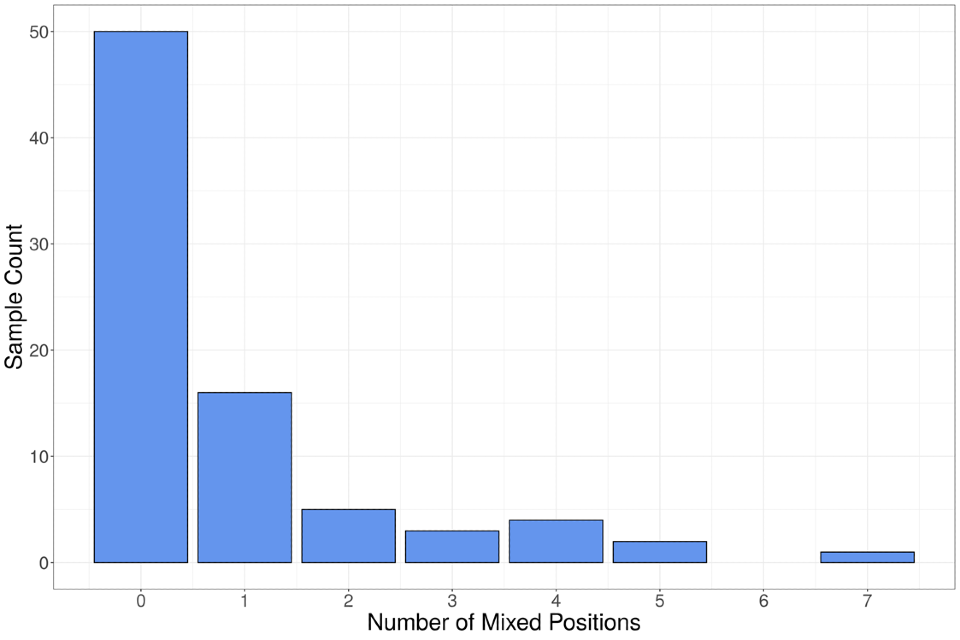
Approximately 38% of samples contain at least one mixed base (Figure 10). Mixtures are called within the sequence data if 2 bases are present at a given position and the total read depth at that position is more than or equal to 20X. Reads must have a MapQ score more than or equal to 30 and individual bases must have a QUAL score more than or equal to 33 to be included in depth calculations. In one set of duplicate sequences, the same 5 mixed positions were observed in both sets of read data. The potential for mixed populations has implications for inferences made about the structure of the B.1 clade. The utility of genomic data in assessing transmission may be reduced as samples from different individuals may have acquired unique mutations in the time between transmission and sampling.
Figure 10. Number of mixed bases observed in UK sequences
The data used in this graph can be found in the accompanying spreadsheet.
Evidence for spontaneous generation of variation within a lesion
Fifty-one of the 61 unique mutations (83.61%) observed at mixed positions are consistent with the action of host APOBEC3. The high-quality thresholds for calling mixed bases, and native nature of the sequencing protocol (that is, no PCR enrichment) combined with the APOBEC3 style mutations supports the hypothesis that diversity is acquired within a lesion. Additionally, sequence data from 2 different swabs taken from the same patient show 2 different sets of mixed bases, suggesting this diversity is acquired within an individual lesion over time.
Sequencing a single lesion may not be representative of the initial viral population acquired by an individual, or similarly of the population subsequently transmitted to contacts.
Detection of minority variants shared with other sequences
Sequencing of a UK sample showed 5 mixed bases at a frequency between 45 and 50% of reads. Four of the 5 mixed positions observed in this sample were present in the consensus sequence of 2 different B.1 subclades. It is not possible at this time to determine the probable cause of this observation, however superinfection, transmission of variation and convergent evolution are all possible explanations. The presence of mixed bases shared with other genomes instead of wildtype or alternate calls in the consensus genomes resulted in uncertainty in the placement of these sequences within phylogenies of the B.1 lineage.
Further sequencing of samples from different lesions or sites (for example, lesion versus blood), and the same lesion over time is required to fully understand the relevance of mixed bases in the genomic data. The detection of mixed bases in 38% of the sequenced samples coupled with the low overall diversity in the B.1 clade resulted in significant uncertainty in any phylogenetic representation of the clade. Uncertainty in the phylogeny will limit the molecular epidemiological inferences that are possible from the genomic data, therefore it is critical, as an initial step, that the extent and mechanisms leading to the generation of intra-host diversity are described.
Sources and acknowledgments
Data sources
Monkeypox virus PCR results are submitted to UKHSA daily by the Rare and Imported Pathogens Laboratory, Porton, UKHSA regional laboratories and NHS laboratories undertaking testing. Data on people testing positive since 6 May 2022 is enhanced with demographic, symptom, epidemiological, and exposure information extracted from the UKHSA Health Protection Team case management system (HPZone), or collected in enhanced surveillance questionnaires.
Enhanced surveillance questionnaires include data collected via rapid sexual health questionnaires administered during the initial weeks of the outbreak (used from 27 to 31 May 2022), questionnaires completed by health protection teams during telephone interviews (used from 1 to 24 June 2022), and self-completed questionnaires sent electronically to cases (used from 25 June 2022 onwards).
Authors of this report
Zahidul Abedin, Carolina Arevalo, Paula Blomquist, Jessica Bridgen, Chloe Byers, Meera Chand, Kristine Cooper, Fergus Cumming, Julie Day, Jake Dunning, Ashley Goddard, Irene Gonsalvez, Alice Graham, Natalie Groves, James Guilder, Susan Hopkins, Luke Hounsome, Christopher I Jarvis, Nicholas Loman, Nicola Love, Richard Myers, Isabel Oliver, Karthik Paranthaman, Mateo Prochazka, Thomas Ward, Nick Watkins, William Welfare, Katie Wrenn.
Contributors
UKHSA Data, Epidemiology and Analytics Cell
UKHSA Research and Science Cell
UKHSA Modelling Cell
UKHSA Genomics Public Health Analysis
UKHSA Sexual Health Liaison Group
UKHSA Monkeypox Incident Management Team
Monkeypox Technical Group
The Monkeypox Technical Group includes members with expertise in clinical infectious diseases, clinical research, epidemiology, genomics, modelling and virology:
- Meera Chand (Chair), UKHSA
- Andre Charlett, UKHSA
- Andrew Rambaut, University of Edinburgh
- Allan Bennett, UKHSA
- Ashley Otter, UKHSA
- Calum Semple, University of Liverpool
- Christophe Fraser, University of Oxford
- Claire Dewsnap, British Association for Sexual Health and HIV
- Claire Gordon, UKHSA
- David Ulaeto, Defence Science and Technology Laboratory
- Erik Volz, Imperial College London
- Emma Thomson, Medical Research Council-University of Glasgow Centre for Virus Research
- Fergus Cumming, UKHSA
- Giri Shankar, Public Health Wales
- Geoffrey L. Smith, University of Cambridge
- Geraldine O’Hara, High Consequence Infectious Diseases Network
- Helen Fifer, UKHSA
- Isabel Oliver, UKHSA
- Jake Dunning, University of Oxford and Royal Free Hospital
- Jason Mercer, University of Birmingham
- Maria Rossi, Public Health Scotland
- Matt Keeling, Warwick University
- Matt Phillips, British Association for Sexual Health and HIV
- Natalie Groves, UKHSA
- Neil Ferguson, Imperial College London
- Nicholas Loman, University of Birmingham and UKHSA
- Obaghe Edeghere, UKHSA
Health and Social Care Northern Ireland
- Richard Myers. UKHSA
- Steven Riley, UKHSA
- Susan Hopkins, UKHSA
- Tommy Rampling, UKHSA
Acknowledgements
The authors are grateful to those teams and groups providing data for these analyses including:
- British HIV Association (BHIVA)
- British Association for Sexual Health and HIV (BASHH)
- Sexual Health Services
- NHS England and Improvement
- High Consequence Infectious Diseases Network
- Public Health Scotland
- Public Health Wales
- Public Health Agency, Northern Ireland
