Investigation into monkeypox outbreak in England: technical briefing 6
Updated 19 December 2024
Applies to England
The UK Health Security Agency (UKHSA) is working with the NHS and the public health agencies of the 4 nations to investigate the monkeypox outbreak. This briefing is produced to share data useful to other public health investigators and academic partners undertaking related work. It includes early evidence and preliminary analyses which may be subject to change.
Data reported in the technical briefing is as of 15 August 2022 (or as specified in the text) to allow time for analysis.
Potential levels of the outbreak in England
The outbreak can be considered to fall into 1 of 4 potential levels of transmission.
Level 1
Incursions from rest of the world – small numbers of imported cases with limited onward transmission.
Level 2
Transmission within a defined sub-population.
Level 3
Transmission within multiple sub-populations or larger sub-population.
Level 4
Wider significant community transmission – with potential for endemic and local epi-zoonotic disease.
These may be refined with better understanding of modes of transmission.
At present, England is judged to be in level 2.
Part 1. Risk assessment as of 15 August 2022
UK trajectory
Nowcast and growth
There is an overall decline in daily cases which suggests that the spread of infection may be reducing. This is subject to uncertainties around ascertainment. Importation is likely to be contributing to case numbers.
Assessment (confidence): Negative daily case growth (moderate)
Geographic spread
The proportion of new cases within London has fallen since late-July.
Assessment (confidence): Cases are increasingly geographically spread (moderate)
Ascertainment
Whilst testing is freely available, testing is likely to be influenced by known risk factors and therefore it is likely that there is under ascertainment. Atypical symptoms, including single or scarce lesions, are reported and there are increasing international reports of subclinical infection.
Assessment (confidence): Insufficient information.
UK transmission
Outbreak level
Level 2 is defined as transmission within a defined sub-population, currently gay, bisexual and men who have sex with men (GBMSM) connected by sexual networks. Enhanced surveillance data does not suggest a change in case mix, although it is available for only 30% (917 out of 3,050) of cases in England and may not be representative of the whole cohort. Out of 3,025 cases with known gender, 99% are men, and there are 36 women. There is no robust evidence of sustained transmission outside some sexual networks of GBMSM, although the increase in cases who are women requires close surveillance.
Assessment (confidence): Level 2 (moderate).
Route of transmission
Whilst the primary reported route is through close or sexual contact, monkeypox virus has been detected in air and environmental samples in the hospital room of infected patients. There are no confirmed instances of airborne transmission. Limited household transmission has been described in the UK. Detailed investigations have found small numbers of cases with no identified route of acquisition.
Assessment (confidence): Transmitting primarily through close or sexual contact (moderate).
Severity
Observed clinical severity
There are no reported deaths in the UK and a small number of deaths reported globally linked to the outbreak. There is significant morbidity amongst people who are admitted to hospital for clinical care reasons, including severe pain and complications due to secondary bacterial infection. Encephalitis has been reported although it appears uncommon.
Assessment (confidence): In current population group, low mortality but significant morbidity (moderate). In wider population, insufficient data.
Virological characterisation
UK genomic data is now available. The majority of outbreak cases are currently in a distinct lineage (B.1) which has mutations of unknown significance when compared with the closest previously characterised monkeypox virus genomes. A small number of cases, including 2 UK cases and some international cases, are in lineage A.2. Phylogeny suggest there may be under sampled circulation of this lineage alongside B.1, and this virus and outbreak requires separate characterisation. There is evidence suggestive of sustained human transmission prior to the 2022 outbreak but not clear-cut evidence of adaptation for improved human transmission. There is no phenotypic data available for this clade to date.
Assessment (confidence): Insufficient data.
International transmission
Routes of transmission reported by other countries
Cases in previously non-endemic countries are reported as primarily in GBMSM. Small number of cases are reported in women and children. In countries with a longer history of monkeypox, apparent wider population transmission is reported with unclear routes. Phylogenetic data suggests that human transmission has been occurring for a number of years in some areas prior to the appearance of the outbreak clade and there is at present no reason to assume that the epidemic as a whole will naturally reduce without interventions globally.
Assessment (confidence): Transmitting primarily by close or sexual contact in non-endemic countries (low), unknown transmission routes in endemic countries.
Part 2. Epidemiology update
2.1 Current epidemiological situation
Cases of monkeypox infection were identified in England from 6 May 2022. As of 15 August 2022, there were 3,195 laboratory-detected cases of monkeypox in the UK. This includes 3,081 confirmed and 114 highly probable cases, collectively referred to as ‘cases’ in this briefing for simplicity and defined respectively as those who tested positive with a monkeypox and orthopox polymerase chain reaction (PCR) test. Positive results for orthopox, the genus of viruses that includes monkeypox, are considered highly probable due to the rarity of other orthopox viruses in the UK.
Of all cases, 75 were in Scotland, 27 were in Northern Ireland, 43 were in Wales and 3,050 were in England.
Table 1. Number of confirmed and highly probable monkeypox cases by devolved administrations, 6 May to 15 August 2022
| Devolved administrations | Total | Confirmed | Highly probable |
|---|---|---|---|
| England | 3,050 | 2,936 | 114 |
| Northern Ireland | 27 | 27 | 0 |
| Scotland | 75 | 75 | 0 |
| Wales | 43 | 43 | 0 |
| Total | 3,195 | 3,081 | 114 |
Figure 1 shows the specimen dates of monkeypox cases over time in England, from the first positive sample taken on 7 March 2022. As reported in technical briefing 5, this case was identified in late July 2022 through retrospective testing of a residual sample; however, the first reported case in England was identified 6 May 2022 in a traveller from an endemic country for monkeypox. An additional small number of cases have also been detected with specimen dates in May 2022 but with symptom onset dates in early April 2022. Some of these early cases had no travel history in their incubation period.
Figure 1. Confirmed and highly probable monkeypox cases by specimen date* in England as of 15 August 2022
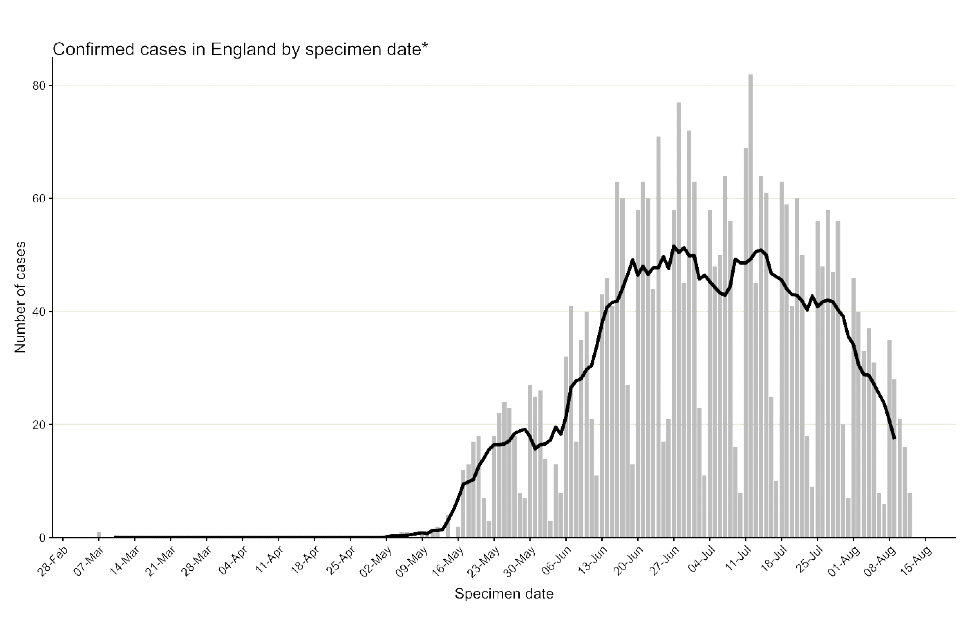
*Where the specimen date is missing, the date the lab received the specimen is used. Where both are missing (mainly among early cases), the date added to the linelist is used (7 out of 3,050).
The data used in this graph can be found in the accompanying spreadsheet.
In England, a high proportion of cases were known to be London residents (overall 71%, 2,141 out of 3,035 with reported home address), see Table 2. The number of cases in other regions has been steadily increasing, particularly in the South East and North West of England (Figure 2). Accordingly, the proportion of cases in London has declined over time, to approximately 58% in August to date (Figure 3).
Where gender information was available, 2,989 out of 3,025 (99%) confirmed cases in England were men, and 36 were women (Figure 4). The median age of confirmed and highly probable cases in the UK was 36 years (interquartile range 31 to 44).
Table 2. Number of confirmed and highly probable monkeypox cases by region of residence, England, 6 May to 15 August 2022
| Region of residence | Total confirmed and highly probable cases | Regional distribution of all cases (% excluding unknown) |
|---|---|---|
| East of England | 109 | 3.6 |
| East Midlands | 43 | 1.4 |
| London | 2,141 | 70.5 |
| North East | 37 | 1.2 |
| North West | 180 | 5.9 |
| South East | 281 | 9.3 |
| South West | 80 | 2.6 |
| West Midlands | 98 | 3.2 |
| Yorkshire and Humber | 67 | 2.2 |
| Unknown | 14 | 0.0 |
| Total | 3,050 | 100.0 |
Figure 2. Cumulative count of confirmed and highly probable monkeypox cases by region of residence in England as of 15 August 2022*
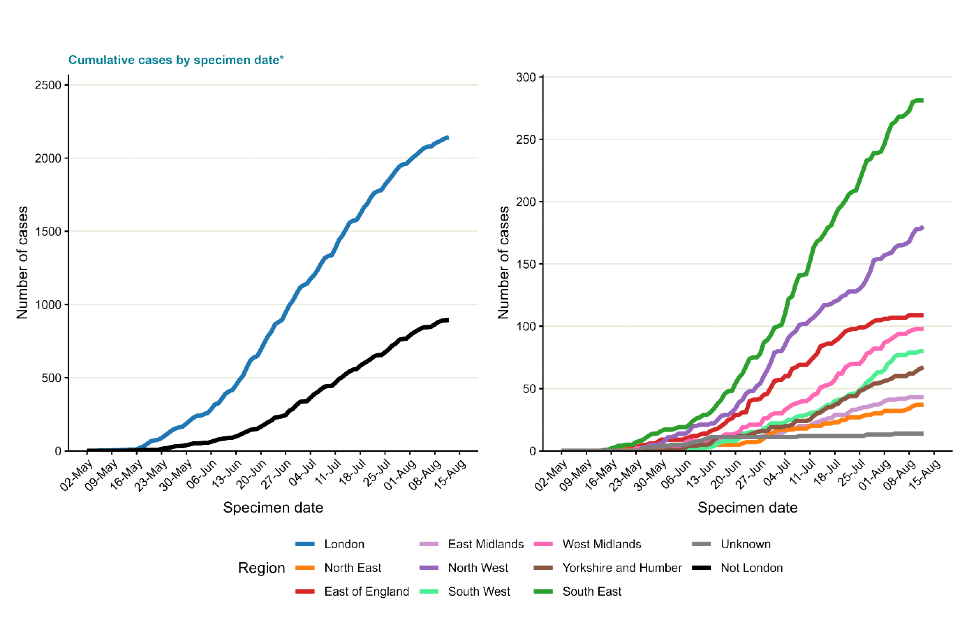
*If the residential postcode is known, then the region of residence is used, otherwise the region of the healthcare facility where the testing occurred at is used.
The case with a retrospective specimen date of March 2022 has been omitted from the chart.
The data used in these graphs can be found in the accompanying spreadsheet.
Figure 3. Seven day rolling average of the percentage of confirmed and highly probable monkeypox cases in London, by specimen date
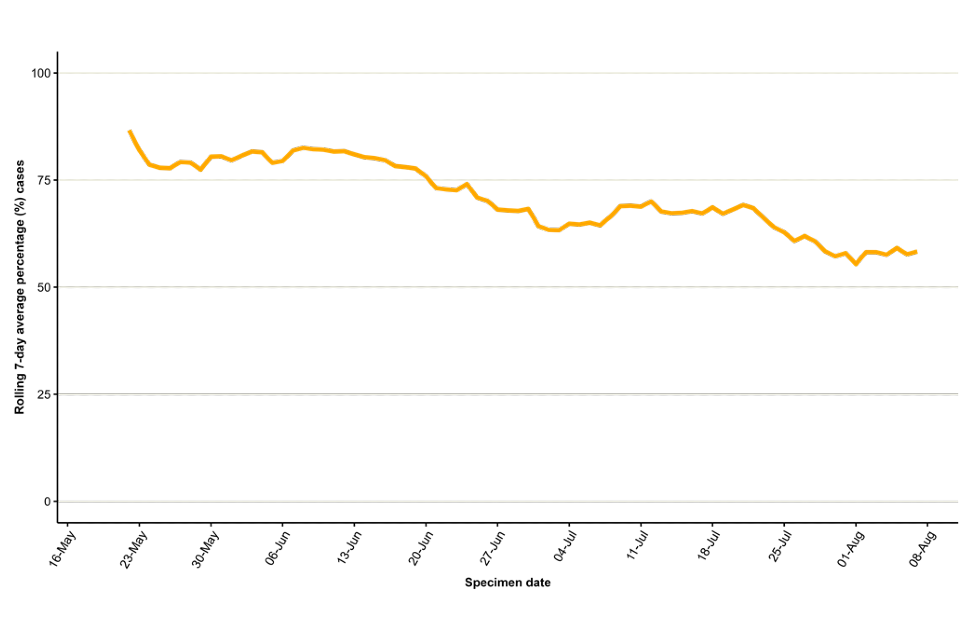
The data used in this graph can be found in the accompanying spreadsheet.
Figure 4. Age and gender distribution of confirmed and highly probable monkeypox cases by lab result date in England as of 15 August 2022
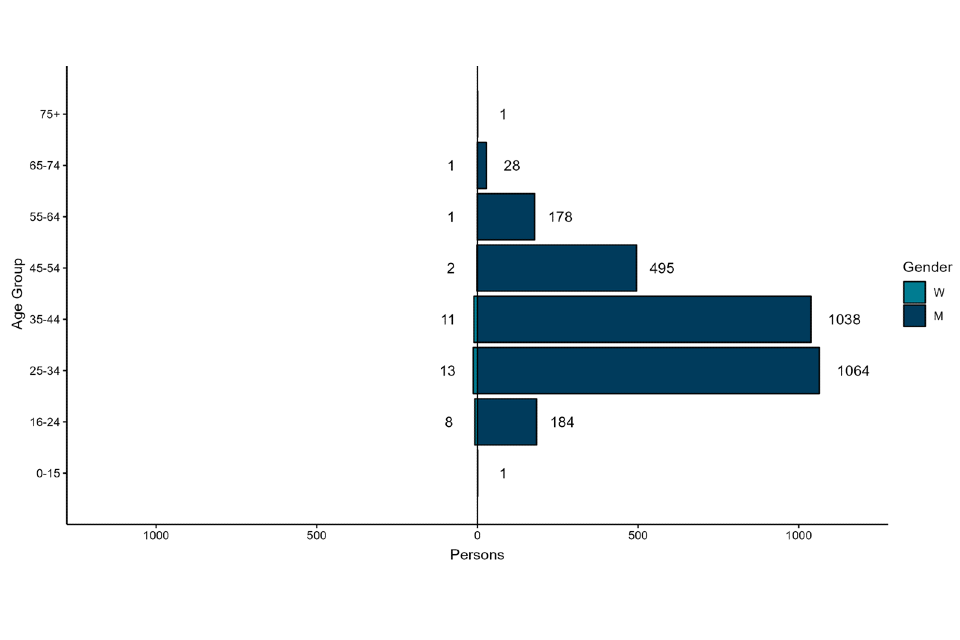
The data used in this graph can be found in the accompanying spreadsheet.
2.2 International travel
In England, 442 (14%) confirmed and highly probable monkeypox cases are known to have travelled internationally within the 21 days prior to symptom onset, as of 15 August 2022. This information is taken from a combination of questionnaire data and data from the public health case management system HPZone.
The majority of these individuals had travelled to Europe (Table 3), and the most frequently visited country was Spain, with 204 (7%) cases visiting prior to symptom onset. These cases do not necessarily represent importations, as travel abroad in 21 days prior to symptom onset does not equate to acquisition abroad, but this may have occurred for some cases.
Table 3. Number of confirmed and highly probable monkeypox cases in England with reported international travel in 21 days prior to symptom onset by United Nations (UN) world region travelled to, as of 15 August 2022
| UN world region | Number of confirmed and highly probable cases with travel history within 21 days prior to symptom onset* | Percentage (%) of total cases | Percentage (%) of cases with pre-symptom travel |
|---|---|---|---|
| Europe | 361 | 11.8 | 81.7 |
| Americas | 37 | 1.2 | 8.4 |
| Asia | 32 | 1.0 | 7.2 |
| Africa | 21 | 0.7 | 4.8 |
| Oceania | 6 | 0.2 | 1.4 |
*Table counts visits to UN world regions by cases. If a case visited multiple regions, they will be counted multiple times in the table. Total proportions may be underestimated as travel data is not available for all cases.
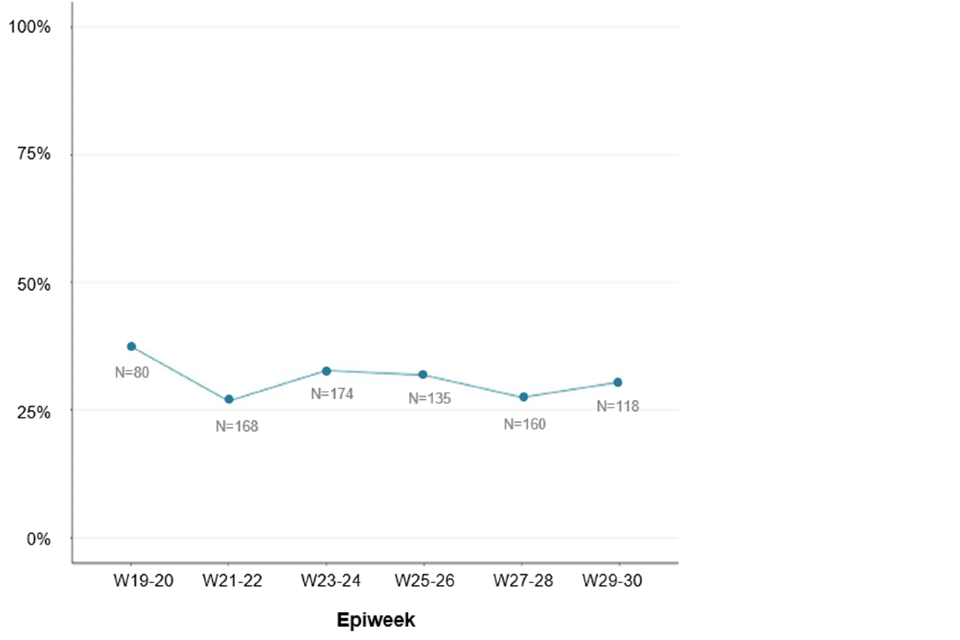
Ascertainment of travel information among all cases is likely to have decreased over time. Therefore, questionnaire responses can be used to monitor the proportion of cases with travel history over time. Travel in the 21 days prior to symptom onset has declined slightly among questionnaire respondents since the beginning of the outbreak, from 38% of 80 respondents with symptom onset dates in weeks 19 to 20, to 27% of 160 respondents in weeks 27 to 28. This may not be a significant or sustained decrease and may also reflect patterns in summer holiday travel in the general population rather than patterns in importation.
Figure 5. Proportion of questionnaire respondents over time with travel in 21 days prior to symptom onset as of 16 August 2022 (N = 835) Numbers next to line represent denominator per 2-week period.
The data used in this graph can be found in the accompanying spreadsheet.
2.3 Findings from enhanced surveillance questionnaires
Of the 3,050 cases identified in England up to 15 August 2022, a total of 917 (30%) had completed enhanced surveillance questionnaires. This includes data collected via rapid sexual health questionnaires administered during the initial weeks of the outbreak (used from 27 to 31 May 2022), questionnaires completed by health protection teams during telephone interviews (used from 1 to 24 June 2022), and self-completed questionnaires sent electronically to cases (used from 25 June 2022 onwards).
Information from enhanced questionnaires should be interpreted with caution, due to the low uptake and because uptake has changed overtime. Uptake was an estimated 50% among cases with symptom onset within the 14-day period starting 9 May, down to 21% for those with symptom onset within the 14-day period starting 18 July. People completing questionnaires may not be representative of all those affected.
Most respondents were of white ethnicity (76%), followed by mixed (10%) and Asian (7%) ethnicities. Additionally, most cases were born in the UK or other European countries, although 10% reported being born in Latin American or Caribbean countries.
Table 4. Ethnicity and region of birth from enhanced surveillance questionnaires in confirmed monkeypox cases in England as of 15 August 2022
N=917
| Ethnicity | Count | Percentage (%) |
|---|---|---|
| White | 701 | 76.4 |
| Mixed | 92 | 10.0 |
| Asian | 61 | 6.7 |
| Black | 37 | 4.0 |
| Other | 1 | 0.1 |
| Unknown | 25 | 2.7 |
| Region of birth | Count | Percentage (%) |
|---|---|---|
| UK | 488 | 53.2 |
| Europe | 197 | 21.5 |
| Latin America and the Caribbean | 90 | 9.8 |
| Asia | 47 | 5.1 |
| Africa | 27 | 2.9 |
| Oceania | 20 | 2.2 |
| Northern America | 14 | 1.5 |
| Unknown | 34 | 3.7 |
As shown in Table 5 and reported in previous technical briefings, monkeypox is predominantly being transmitted in interconnected sexual networks of GBMSM: the majority of cases report multiple sexual partners in the last 3 months, history of a sexually transmitted infection (STI) in the last year, and/or use of human immunodeficiency virus pre-exposure prophylaxis (HIV PrEP).
Table 5. Selected epidemiological metrics from enhanced surveillance questionnaires in confirmed monkeypox cases in England as of 15 August 2022
N=917, some metrics have smaller denominators due to missing values
| Metric | Count | Denominator (excluding cases with missing responses) | Percentage (%) |
|---|---|---|---|
| Gay, bisexual, or men who have sex with men | 857 | 886 | 96.7% |
| History of STI in the last year | 481 | 899 | 53.5% |
| 4 to 9 sexual partners in the last 3 months | 312 | 904 | 34.5% |
| 10+ sexual partners in last 3 months | 267 | 904 | 29.5% |
| Living with HIV | 228 | 867 | 26.3% |
| Ever used PrEP (among HIV negative) | 490 | 624 | 78.5% |
Figure 6 shows trends of the selected epidemiological metrics over time, among cases with completed questionnaires and with symptom onset dates between epidemiological weeks 19 to 30 (from 8 May 2022 to 30 July 2022). Cases with onset dates before or after these dates were excluded from these analyses due to small numbers. These metrics were consistent over time, suggesting transmission of monkeypox virus in England has continued to occur in defined sexual networks of GBMSM.
More detailed analyses of questionnaire data, in combination with manual review of case management notes, was undertaken to describe the small number of cases that are known to not be GBMSM including examining possible routes of acquisition. Of 36 cases who were women aged 16 and above, 12 (38%) of 32 with available information were transgender women. Of the 32 women, 15 had evidence of possible transmission during sexual contact (due to sex with a reported case or high numbers of anonymous sexual partners through sex work), 8 appeared to have non-sexual routes of acquisition (either likely household transmission or no reported sexual activity in 21 days prior), and 9 had unclear possible routes of acquisition or no information. Additionally, there were 20 cisgender men who reported being heterosexual and reported not having had sex with another man in the past 21 days. Of these, a small number had evidence of possible transmission during sexual contact with cisgender women.
Figure 6. Trends of selected epidemiological metrics in monkeypox cases with completed questionnaires, by week of symptom onset in England, epidemiological weeks 19 to 30 (8 May 2022 to 30 July 2022)
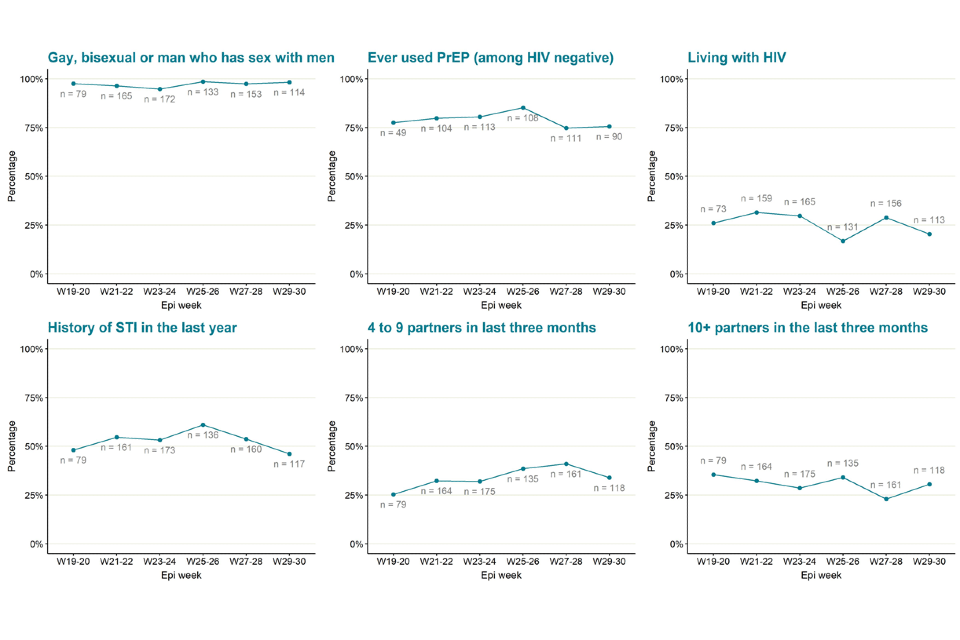
The data used in these graphs can be found in the accompanying spreadsheet.
Due to delays between symptom onset and questionnaire completion, data for the most recent epidemiological weeks may change and should be interpreted with caution.
2.4 Testing data
Testing for monkeypox in the UK is conducted by the UKHSA Rare and Imported Pathogen Laboratory (RIPL) as well as NHS and private laboratories that have begun using monkeypox or orthopox PCR tests since early July 2022.
Testing analyses are currently only available for RIPL, so these are not representative of all testing across the UK. In the most recent 2 weeks of data (30 July to 12 August 2022), RIPL positive tests made up 38% of all positives, and under-represented testing in London.
Up to 12 August 2022, 5,399 monkeypox tests were carried out in RIPL, of which 44% (2,355) were positive.
Figure 7 shows positivity by sex. Positivity in females is low but has increased slightly in recent weeks (based on small numbers in each time period), whereas positivity in males was higher between weeks 25 and 28, but more recently has decreased to below 40%. This may align with decreasing case numbers over time but may not reflect total positivity trends across all laboratories.
Figure 7. Monkeypox test positivity over time among samples tested by UKHSA RIPL by sex, 1 May to 12 August 2022
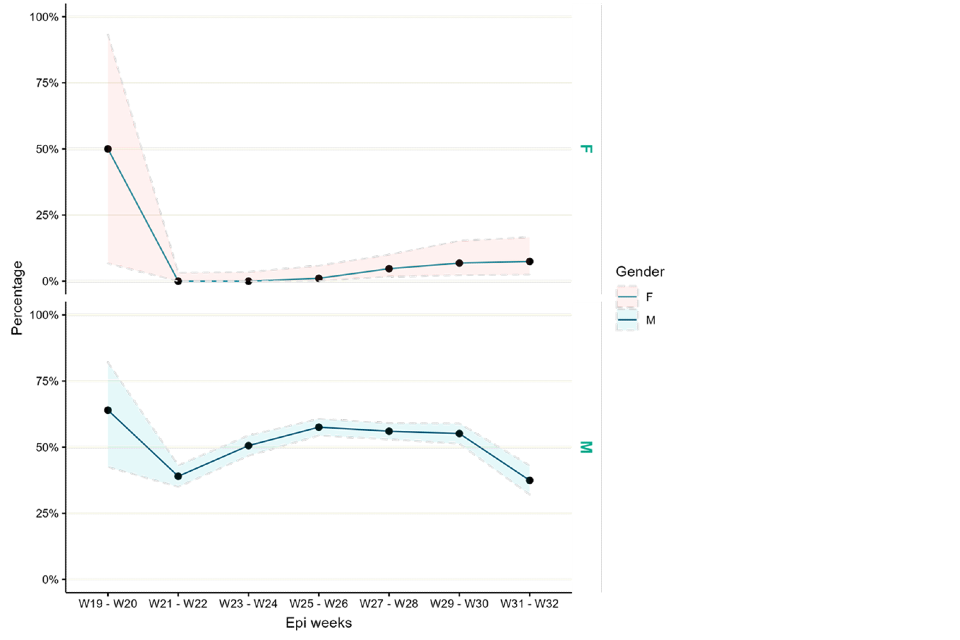
Supplementary data is not available for this figure.
Part 3. Monkeypox vaccination
As of 17 August 2022, a total of 33,199 administered doses of vaccine have been recorded, of which 29,803 doses have been administered as pre-exposure vaccination, primarily to gay, bisexual and other men who have sex with men who are at highest risk of exposure. A further 1,767 doses have been given to healthcare workers managing monkeypox cases and 1,629 doses have been given to close contacts of cases.
The recommended groups for vaccination are set out in UKHSA guidance.
For reference, as of 10 August 2022, a total of 25,325 vaccines had been administered via sexual health services to people at highest risk of infection. A further 2,295 doses had been administered to healthcare workers or community contacts of cases (data up to 8 August 2022). This information is included to provide context to previous announcements.
Part 4. Transmission dynamics
Nowcasting by both specimen date and reporting date continues to indicate and declining epidemic. However, there is a difference for cases outside of London, where the indication is zero growth.
4.1 Nowcasting by specimen date
As the epidemic has grown, the availability of data with recorded symptom onset date has declined. This biases the epidemic curve by symptom onset date, since the proportion of cases being recorded will decay over time, even when adjusting for reporting delays. Therefore, here we model the epidemic using specimen date, which is the date the sample is collected from a patient. This reflects an epidemic curve lagged by the delay between infection and seeking healthcare.
Visualising the epidemic curve by specimen date is affected by the delay from specimen date to the case being reported to UKHSA. To correct for this delay, nowcasting methods adjust the observed data by the distribution of reporting delays to create predictions of the actual frequency. We use a generalised additive model to nowcast current cases and apply this to non-travel associated cases in England.
The nowcasting suggests that the growth in confirmed daily cases is negative overall (Figure 8), though with high uncertainty. The halving time is estimated at 24 days (though with confidence intervals that allow both growth and decay). The nowcast estimate of cases is around 20 per day. The epidemic inside London has declined since mid-July (Figure 9) but is now either flat or growing. The epidemic outside of London has been flat since late-July and is now a similar size to the London epidemic. Current trends, both regionally and nationally, have very high uncertainty.
Figure 8. Nowcast growth rate and incidence of monkeypox cases in England. 8a shows estimates by specimen date, 8b shows estimates by report date
The charts exclude cases associated with travel. The shaded area is the 90% Credible Interval (CrI), the dark shaded area is the 50% CrI and the lighter area is the 90% CrI.
Figure 8a
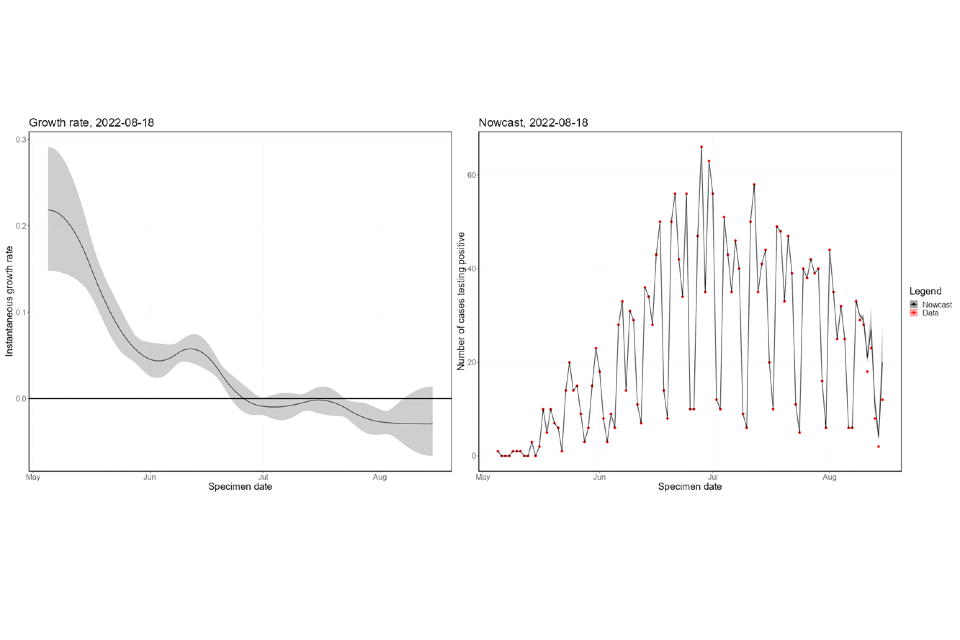
Figure 8b
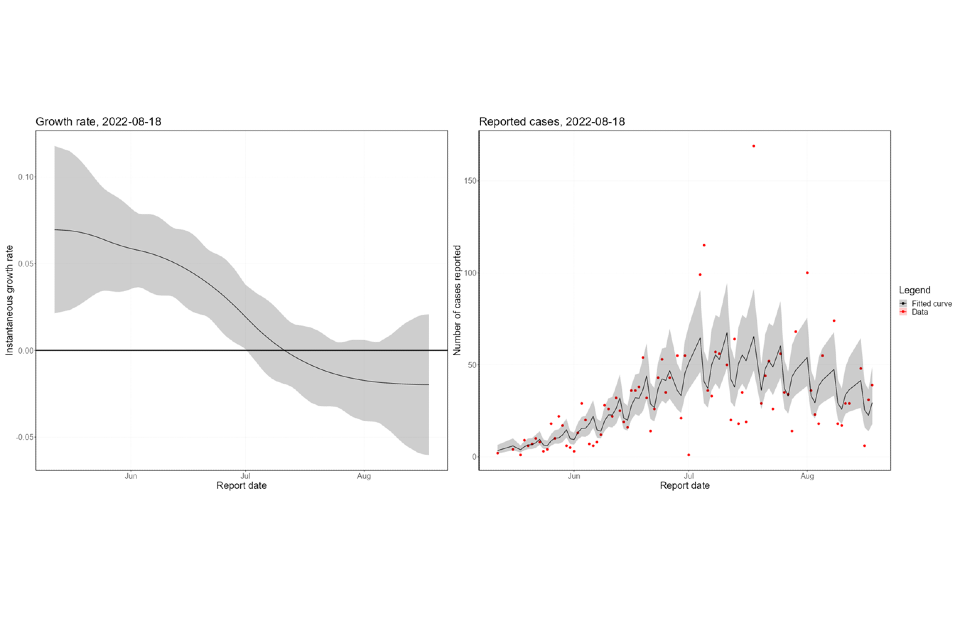
Supplementary data is not available for these figures.
Figure 9. Estimates of nowcast growth rate and incidence by specimen date inside and outside London
The charts exclude cases associated with travel. The shaded area is the 90% Credible Interval (CrI), the dark shaded area is the 50% CrI and the lighter area is the 90% CrI.
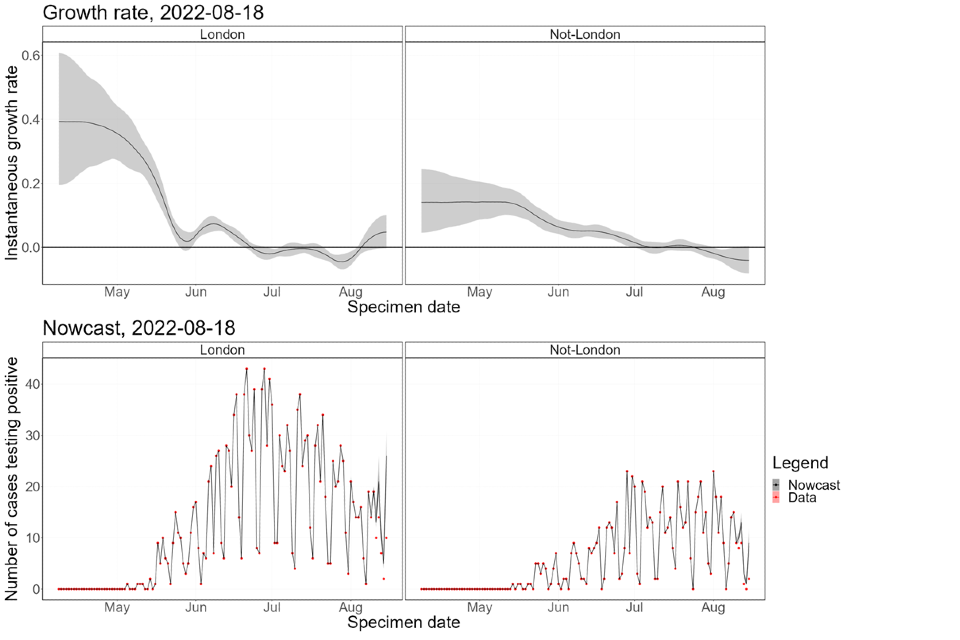
4.2 Temporal trends in gender distribution
Thus far, the majority of cases have occurred in defined networks of GBMSM. To assess whether the outbreak is moving away from this initial subpopulation, we investigate temporal trends in the proportion of cases that do not identify as women. Assuming Monkeypox is being transmitted in sexual networks of GBMSM, the proportion of cases that identify as women should remain constant (allowing for random fluctuations in the observed proportion).
A small number of sporadic transmission events from GBMSM networks to women is expected. However, if the proportion of cases in women is increasing, this could suggest that there is sustained transmission outside GBMSM networks. If the proportion of cases in women with no known links to GBMSM networks is increasing, that could be even stronger evidence of sustained onward transmission.
To assess the evidence, we construct a null hypothesis of no change in the transmission network based on the gender-distribution of cases before July. We then adjust this observed proportion to the number of cases in the current week, to create a 95% confidence region where we would expect the currently observed gender-distribution to lie if it follows the null hypothesis. By overlaying the currently observed gender-distribution, we can identify statistically significant perturbations. Note that although statistically significant, these perturbations may not reflect underlying transmission changes, since it could also be affected by changes in the relative ascertainment of cases in women relative to men, or unknown cases. We plot the statistical test as a rolling function of time for each week of the outbreak.
The proportion of non-contact-tracing-linked female cases has increased over the last few weeks, which is a statistically significant perturbation in the distribution (Figure 10). Removing female cases with known links to MSM communities and trans women (n=15) from the count of female cases accounts for most of this overall trend, but there does seem to be some increase in unlinked female cases. This is currently not significant at the 5% level but continues under close surveillance.
Figure 10. Binomial probability of male cases using a binomial exact test
The purple shaded area is the null hypothesis (which implies no change in the rate). The solid black line is the proportion observed in the latest week, plotted by the first day of the focal week. Plot a) removes 15 cases (at time of analysis) which were known to have a link to a GBMSM primary case or are trans women. Plot b) analysis including all women.
Figure 10a
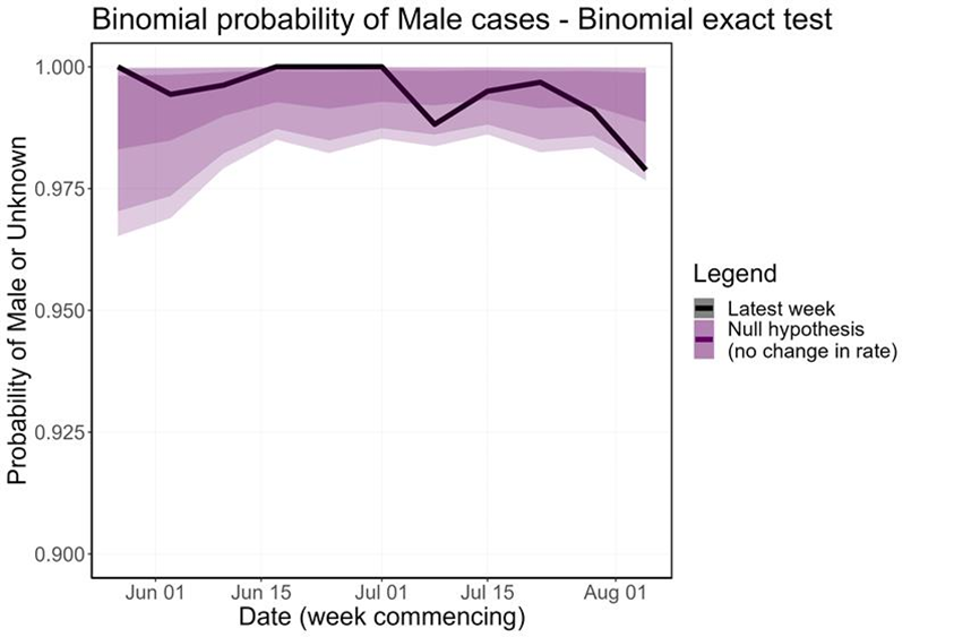
Figure 10b
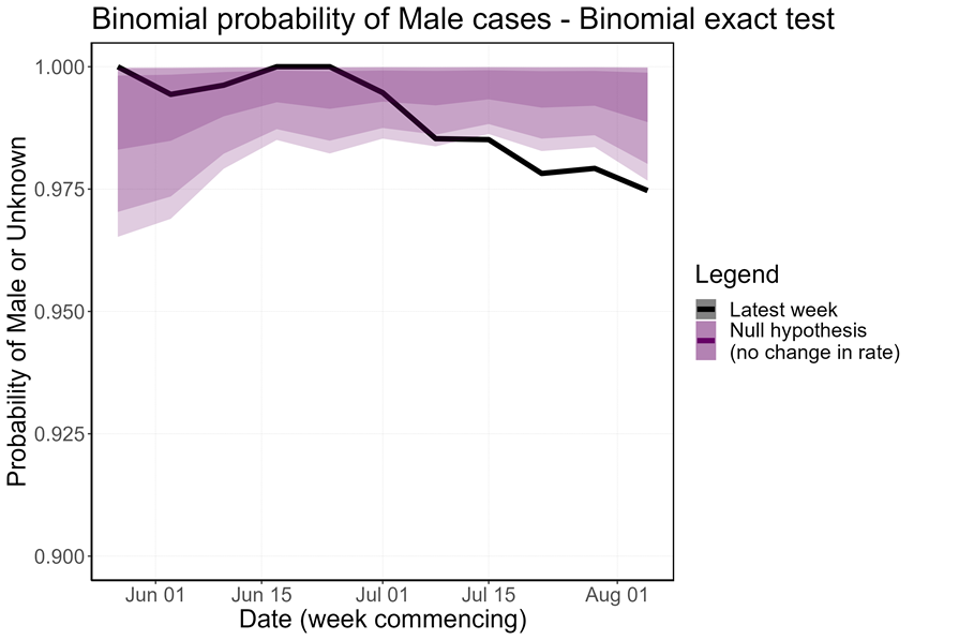
Supplementary data is not available for this figure.
4.3 Modelling data on importation
Simulations can help us understand the contribution importations may be making to the overall picture in the UK. To better understand the contribution of importations, we have run simulations of imported-infection outbreaks, using different assumptions about reproduction number (Rt) and the number of imports, to show what reasonable numbers of autochthonous infections we may expect to see.
For the purpose of this analysis, we consider all infections that are 2 or more generations from the imported infection to be autochthonous infections. The modelling used simulations based on a negative binomial assuming a k of 1. The modelling is done for 4 different monthly imported infections scenarios (10, 30, 60, 90) and for 3 different Rt scenarios (0.5, 0.75, 0.95).
Figure 11. Probability of endemic transmission. Median and 90% quantile number of endemic cases (that is the number of endemic cases seen in 50% and 90% of our simulations) we would expect to see as a result of importations even under domestic eradication.
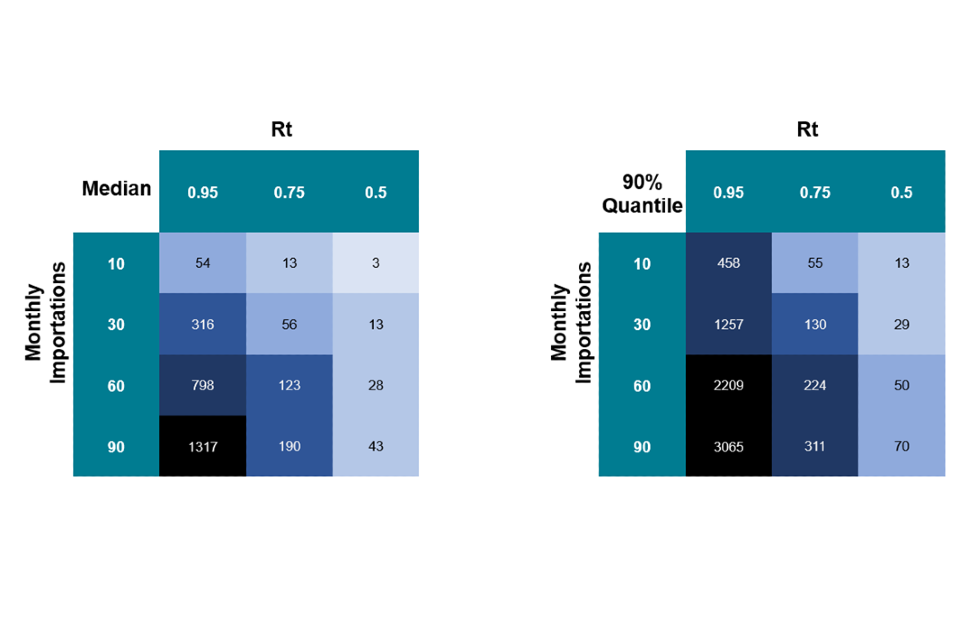
Approximate values based on numerical simulations – 50K runs. One step = One generation time. Supplementary data is not available for this figure.
The main takeaway from this analysis is that even when Rt is less than 1, we can expect to see ongoing autochthonous cases as a result of importations. For example, assuming a k = 1, to have a 90% chance of seeing fewer than 100 autochthonous infections per month due to importations, we would need an Rt in the range 0.75 to 0.5 and less than 10 to 30 importations per month.
Caveats
This analysis should be interpreted as an illustrative example of the levels of autochthonous infections we may expect to detect. Crucially, these analyses are not predictions or forecasts, and are not fitted to outbreak data.
The assumptions presented here (for example, Rt) should be interpreted as an average which may hide considerable variation. For example, an Rt of 0.75 may be an average of a small number of imported cases that enter into a highly dense network (resulting in a large number of additional infections) and a larger number of imported cases in a family, likely to result in a smaller number of additional infections.
Modelling infections means that these results are likely to be higher than the observed case numbers, as surveillance systems are unlikely to achieve 100% ascertainment.
Finally, there is currently high uncertainty around the level of dispersion (k) in the secondary case distribution. Lower k values (more overdispersion) would make outliers more likely. In other words, if k is substantially lower than the assumption used in these simulations, observing very high numbers of infections is more likely (but it does relatively little to the 90% quantile).
Sources and acknowledgments
Data sources
Monkeypox virus PCR results are submitted to UKHSA daily by the Rare and Imported Pathogens Laboratory, Porton, UKHSA regional laboratories and NHS laboratories undertaking testing. Data on people testing positive since 6 May 2022 is enhanced with demographic, symptom, epidemiological, and exposure information extracted from the UKHSA Health Protection Team case management system (HPZone), or collected in enhanced surveillance questionnaires. Enhanced surveillance questionnaires include data collected via rapid sexual health questionnaires administered during the initial weeks of the outbreak (used from 27 to 31 May 2022), questionnaires completed by health protection teams during telephone interviews (used from 1 to 24 June 2022), and self-completed questionnaires sent electronically to cases (used from 25 June 2022 onwards).
Authors of this report
Zahidul Abedin, Paula Blomquist, Jessica Bridgen, Chloe Byers, Meera Chand, Kristine Cooper, Fergus Cumming, Julie Day, Ashley Goddard, Irene Gonsalvez, Alice Graham, James Guilder, Susan Hopkins, Luke Hounsome, Jamie Lopez-Bernal, Nicola Love, Richard Myers, Isabel Oliver, Karthik Paranthaman, Mateo Prochazka, James Sedgwick, Thomas Ward, Nick Watkins, William Welfare, Katie Wrenn.
Contributors
UKHSA Data, Epidemiology and Analytics Cell
UKHSA Research and Science Cell
UKHSA Modelling Cell
UKHSA Genomics Public Health Analysis
UKHSA Sexual Health Liaison Group
UKHSA Monkeypox Incident Management Team
Monkeypox Technical Group
The Monkeypox Technical Group includes members with expertise in clinical infectious diseases, clinical research, epidemiology, genomics, modelling, and virology:
- Meera Chand (Chair), UKHSA
- Andre Charlett, UKHSA
- Andrew Rambaut, University of Edinburgh
- Allan Bennett, UKHSA
- Ashley Otter, UKHSA
- Calum Semple, University of Liverpool
- Christophe Fraser, University of Oxford
- Claire Dewsnap, British Association for Sexual Health and HIV
- Claire Gordon, UKHSA
- David Ulaeto, Defence Science and Technology Laboratory
- Erik Volz, Imperial College London
- Emma Thomson, Medical Research Council-University of Glasgow Centre for Virus Research
- Fergus Cumming, UKHSA
- Giri Shankar, Public Health Wales
- Geoffrey L. Smith, University of Cambridge
- Geraldine O’Hara, High Consequence Infectious Diseases Network
- Health and Social Care Northern Ireland
- Helen Fifer, UKHSA
- Isabel Oliver, UKHSA
- Jake Dunning, University of Oxford and Royal Free Hospital
- Jason Mercer, University of Birmingham
- Maria Rossi, Public Health Scotland
- Matt Keeling, Warwick University
- Matt Phillips, British Association for Sexual Health and HIV
- Natalie Groves, UKHSA
- Neil Ferguson, Imperial College London
- Nicholas Loman, University of Birmingham and UKHSA
- Obaghe Edeghere, UKHSA
- Richard Myers, UKHSA
- Steven Riley, UKHSA
- Susan Hopkins, UKHSA
- Tommy Rampling, UKHSA
Acknowledgements
The authors are grateful to those teams and groups providing data for these analyses including:
- British HIV Association (BHIVA)
- British Association for Sexual Health and HIV (BASHH)
- Sexual Health Services
- NHS England and Improvement
- High Consequence Infectious Diseases Network
- Public Health Scotland
- Public Health Wales
- Public Health Agency, Northern Ireland
