Investigation into monkeypox outbreak in England: technical briefing 8
Updated 19 December 2024
Applies to England
The UK Health Security Agency (UKHSA) is working with the National Health Service (NHS) and the public health agencies of the 4 nations to investigate the monkeypox outbreak. This briefing is produced to share data useful to other public health investigators and academic partners undertaking related work. It includes early evidence and preliminary analyses which may be subject to change.
Data reported in the technical briefing is as of 16 September 2022 (or as specified in the text) to allow time for analysis.
Potential levels of the outbreak in England
The outbreak can be considered to fall into 1 of 4 potential levels of transmission.
Level 1
Incursions from rest of the world – small numbers of imported cases with limited onward transmission.
Level 2
Transmission within a defined sub-population.
Level 3
Transmission within multiple sub-populations or larger sub-population.
Level 4
Wider significant community transmission – with potential for endemic and local epi-zoonotic disease.
These may be refined with better understanding of modes of transmission.
At present, England is judged to be in level 2.
Part 1. Risk assessment as of 23 September 2022
UK trajectory
Nowcast and growth
Incidence remains low.
It is likely that multiple factors, including but not limited to vaccination, are contributing to the decline in transmission (moderate confidence). Reduction in some other sexually transmitted infections, as well as modelling, suggests behavioural modification may have been a factor (low confidence).
Assessment (confidence): Approximately flat daily growth rate at low levels of incidence (high).
Geographic spread
The proportion of new cases which are in London has decreased from 80% at the start of the outbreak to 50% in mid-August, but has increased again more recently suggesting the slow-down in cases has been more prominent outside London.
Assessment (confidence): Approximately stable geography within England (moderate).
Ascertainment
Whilst testing is freely available, testing is likely to be influenced by known risk factors and therefore it is likely that there is under ascertainment. Atypical symptoms, including single or scarce lesions, are reported and there are increasing international reports of subclinical infection.
Assessment (confidence): Insufficient information.
UK transmission
Outbreak level
Level 2 is defined as transmission within a defined sub-population, currently gay, bisexual and men who have sex with men (GBMSM) connected by sexual networks. Enhanced surveillance data does not suggest a change in case mix, although it is available for only 31% (1,050 out of 3,412) of cases in England and may not be representative of the whole cohort. Out of 3,390 cases with known gender, 99% are men, and there are 45 women. There is no robust evidence of sustained transmission outside some sexual networks of GBMSM.
Assessment (confidence): Level 2 (moderate).
Route of transmission
Whilst the primary reported route is through close or sexual contact, monkeypox virus has been detected in air and environmental samples in the hospital room of infected patients. There are no confirmed instances of airborne transmission. Limited household transmission has been described in the UK. Detailed investigations have found small numbers of cases with no identified route of acquisition. There are preliminary indications from a small number of cases that transmission may be occurring early in infection, which may include before individuals have identified that they are symptomatic.
Assessment (confidence): Transmitting primarily through close or sexual contact (moderate).
Severity
Observed clinical severity
There are no reported deaths in the UK and a small number of deaths reported globally linked to the outbreak. There is significant morbidity amongst people who are admitted to hospital for clinical care reasons, including severe pain and complications due to secondary bacterial infection. Encephalitis has been reported although it appears uncommon.
Based on genomic surveillance the majority of the outbreak falls in lineage B.1 and these clinical severity attributes cannot be extended to other lineages of monkeypox. There are insufficient data to comment on severity for other lineages.
Assessment (confidence): In current population group, low mortality but significant morbidity (moderate). In wider population, insufficient data.
Virological characterisation
The outbreak falls within Clade IIb, within which the majority of sequenced cases are lineage B.1 or descendants. A small amount of diversity has developed within lineage B.1 both in and out of the UK.
A small number of genomes from other clade IIb lineages have also been detected in 2022. The most prominent additional lineage is A.2. These additional lineages require separate characterisation.
There are very limited laboratory data and it is not currently possible to determine whether the differences between lineages are likely to have biological significance.
There is evidence suggestive of sustained human transmission prior to the 2022 outbreak but not clear-cut evidence of adaptation for improved human transmission.
Assessment (confidence): The primary outbreak lineage is B.1, however additional lineages may be co-circulating (moderate).
International transmission
Routes of transmission reported by other countries
Cases in previously non-endemic countries are reported as primarily in GBMSM. Small number of cases are reported in women and children. In countries with a longer history of monkeypox, apparent wider population transmission is reported with unclear routes. Phylogenetic data suggests that human transmission has been occurring for a number of years in some areas prior to the appearance of the outbreak clade.
Assessment (confidence): Transmitting primarily by close or sexual contact in non-endemic countries (low), unknown transmission routes in endemic countries.
Part 2. Epidemiology update
2.1 Current epidemiological situation
As of 16 September 2022, there were 3,585 laboratory-detected cases of monkeypox in the UK. This includes 3,439 confirmed and 146 highly probable cases. Definitions of cases can be found in previous technical briefings.
Note that whilst data cleaning is carried out routinely, quality assurance samples may have been included from laboratory surveillance systems which will be corrected in subsequent reports.
Table 1. Number of confirmed and highly probable monkeypox cases by devolved administrations, 6 May 2022 to 16 September 2022
| Devolved administrations | Total | Confirmed | Highly probable |
|---|---|---|---|
| England | 3,412 | 3,266 | 146 |
| Northern Ireland | 34 | 34 | 0 |
| Scotland | 93 | 93 | 0 |
| Wales | 46 | 46 | 0 |
| Total | 3,585 | 3,439 | 146 |
Figure 1 shows the specimen dates of monkeypox cases over time in England. There is a clear peak in mid-July, with over more than 60 cases per day, followed by a decline to less than 15 cases per day during the beginning of September.
Figure 1. Confirmed and highly probable monkeypox cases by specimen date* in England as of 16 September 2022
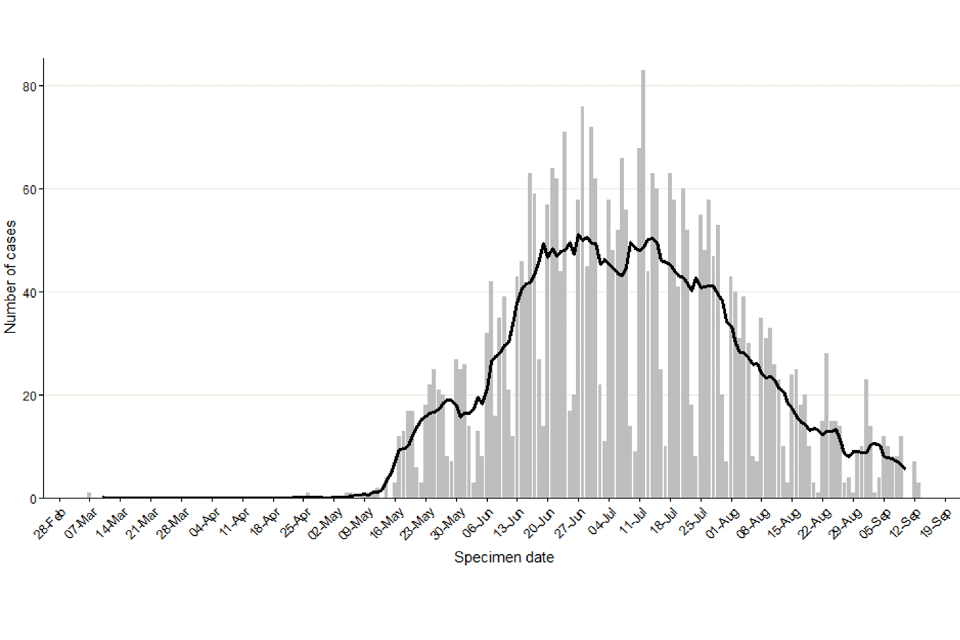
*Where the specimen date is missing, the date the laboratory received the specimen is used. Where both are missing (mainly among early cases), the date added to the case line list is used (5 out of 3,412). The data used in this graph can be found in the accompanying spreadsheet.
As of 16 September 2022, 69% of England cases were known to be London residents (2,359 out of 3,399 with reported home address) (Table 2). This percentage had decreased from more than 80% in May 2022 to 50% in mid-August 2022 (Figure 2) but has increased more recently suggesting the slow-down in cases has been more prominent outside London.
Table 2. Number of confirmed and highly probable monkeypox cases by region of residence, England, 6 May 2022 to 16 September 2022
| Region of residence | Total confirmed and highly probable cases | Regional distribution of all cases (% excluding unknown) |
|---|---|---|
| East of England | 115 | 3.4 |
| East Midlands | 59 | 1.7 |
| London | 2,359 | 69.4 |
| North East | 47 | 1.4 |
| North West | 216 | 6.4 |
| South East | 311 | 9.1 |
| South West | 90 | 2.6 |
| West Midlands | 124 | 3.6 |
| Yorkshire and Humber | 78 | 2.3 |
| Unknown | 13 | 0.0 |
| Total | 3,412 | 99.9 |
Figure 2. Regional distribution of confirmed and highly probable monkeypox cases as of 16 September 2022
Figure 2a. Cumulative count of confirmed and highly probable monkeypox cases by region of residence in England*
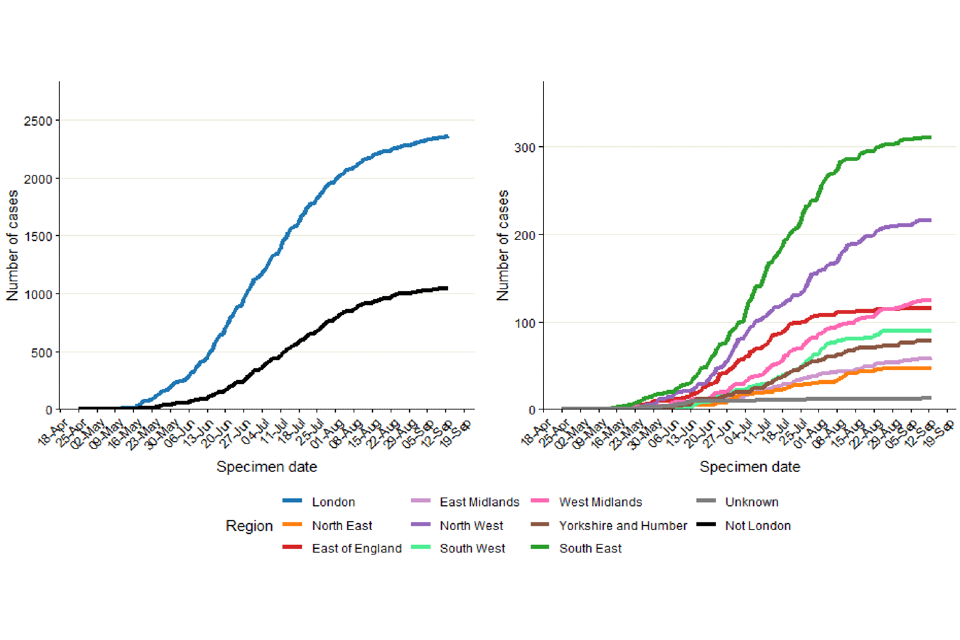
Figure 2b. Percentage of confirmed and highly probable monkeypox cases in London, by specimen date
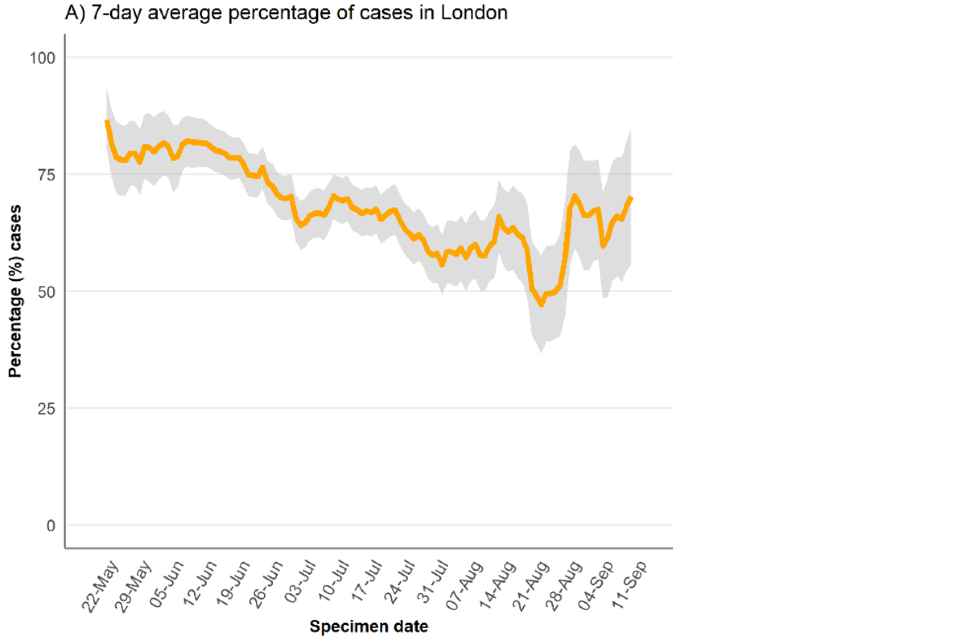
*If the residential postcode is known, then the region of residence is used, otherwise the region of the healthcare facility where the testing occurred at is used. The case with a retrospective specimen date of March 2022 has been omitted from the chart.
The data used in these graphs can be found in the accompanying spreadsheet.
Where gender information was available, 3,345 out of 3,390 (99%) confirmed cases in England were men, and 45 were women (Figure 3). The median age of confirmed and highly probable cases in the UK was 36 years (interquartile range 30 to 44).
Figure 3. Age and gender distribution of confirmed and highly probable monkeypox cases by lab result date in England as of 16 September
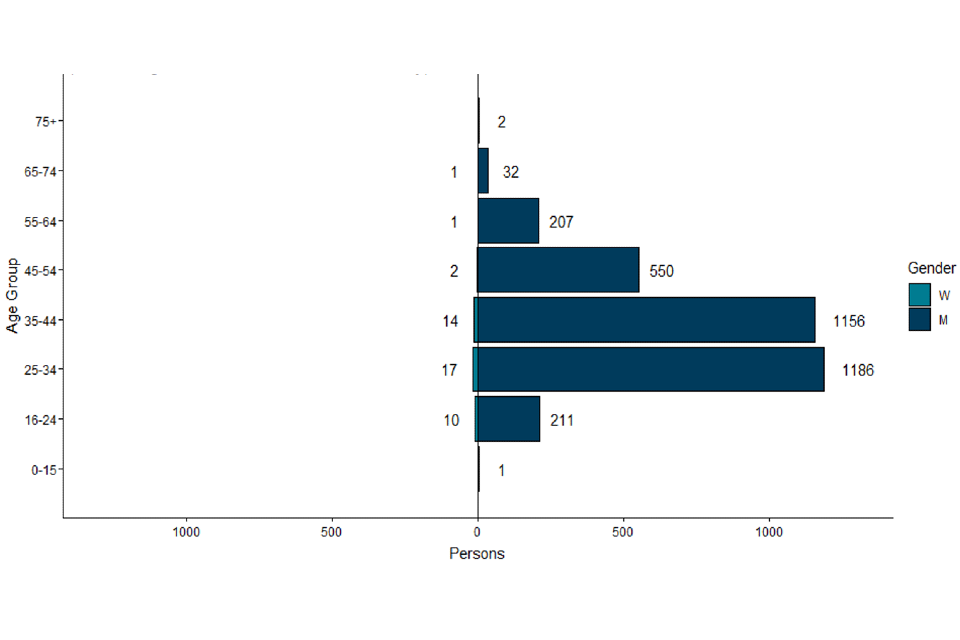
The data used in this graph can be found in the accompanying spreadsheet.
2.2 International travel
In England, 495 confirmed and highly probable monkeypox cases are known to have travelled internationally within the 21 days prior to symptom onset, as of 16 September 2022. The majority of these individuals had travelled to Europe (Table 3), and the most frequently visited country was Spain. Additionally, 7 cases were known to have travelled to endemic countries in West and Central Africa. Travel abroad in the 21 days prior to symptom onset does not equate to acquisition abroad, but this may have occurred for some cases.
Questionnaire data alone is used for estimating proportions of cases that have travelled, as ascertainment of travel information among all cases has changed over time. As of 16 September 2022, 30% of 1,034 questionnaire respondents reported travel in the 21 days prior to symptom onset. Among 998 respondents who provided information about sexual activity abroad, 19% (n=190) reported sex abroad. This may mean acquisition abroad was more likely though it is not confirmed.
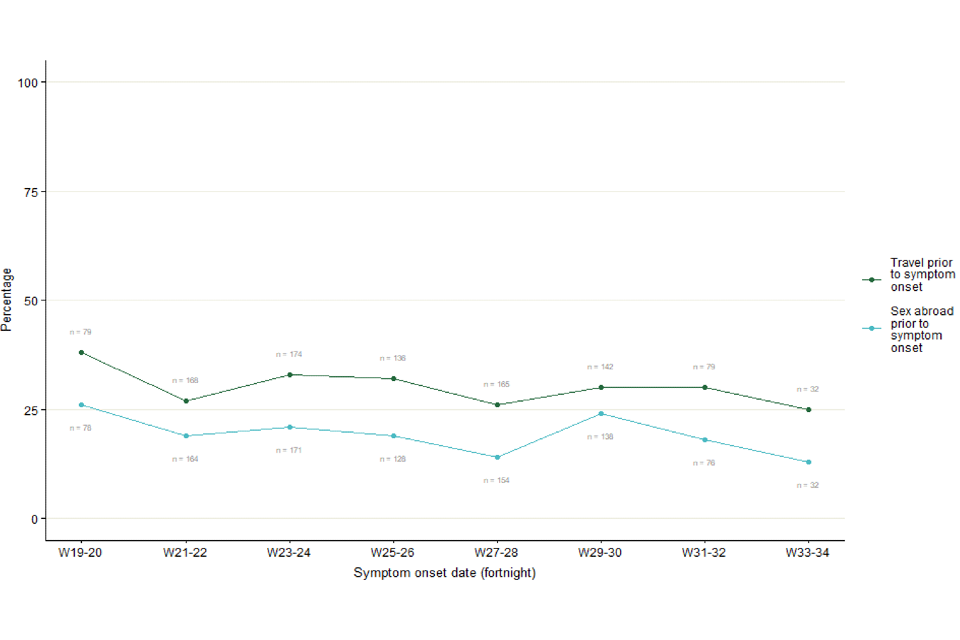
As shown in Figure 4, travel in the 21 days prior to symptom onset has been relatively stable over time, ranging between 25% (8 out of 32) in week 33 to 34, and 38% (30 out of 79) in week 19 to 20 of respondents.
Table 3. Number of confirmed and highly probable monkeypox cases in England with reported international travel in 21 days prior to symptom onset by United Nations (UN) world region travelled to, as of 16 September 2022
| UN world region | Number of confirmed and highly probable cases with travel history within 21 days prior to symptom onset* | Percentage (%) of total cases | Percentage (%) of cases with pre-symptom travel |
|---|---|---|---|
| Europe | 405 | 11.9 | 81.8 |
| Americas | 42 | 1.2 | 8.5 |
| Asia | 34 | 1.0 | 6.9 |
| Africa | 23 | 0.7 | 4.6 |
| Oceania | 6 | 0.2 | 1.2 |
*Table counts visits to UN world regions by cases. If a case visited multiple regions, they will be counted multiple times in the table. Total proportions may be underestimated as travel data is not available for all cases.
Figure 4. Proportion of questionnaire respondents over time with travel in 21 days prior to symptom onset, from week 19 to week 34, as of 16 September 2022. Numbers next to line represent denominator per 2-week period.
The data used in this graph can be found in the accompanying spreadsheet.
2.3 Findings from enhanced surveillance questionnaires
Of the 3,412 cases identified in England by 16 September 2022, a total of 1,050 (31%) had completed enhanced surveillance questionnaires by 18 September 2022.
Information from enhanced questionnaires should be interpreted with caution, due to the low uptake and because uptake has changed over time. In particular, response rates have fallen since the transition to self-completed questionnaires (used from 25 June 2022 onwards): from 56% uptake (165 questionnaires) among 291 cases with specimen dates in the first 2 weeks of May 2022 down to 24% (n=52) among the 218 cases with specimen dates in the three-week period from 29 August 2022, although these are estimates as some questionnaires are filled in anonymously. People completing questionnaires may not be representative of all those affected.
Most respondents were of white ethnicity (77%), followed by mixed (9%) and Asian (7%) ethnicities (Table 4). Additionally, most cases were born in the UK or other European countries, although 9% reported being born in Latin American or Caribbean countries.
Table 4. Ethnicity and region of birth from enhanced surveillance questionnaires in confirmed monkeypox cases in England as of 18 September 2022
| Ethnicity | Count | Percentage (%) |
|---|---|---|
| White | 812 | 77.3% |
| Mixed | 95 | 9.0% |
| Asian | 70 | 6.7% |
| Black | 43 | 4.1% |
| Other | 1 | 0.1% |
| Unknown | 29 | 2.8% |
| Region of birth | Count | Percentage (%) |
|---|---|---|
| UK | 565 | 53.8% |
| Europe | 222 | 21.1% |
| Latin America and the Caribbean | 95 | 9.0% |
| Asia | 55 | 5.2% |
| Africa | 32 | 3.0% |
| Oceania | 22 | 2.1% |
| Northern America | 19 | 1.8% |
| Unknown | 40 | 3.8% |
As shown in Table 5 and reported in previous technical briefings, monkeypox is predominantly being transmitted in interconnected sexual networks of GBMSM: the majority of cases report multiple sexual partners in the last 3 months, history of a sexually transmitted infection (STI) in the last year, and/or use of human immunodeficiency virus pre-exposure prophylaxis (HIV PrEP).
Table 5. Selected epidemiological metrics from enhanced surveillance questionnaires in confirmed monkeypox cases in England as of 18 September 2022
N=1,050, some metrics have smaller denominators due to missing values
| Metric | Count | Denominator (excluding cases with missing responses) | Percentage (%) |
|---|---|---|---|
| Gay, bisexual, or men who have sex with men | 983 | 1,016 | 96.8% |
| History of STI in the last year | 542 | 1,029 | 52.7% |
| 4 to 9 partners in last 3 months | 354 | 1,035 | 34.2% |
| 10+ sexual partners in last 3 months | 299 | 1,035 | 28.9% |
| Living with HIV | 257 | 993 | 25.9% |
| Ever used PrEP (among HIV negative) | 555 | 721 | 77.0% |
Figure 5 shows trends of the selected epidemiological metrics over time, among cases with completed questionnaires and with symptom onset dates between epidemiological weeks 19 to 34 (from 8 May 2022 to 27 August 2022). Trends continue to remain the same as reported in technical briefing 7.
Detailed analyses of questionnaire data, in combination with manual review of case management notes, was undertaken to describe the small number of cases that are known to be not GBMSM including examining possible routes of acquisition. Of 45 cases who were women aged 16 and above, 16 out of 44 (36%) with available information were transgender women.
Additionally, 22 had evidence of possible transmission during sexual contact (due to sex with a reported case or high numbers of anonymous sexual partners through sex work), 9 appeared to have non-sexual routes of acquisition (either likely household transmission or no reported sexual activity in 21 days prior), and 14 had unclear possible routes of acquisition or no information.
Additionally, there were 25 cisgender men who reported being heterosexual and reported not having had sex with another man in the past 21 days. Of these, a small number had evidence of possible transmission during sexual contact with cisgender women.
Figure 5. Trends of selected epidemiological metrics in monkeypox cases with completed questionnaires, by week of symptom onset in England, epidemiological weeks 19 to 34 (8 May 2022 to 27 August 2022). Data labels give total number of questionnaire responses for the relevant time period.
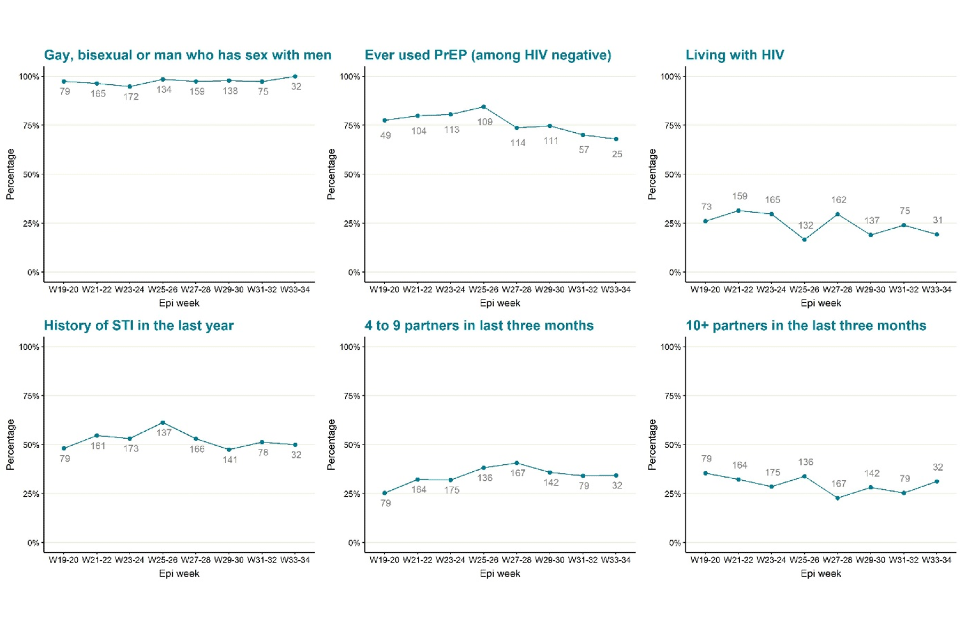
The data used in these graphs can be found in the accompanying spreadsheet. Due to delays between symptom onset and questionnaire completion, data for the most recent epidemiological weeks may change and should be interpreted with caution.
2.4 Testing data
Testing for monkeypox in the UK is conducted by the UKHSA Rare and Imported Pathogen Laboratory (RIPL) as well as NHS and private laboratories that have begun using monkeypox or orthopox polymerase chain reaction (PCR) tests since early July 2022.
Full testing data is available for RIPL. Up to 18 September 2022, 6,001 individuals were tested for monkeypox in this laboratory, of which 41% (2,486) were positive. Further analyses are not included in this technical briefing as RIPL positive tests made up only 45% of all positives in the most recent 2 weeks of data (2 September to 16 September 2022) and the representativeness of RIPL has changed over time.
Part 3. Trends in other infections primarily transmitted in sexual networks of GBMSM
Following the rapid increase in confirmed and highly probable monkeypox cases since May 2022, the number of cases decreased throughout August 2022. We compared the temporal trends in diagnoses of monkeypox with those of Shigella spp and lymphogranuloma venereum (LGV), 2 other infections with a similarly disproportionate burden in GBMSM in highly interconnected sexual networks, to assess whether the reductions in monkeypox cases could be as a result of reduced transmission due to behaviour change in GBMSM.
As can be seen in Figure 6, the decrease observed in the number of monkeypox diagnoses occurred shortly after similar decreases observed in the number of shigellosis and LGV diagnoses. These similar trends suggest that changes in sexual behaviour among some GBMSM may have contributed to the decrease in reported cases of these infections in August 2022. Shigella spp and LGV have shorter incubation periods than monkeypox, which could explain the lag between the observed decreases.
Figure 6. Laboratory diagnoses of Shigella spp, LGV (primary y-axis) and monkeypox (secondary y-axis) in England, from 1 January 2021 to 3 September 2022 (epidemiological week 35)

*Shigella and monkeypox are not categorised as sexually transmitted infections rather infections that can be transmitted during close contact and which are seen within sexual networks of GBMSM. The data used in this graph can be found in the accompanying spreadsheet.
Note: A different scale is used on the secondary vertical axis for monkeypox. Data from epidemiological week 35 2022 is provisional and may be incomplete. Data for diagnoses of shigellosis is extracted from the Second Generation Surveillance System (SGSS) and includes figures for adult men without a recent history of foreign travel; this is a validated epidemiological proxy for domestic Shigella transmission in GBMSM. Data for LGV is extracted from SGSS (for London) and MOLIS (the rest of England) and is restricted to adult men. All data was extracted on 12 September 2022.
Part 4. Monkeypox vaccination
4.1 Consensus estimates of the number of people eligible for monkeypox vaccination in the UK
In August 2022, members of the Sexual Health Liaison Group of UKHSA’s Monkeypox Incident Management Team met with representatives of the group who developed the Monkeypox Consensus Statement to jointly agree assumptions to estimate the number of people most at risk of monkeypox and, therefore, eligible for targeted pre-exposure vaccination.
UKHSA’s estimate of GBMSM regularly attending sexual health services (SHS) and eligible for pre-exposure vaccination is based on data submitted by SHS in England to the GUMCAD Sexually Transmitted Infections (STI) Surveillance System from April 2021 to March 2022 (further details in Table 6 below). The following 2 proxy measures of high risk of monkeypox were used to assume eligibility for vaccination in line with the criteria in the Green Book:
- diagnosed with a bacterial STI in the past 12 months (irrespective of HIV status), or
- coded either as eligible for HIV pre-exposure prophylaxis (PrEP) or having been prescribed PrEP
Both criteria are reliable indicators of future STI risk, and they were independently recommended by clinical stakeholders as proxy markers of being at increased risk of exposure to monkeypox.
This number of GBMSM was scaled up, using Office for National Statistics (ONS) population data on the number of men identifying as gay or bisexual, to account for those accessing SHS in Northern Ireland, Scotland and Wales. Further uplifts were applied to this UK figure, based on research evidence, to account for GBMSM who do not regularly seek care at SHS; and on stakeholder expert opinion, to account for GBMSM who are not in contact with SHSs and are unlikely to participate in research studies. After taking into account healthcare workers and selected outreach staff eligible for vaccination due to an increased risk of exposure to monkeypox, the estimated total number of people eligible for vaccination in the UK is 111,000.
Table 6. Estimates of the number of people eligible for monkeypox vaccination as of 14 September 2022
| Eligibility criteria | Count | |
|---|---|---|
| (a) | Estimated number of GBMSM at high risk of monkeypox regularly attending SHS in England | 48,500 |
| (b) | Estimated number of GBMSM at high risk of monkeypox regularly attending SHS in the other Nations of the UK (15% of a) | 7,500 |
| (c) | Estimated number of GBMSM at high risk of monkeypox who do not regularly attend SHS (UK) (60% of [a + b]) | 33,500 |
| (d) | Additional uplift to account for GBMSM at high risk of monkeypox who are not in contact with SHS and are unlikely to participate in research studies (15% of [a + b + c]) | 13,500 |
| (e) | Healthcare workers – SHS | 5,000 |
| (f) | Healthcare workers – High Consequence Infectious Diseases (HCID) centres | 2,000 |
| (g) | Outreach workers and volunteers working in hostels or sex-on-premises venues | 1,000 |
| Total | 111,000 |
Sources
a) GUMCAD (STI) Surveillance System. Provisional data from 1 April 2021 to 31 March 2022 (see section “Assumptions used to estimate the number of GBMSM regularly attending SHS and at high risk of monkeypox” for further details).
b) ONS data on sexual orientation used to derive a multiplier of 1.15 applied to the England number to reach a UK estimate.
c) The Reducing Inequalities in Sexual Health during the coronavirus (COVID-19) pandemic (RiiSH-COVID) behavioural survey of GBMSM (based on behaviours from August 2021 to December 2021; wave 4, unpublished) suggests that 37.4% of GBMSM with high-risk behaviours (4 or more sex partners in the last 4 months) did not attend SHS over that period; to account for all GBMSM at high risk of monkeypox irrespective of them regularly attending SHS, this equates to an uplift of 60% to the sum of (a) + (b).
d) Expert opinion, experience of recent monkeypox outreach event where an estimated 15% of GBMSM were not currently linked to any SHS. This included recently arrived migrant GBMSM who did not know how to access SHS or were fearful of accessing SHS because of immigration issues (without knowing that they are open access services available to everyone irrespective of nationality or immigration status). An uplift of 15% was applied to the sum of (a)+(b)+(c).
e) Workforce estimate provided by British Association for Sexual Health and HIV (BASHH).
f) Estimate provided by HCID network based on 11 HCID centres each with approximately 80 staff eligible for pre-exposure prophylaxis vaccination and a further 24 HCID centres each with approximately 40 staff eligible for pre-exposure prophylaxis vaccination.
g) Expert opinion – commissioners and providers of sexual health outreach, figure provided by the Monkeypox Consensus Statement Group.
To account for uncertainty in these estimates, all numbers have been rounded up to the nearest 500; the total is the sum of these rounded numbers.
Other considerations for vaccine eligibility
Male sex workers who have sex with other men, were considered to be among those most at risk of monkeypox and, therefore, eligible for targeted pre-exposure vaccination. A separate estimate for male sex workers has not been made as it is assumed that those eligible for vaccination based on the criteria in the Green Book and will be included by the method used and sub-groups defined above to create the estimated total.
UKHSA has advised that others who have frequent close and intimate contact with GBMSM at high risk of monkeypox may be vaccinated irrespective of their gender identity. This also would include staff who work in sex on premises venues, such as saunas, if they are regularly exposed to items (for example, linens) or surfaces likely to be contaminated with body fluids or skin cells. This offer could be combined with supplementary approaches to provide outreach vaccination to GBMSM at high risk of monkeypox who may not be in contact with SHS.
Assumptions used to estimate the number of GBMSM regularly attending SHS and at high risk of monkeypox
Provisional data on the number of GBMSM attending SHS in England between 1 April 2021 to 31 March 2022 were obtained from the GUMCAD STI surveillance system, a depersonalised dataset of all attendances at SHS in England.
At the time of publication of this technical briefing, this is the most up to date dataset of SHS attendances in England, but it is important to note that:
- the start of this time period (April 2021 to June 2021) coincides with the progressive easing of the third national lockdown in response to COVID-19 (SHS service delivery was still lower than pre-COVID-19 pandemic levels in Spring 2021)
- this time period also coincides with a rebound in SHS service provision after the easing of COVID-19 control measures in Summer 2021 and with the scale up of routine commissioning of HIV PrEP at SHS in England
Based on the proxy measures of high risk of monkeypox described above, the total number of GBMSM with these proxy markers of high risk of monkeypox that regularly attend SHS in England was calculated.
GUMCAD data is submitted quarterly by SHS in England to UKHSA. The GUMCAD estimates described above will therefore be reviewed when updated data became available.
The assumptions used to estimate the total number of people eligible for vaccination will be reviewed as further evidence becomes available.
4.2 Vaccines administered
As of 20 September 2022, a total of 45,049 administered doses of vaccine have been recorded, of which 40,426 doses have been administered as pre-exposure vaccination, primarily to gay, bisexual, and other men who have sex with men who are at highest risk of exposure. A further 2,103 doses have been given to healthcare workers managing monkeypox cases and 2,520 doses have been given to close contacts of cases.
Vaccination has been targeted to London where around 69% of the cases have been seen (Table 2). The recommended groups for vaccination are set out in UKHSA guidance.
Vaccination doses given by cohort and region are shown in Table 7.
Epidemiological curves by region, overlaying vaccine coverage are shown in Figures 7 and 8.
Table 7. Vaccination doses given by cohort and region as of 20 September 2022
| Region | GBMSM* | Staff | Community contacts (Post-exposure prophylaxis) | Total |
|---|---|---|---|---|
| East of England | 678 | 78 | 16 | 772 |
| London | 28,939 | 1,511 | 1,570 | 32,020 |
| Midlands | 1,668 | 158 | 505 | 2,331 |
| North East, Yorkshire and the Humber (NEY) | 1,419 | 192 | 201 | 1,812 |
| North West | 3,474 | 53 | 96 | 3,623 |
| South East | 2,702 | 63 | 30 | 2,795 |
| South West | 1,546 | 48 | 102 | 1,696 |
| Total | 40,426 | 2,103 | 2,520 | 45,049 |
*Also includes ancillary and other workforce in sex-on-premises venues.
Figure 7. Vaccination doses given in all cohorts by week* in (a) London and (b) other regions
Figure 7a (Note: London’s graph has a different scale)
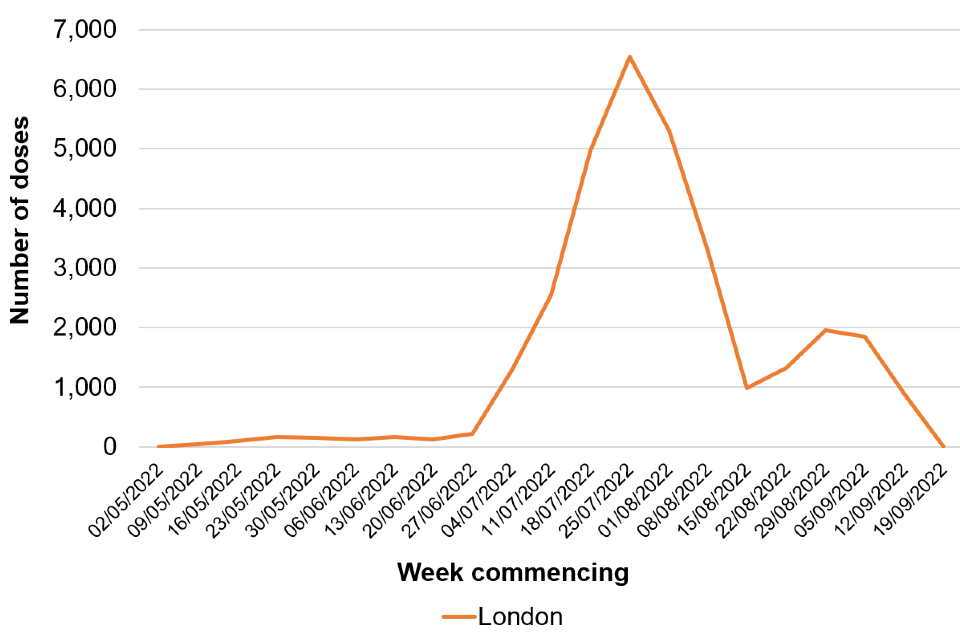
*Data from most recent week incomplete and likely to be lagged
Figure 7b
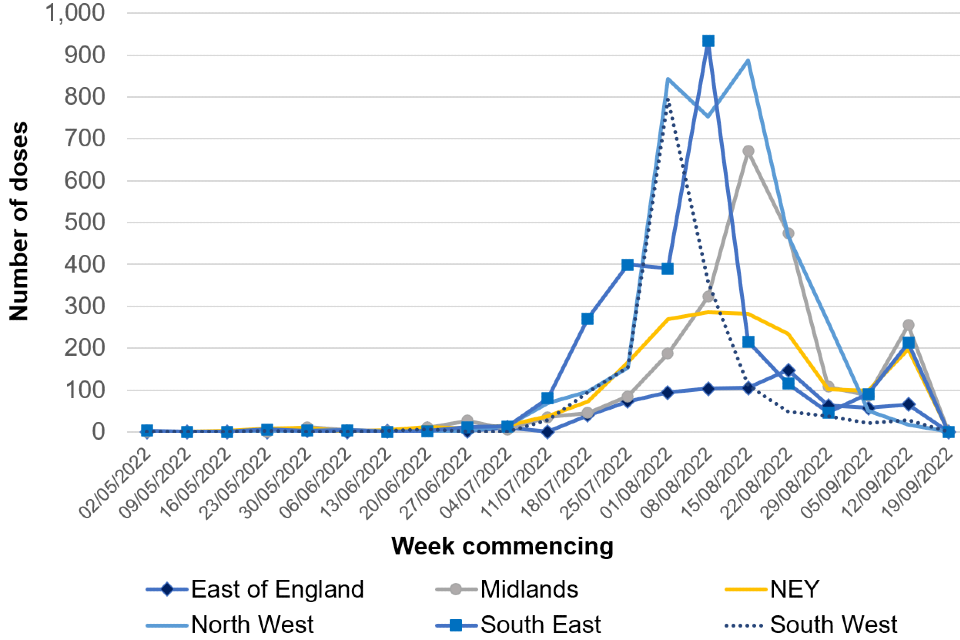
*Data from most recent week incomplete and likely to be lagged.
The data used in these graphs can be found in the accompanying spreadsheet.
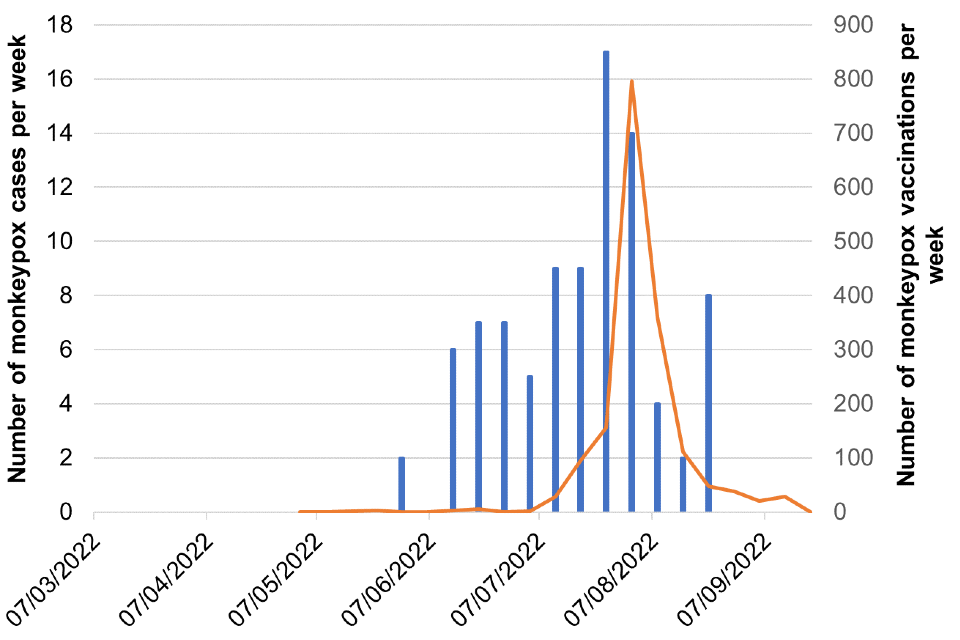
Figure 8. Cases and vaccination doses given by week as of 20 September 2022
Blue bars = number of cases, orange line = vaccination events
Figure 8a. East of England
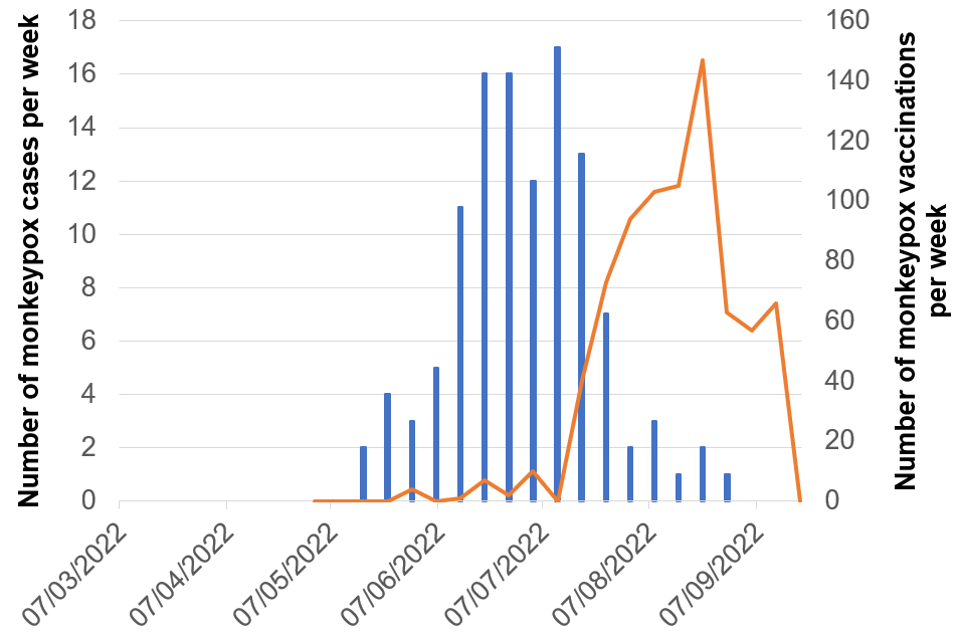
Figure 8b. London
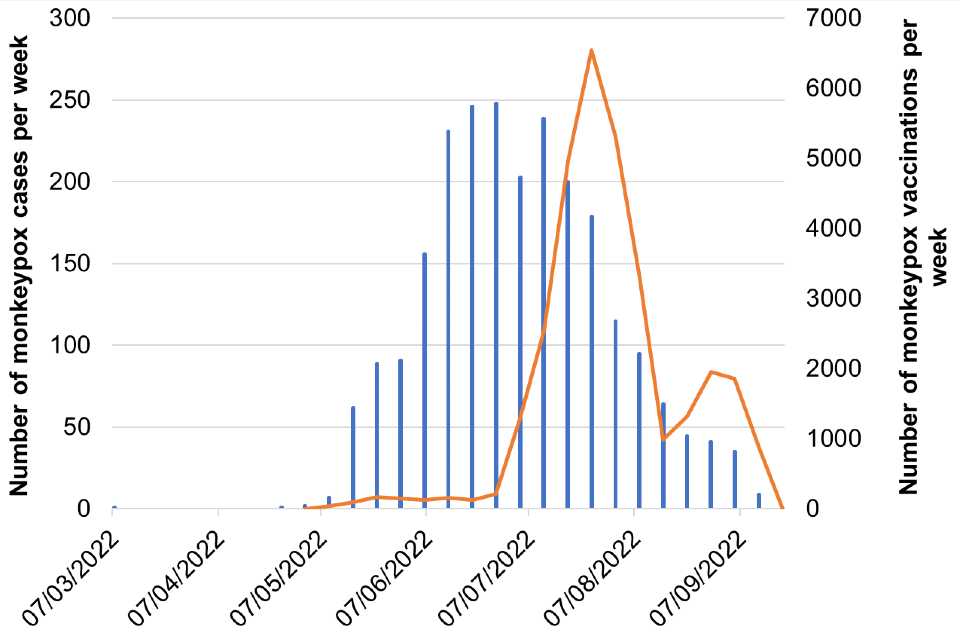
Figure 8c. Midlands
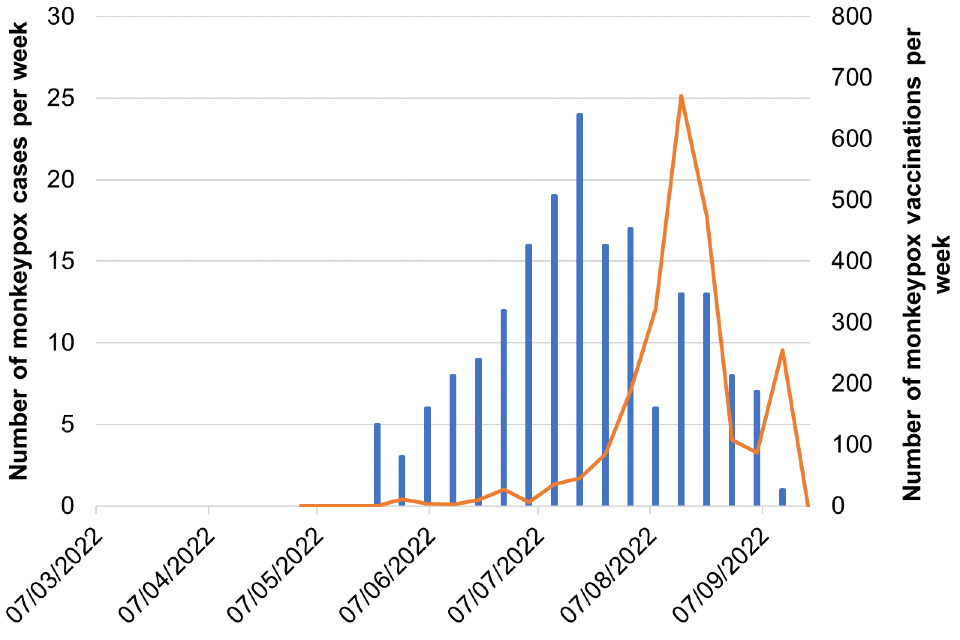
Figure 8d. North East and Yorkshire

Figure 8e. North West
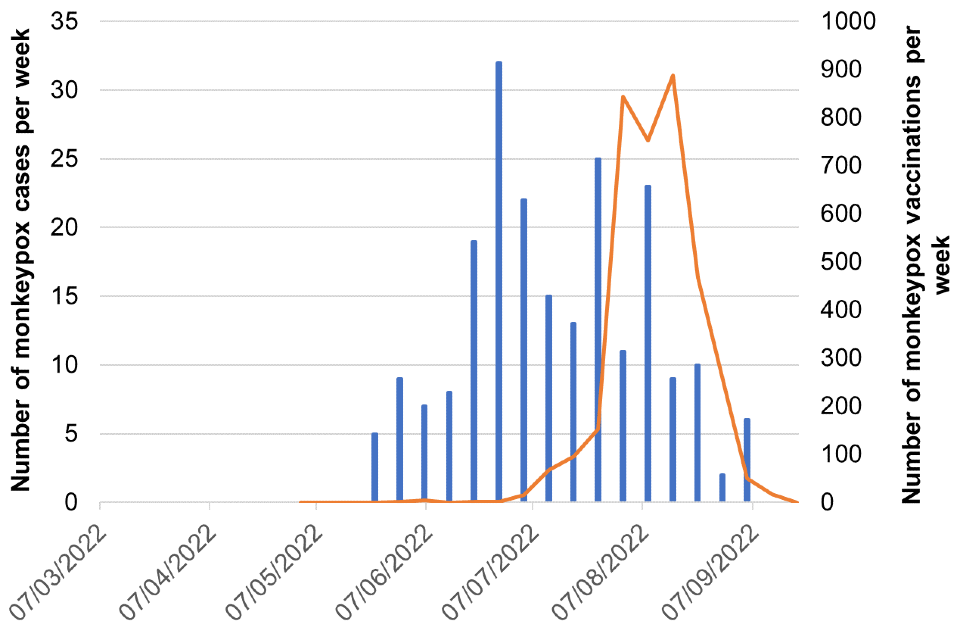
Figure 8f. South East
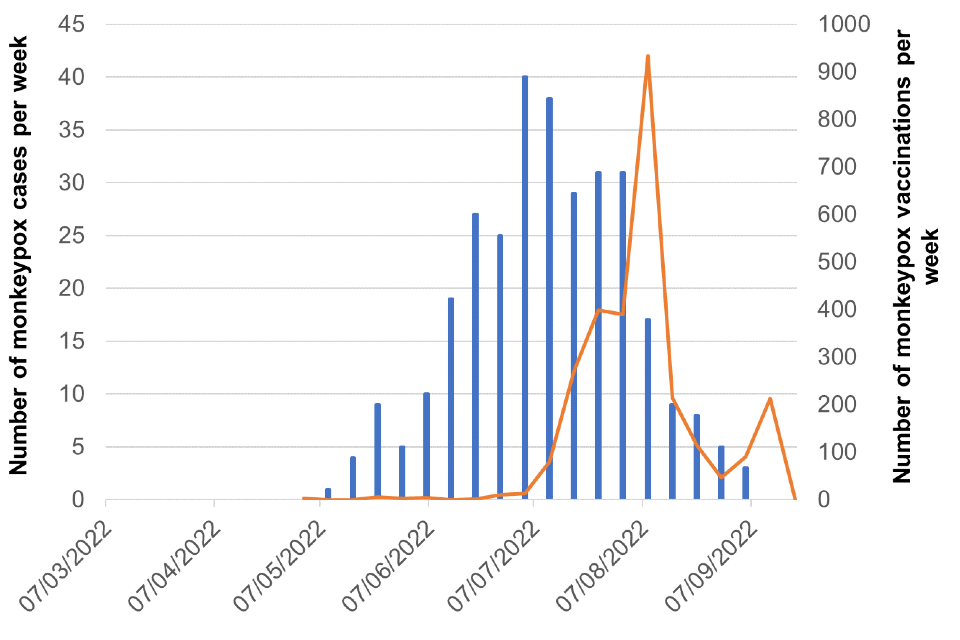
Figure 8g. South West
Most recent weeks subject to reporting delays.
The data used in these graphs can be found in the accompanying spreadsheet.
Part 5. Transmission dynamics
5.1 Modelling consensus from UKHSA and Joint Universities Pandemic and Epidemiological Research (JUNIPER) research challenge meeting
UKHSA and the JUNIPER Consortium convened 6 independent infectious disease modelling groups from 5 different institutions to discuss results of modelling work on the current monkeypox outbreak and understand the implications for public health management of the outbreak.
Modellers used a number of methodologies for modelling transmission of monkeypox virus through GBMSM and other groups. Imperfect data and uncertainty in the natural history and epidemiology of monkeypox virus contribute to uncertainty in these results. The following main conclusions represent a consensus between modelling groups:
- In the absence of vaccination or other mitigations, modelling suggests the final size of the monkeypox virus outbreak could have been between 0.4% and 12% of the sexually active GBMSM population in England infected. The various models run over different time period which may affect final size estimates. (Moderate confidence: This finding comes from a range of models, however, is very sensitive to model inputs and methods. Different models make slightly different assumptions and run over different time periods.). To date, the reported number of cases equates to about 0.5% of the estimated sexually active GBMSM population assuming 15% of the GBMSM population is abstinent based on Whittles and others (2017).
- Vaccination in England is likely to be reducing numbers of new cases of monkeypox virus in GBMSM substantially, with one model estimating that vaccines administered by the end of August could be reducing new weekly cases by around 47% already. (Low confidence: This estimate is based on uncertain assumptions, for example, that 80% of vaccines delivered have been to GBMSM with highest-risk behaviours).
- The effectiveness of vaccination is likely to be enhanced with careful targeting towards individuals most at risk. Modelling of multiple scenarios suggests that vaccinating 25% of GBMSM with the highest risk behaviour reduces cumulative incidence post vaccination by around 70% (Moderate confidence: This finding is sensitive to model inputs and assumptions).
- Models of sexual behaviour in GBMSM, women, and men who have sex exclusively with women do not predict exponential growth in case incidence outside of GBMSM (High confidence: This finding is supported by several models. There is some potential for under-ascertainment of female cases).
- The causes of the decline in case incidence in England are highly uncertain but changing sexual behaviour in GBMSM is identified as one of the most credible potential reasons by several modelling groups (Low confidence: Not all models supported this, and there are other plausible factors).
Appendix 2 contains details of the questions posed to modelling groups, the methodologies used, further details on the main conclusions and known uncertainties surrounding these results. Preprints of 2 of the groups’ research are publicly available (Brand and others (2022) and Endo and others (2022).
Part 6. Genomic surveillance
6.1 UK and international phylogeny
The phylogeny of monkeypox virus is separated into Clade I (formerly known as central African or Congo Basin clade; reportedly associated with more severe outcomes), Clade IIa (formerly known as West African clade; outbreak associated with imported animals), and Clade IIb, which contains the lineage associated with the current outbreak as well as some other genomes. All UK genomes in 2022 fall into Clade IIb.
Of 3,439 confirmed cases in the UK, sequencing has been attempted for 279. A total of 222 cases have at least one sequence that passed quality control (QC) (70% genome coverage more than or equal to 30X and 90% genome coverage more than or equal to 20X). Two hundred cases have one sequence and 22 cases have more than one sequence available. Cases are chosen for sequencing if at least one sample has a monkeypox PCR cycle threshold below 20, or the case is considered a potential high consequence infectious disease case due to recent travel to West or Central Africa.
Of the 222 UK cases with at least one sequence available, all cases fall within Clade IIb. Two hundred and nineteen are lineage B.1, 2 are lineage A.2 and one is lineage A.3. One further A.2 sequence has been analysed through a currently unvalidated method and is being confirmed. All non B.1 lineages are associated with travel.
Both globally and within the UK, the primary outbreak lineage remains B.1 (Figure 9). Within B.1 in the UK, a small amount of diversity has accrued (Figure 10).
Figure 9. Phylogenetic tree of Clade IIb sequences
All UK sequences that have passed QC are included in the tree. Clade IIb genomes in Genbank that are not from the 2022 UK cases and are at least 180 kb (kilobases) in length are also included.
The sequences are processed using squirrel to align to reference NC_063383 and mask repetitive regions. The 5’ inverted terminal repeat (ITR) sequence is also masked. The tree was generated using the Jukes-Cantor method in iqtree. Sequences are coloured by WHO region and lineages B.1, A.2, and A.3 are labelled.
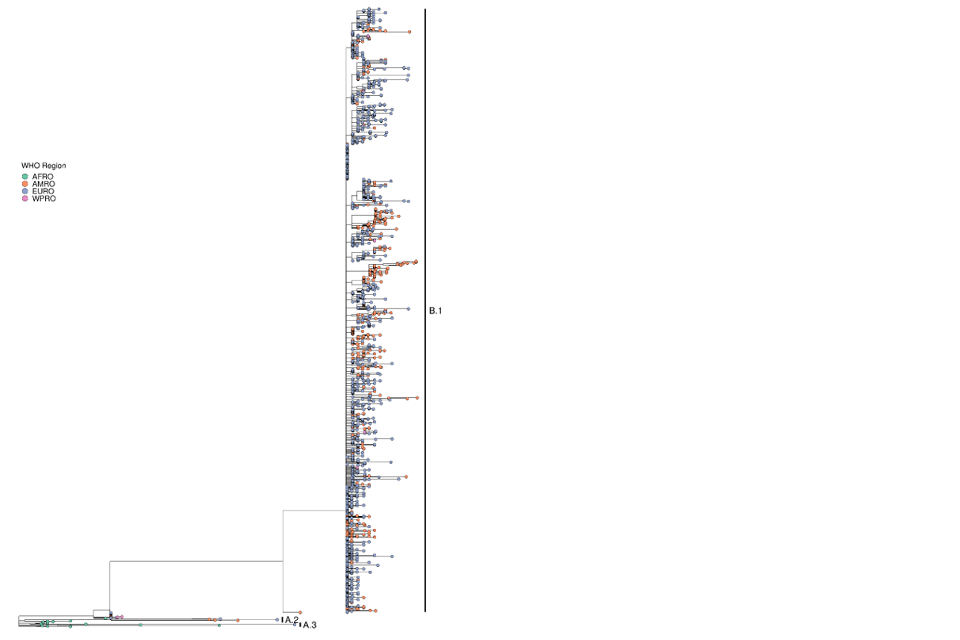
Supplementary data is not available for this figure.
A large format version of this figure is also available.
Figure 10. Phylogeny of Clade IIb UK sequences
Sequences are coloured by lineage and labelled by the sample identifier used in the isolate field for Genbank. The sequences are processed using squirrel to align to NC_063383 and mask repetitive regions. The 5’ ITR is also masked. The tree was generated using the Jukes-Cantor method in iqtree.
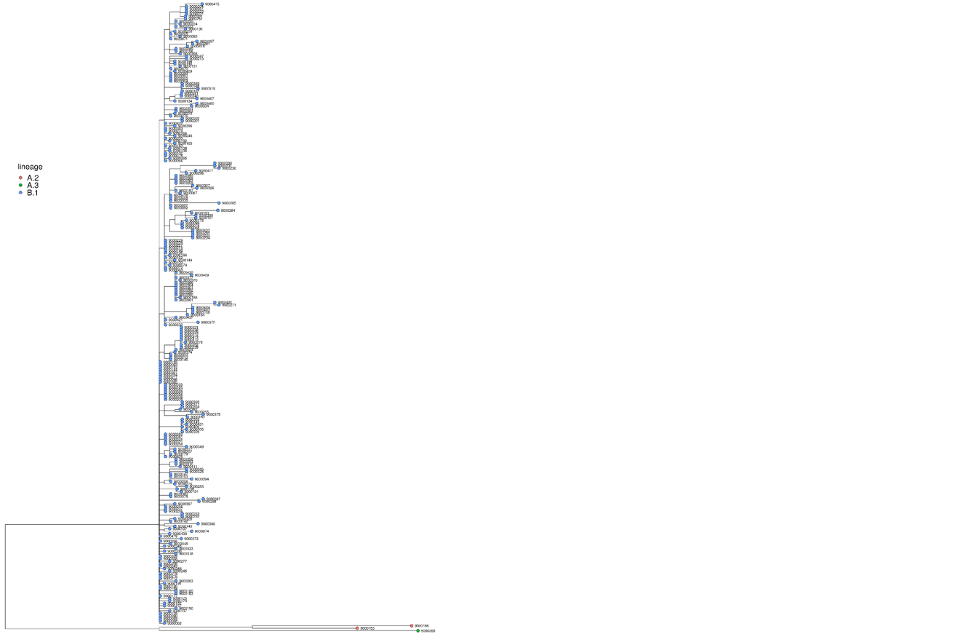
Supplementary data is not available for this figure.
A large format version of this figure is also available.
6.2 In host diversity
In host diversity is being monitored through routinely available samples to understand its potential impact on genetic surveillance and analysis of the monkeypox outbreak. As yet sample numbers are too limited to draw conclusions, but interim findings are reported here.
Twenty-one cases have more than one sequenced sample available. Of those, full sample site data is available for 9 cases (8 cases with 2 sequences, 1 case with three sequences). A further 3 cases have at least 2 sequences available with known differing sample dates, although sample site is not able to be distinguished currently (2 cases with 3 sequences, 1 case with 2 sequences).
Of the 9 cases with sample site data available, 4 cases have no mutations differing between sequences. The maximum diversity between sequences from the same case is 2 mixed bases and 2 mutations. Comparisons of the other 4 sequences included a combination of mixed bases and mutations. The maximum number of mixed bases between any of these 2 sets of sequences is 4.
Of the 3 cases with different sequence sample dates, the difference between sample dates is 5 to 6 days. There are no mutational differences between sequences from the same case.
Sources and acknowledgments
Data sources
Monkeypox virus PCR results are submitted to UKHSA daily by the Rare and Imported Pathogens Laboratory, Porton, UKHSA regional laboratories and NHS laboratories undertaking testing. Data on people testing positive since 6 May 2022 is enhanced with demographic, symptom, epidemiological, and exposure information extracted from the UKHSA Health Protection Team case management system (HPZone), or collected in enhanced surveillance questionnaires.
Enhanced surveillance questionnaires include data collected via rapid sexual health questionnaires administered during the initial weeks of the outbreak (used from 27 to 31 May 2022), questionnaires completed by health protection teams during telephone interviews (used from 1 to 24 June 2022), and self-completed questionnaires sent electronically to cases (used from 25 June 2022 onwards).
Data on estimated number of GBMSM at high risk of monkeypox regularly attending SHS in England was collected from GUMCAD (STI) Surveillance System. Estimated number of GBMSM at high risk of monkeypox regularly attending SHS in the other Nations of the UK collected from ONS data on sexual orientation.
Authors of this report
Zahidul Abedin, Paula Blomquist, Jessica Bridgen, Chloe Byers, Lorenzo Cattarino, Meera Chand, Andre Charlett, Kristine Cooper, Fergus Cumming, Julie Day, Helen Fifer, Kate Folkard, Eileen Gallagher, Ashley Goddard, Irene Gonsalvez, Alice Graham, Freddy Green, Natalie Groves, James Guilder, Susan Hopkins, Luke Hounsome, Timothy Laurence, Leo Loman, Harry Long, Jamie Lopez-Bernal, Nicola Love, Hamish Mohammed, Richard Myers, Christopher Overton, Karthik Paranthaman, Mateo Prochazka, Andrew Rambaut, Natasha Ratna, Steven Riley, James Sedgwick, Katy Sinka, Roberto Vivancos, Thomas Ward, Nick Watkins, William Welfare, Katie Wrenn.
Contributors
- UKHSA Data, Epidemiology and Analytics Cell
- UKHSA Research and Science Cell
- UKHSA Modelling Cell
- UKHSA Genomics Public Health Analysis
- UKHSA Sexual Health Liaison Group
- UKHSA Monkeypox Incident Management Team
- JUNIPER consortium
- Imperial College London
- London School of Hygiene and Tropical Medicine
- International University of Health and Welfare
- University College London
- University of Bristol
- University of Manchester
- University of Edinburgh
- University of Warwick
Monkeypox Technical Group
The Monkeypox Technical Group includes members with expertise in clinical infectious diseases, clinical research, epidemiology, genomics, modelling, and virology:
- Meera Chand (Chair), UKHSA
- Andre Charlett, UKHSA
- Andrew Rambaut, University of Edinburgh
- Alison Rodger, University College London
- Allan Bennett, UKHSA
- Ashley Otter, UKHSA
- Calum Semple, University of Liverpool
- Christophe Fraser, University of Oxford
- Claire Dewsnap, British Association for Sexual Health and HIV
- Claire Gordon, UKHSA
- Colin Brown, UKHSA
- David Ulaeto, Defence Science and Technology Laboratory
- Erik Volz, Imperial College London
- Emma Thomson, Medical Research Council-University of Glasgow Centre for Virus Research
- Fergus Cumming, UKHSA
- Giri Shankar, Public Health Wales
- Geoffrey L. Smith, University of Cambridge
- Geraldine O’Hara, High Consequence Infectious Diseases Network
- Health and Social Care Northern Ireland
- Helen Fifer, UKHSA
- Isabel Oliver, UKHSA
- Jake Dunning, University of Oxford and Royal Free Hospital
- Jason Mercer, University of Birmingham
- Maria Rossi, Public Health Scotland
- Matt Keeling, Warwick University
- Matt Phillips, British Association for Sexual Health and HIV
- Natalie Groves, UKHSA
- Neil Ferguson, Imperial College London
- Nicholas Loman, University of Birmingham and UKHSA
- Obaghe Edeghere, UKHSA
- Richard Myers, UKHSA
- Steven Riley, UKHSA
- Susan Hopkins, UKHSA
- Tommy Rampling, UKHSA
Acknowledgements
The authors are grateful to those teams and groups providing data for these analyses including:
- British HIV Association (BHIVA)
- British Association for Sexual Health and HIV (BASHH)
- Sexual Health Services
- NHS England and Improvement
- High Consequence Infectious Diseases Network
- Public Health Scotland
- Public Health Wales
- Public Health Agency, Northern Ireland
- Representatives of the Monkeypox Consensus Coalition
Appendix 1. Glossary of acronyms and terms
| Acronym or term | Definition |
|---|---|
| APOBEC3 | Apolipoprotein B mRNA editing enzyme catalytic polypeptide 3 |
| Assortative | A network model is assortative just if high-degree nodes (that is, nodes with high numbers of edges) are more likely to form edges with other high-degree nodes |
| BASHH | British Association for Sexual Health and HIV |
| Clade | A group of organisms evolved from the same ancestor |
| CrI | Credible interval |
| EMIS | European Men’s Internet Survey, 2017 |
| GBMSM | Gay, bisexual or other men who have sex with men |
| GUMCAD | Genitourinary Medicine Clinic Activity Dataset, the mandatory surveillance system for STIs |
| HCID | High consequence infectious disease, defined according to set criteria. |
| HIV | Human immunodeficiency virus |
| ITR | Inverted terminal repeat sequence |
| Jukes-Cantor method | A model which computes probability of substitution (nucleotides, codons, amino acids) from one state to another |
| JUNIPER consortium | Joint Universities Pandemic and Epidemiological Research, a consortium of modelling groups from 8 universities: Warwick, Cambridge, Exeter, Lancaster, Manchester, Oxford, London School of Hygiene and Tropical Medicine and Bristol. |
| Kb | Kilobase, a unit of measurement in molecular biology equal to 1000 base pairs of deoxyribonucleic acid (DNA) or ribonucleic acid (RNA) |
| Lineage | A sequence of species, each of which has evolved from its predecessor |
| MPXV | Monkeypox virus |
| MSEW | Men who have sex exclusively with women |
| MSM | Men who have sex with men |
| MSMAW | Men who have sex with men and women |
| NATSAL | National Survey of Sexual Attitudes and Lifestyles |
| NIHR | National Institute for Health Research |
| HPRU | Health Protection Unit |
| PCR | Polymerase chain reaction |
| Phylogeny | The study of the evolutionary history of a species or group of genetically related organisms |
| PrEP | HIV pre-exposure prophylaxis |
| RiiSH-COVID | Reducing Inequality in Sexual Health survey of GBMSM, implemented during the COVID-19 pandemic |
| R0 | The basic reproduction number of the relevant infection, that is, the expected number of cases directly generated by one case in a population where all individuals are susceptible to that infection |
| Rt | The average number of cases directly generated by one case at time t |
| SAR | Secondary attack rate: the proportion of an index case’s contacts that are infected. In models it refers to infections by the index case only. |
| SEIRV | Susceptible, Exposed, Infected, Recovered, Vaccinated |
| SHS | Sexual health services |
| SNP | Single nucleotide polymorphism, a substitution of a single nucleotide base at a specific position in the genome |
| STI | Sexually transmitted infection |
| Stochastic | Having a random probability distribution that may be analysed statistically but may not be predicted precisely |
| US CDC | US Centers for Disease Control and Prevention |
Appendix 2. UKHSA and JUNIPER modelling meeting
Appendix 2.1. Introduction
UKHSA and the JUNIPER Consortium convened 6 independent infectious disease modelling groups from 5 different institutions to address a series of research questions relevant to the current monkeypox outbreak:
- What are credible ranges of the final size of monkeypox virus (MPXV) in GBMSM in England without vaccination (that is, expected cumulative proportion of GBMSM infected)?
- What impact will vaccination have on MPXV incidence in England (different scenarios could involve targeting vaccination at groups with riskier behaviour)?
- What probability do people put on MPXV growing exponentially in the men who have sex exclusively with women (MSEW), or women part of the sexual network in England?
- What are the implications of adding non-sexual close contacts into network models (for example, household) in England? How consistent could this be with historic patterns of transmission?
- How long temporally does modelling imply there will be domestic transmission of MPXV in England?
Preprints of 2 of the groups’ research are publicly available:
A table of methods and written summary are included in Appendix 2.2, the main conclusions to date are covered in Appendix 2.3 and known uncertainties are covered in Appendix 2.4.
Appendix 2.2. Methods
Table 8. Summary of model progress to date, each line is a model by a different group, all models are work in progress
| Model focus | Network structure | Data used to inform the network | MPX model structure | Disease model parameters | Disease model calibration methods | Other model features |
|---|---|---|---|---|---|---|
| GBMSM sexual contacts | Stochastic pairs approximation model to simulate the behaviour of a network (Miller and Volz, 2013; Volz and Meyers, 2007) The network allows for longer term, medium term and one off partnerships | US Data from Anderson and others (2021) and Jenness and others (2016) for sexual contact patterns and ONS for Gay and Bisexual identifying population for network size | SEIRV | Incubation period and infectious period matched to UKHSA estimates, so the mean incubation period is 8 days and mean infectious period is 4. Susceptible (S) to Exposed (E) transition rate based on a random walk and calibrated. Allows for stochastic continued importations. | Fit to England monkeypox (MPX) case line list using an Mif2 particle filtering algorithm from the pomp R package | Case ascertainment was included in the model by assuming observation was a partially observed Markov process. Case ascertainment was constrained to be between 20% to 80%. |
| GBMSM, and women and MSEW | Stochastic branching process (assortative mixing) Degree distribution follows a truncated Weibull distribution to account for heavy skew in partnership numbers. | Natsal, Natsal-2, Natsal-3, Natsal-COVID, illustrative 500,000 population for GBMSM in England | Basic transmission model with susceptible depletion. | The model was run for a range of values of the secondary attack rate (between 0.1 to 0.5) | Not calibrated to case data, ran a wide range of parameter values | The model assumes perfect case ascertainment. |
| GBMSM sexual contacts | Dynamic network using a separable temporal exponential random graph model framework using EpiModel R package The network consists of steady and non-steady (including one-time) partnerships. Krivitsky and Handock (2014) and Krivitsky and Morris (2017) were used to inform network structure | NATSAL-3 for sustained sexual contacts and European Men’s Internet Survey (EMIS) to inform the one-time partnership rate and geographical cluster distribution. | SEIR (plans to be SEIRV before publication) | A grid of parameters was run: infection per contact probability 0.1 to 0.5 and infectious period 2 to 28 days | Not calibrated to case data, ran a wide range of parameter values, calibration planned | Individuals in the model belong to geographical clusters, whose distribution followed the distribution of EMIS participants across urban conurbations in the UK. Partnerships have a data-informed probability of occurring between individuals resident in the same geographical structure, but there are not spatial effects in the model. |
| GBMSM and women / MSEW sexual contacts | Static network constructed with preferential attachment | Reducing Inequalities in Sexual Health Wave 4 Survey (unpublished) for sexual contacts illustrative 50,000 node network | SEIRV | Incubation period based on UKHSA estimates. S to E transition rate is fit for the early period and late period, and infectious period are both calibrated based on the England case line list. | Fit to England MPX case line list using Approximate Bayesian Computation Sequential Monte Carlo using pyABC Python package | The model assumes perfect case ascertainment |
| All population sexual and non-sexual contacts | Compartmental model with whole population mixing and sexual mixing within GBMSM populations MSM partitioned into 10 subpopulations to capture partner number overdispersion | Distribution fit based on NATSAL-3 and GRASP by Whittles and others (2019) | SEIRV | The incubation period is assumed to have a mean of 8.8 days based on Miura and others (2022) Vaccine effectiveness is assumed to be between 70% to 85% from one dose All parameters are estimated through calibration | Fit to publicly available England MPX data using Approximate Bayesian Computation Sequential Monte Carlo using ApproxBayes Julia package | Vaccination rollout and effectiveness model Case detection model means all cases have an observation probability, which is estimated to be between 30% and 95%, this is likely driven predominantly by the priors. |
| GBMSM sexual contacts | Static network similar to a configuration model with either no-enforced assortativity or maximum-enforced assortativity with respect to number of contacts. The network is going to be a stochastic block model | Reducing Inequalities in Sexual Health Wave 4 Survey (unpublished) for GBMSM contacts, and Natsal-3 data for MSEW networks. Illustrative 20,000 and 50,000 node networks. | SEIRV | The disease model has only been initialised with illustrative values to date. | Not calibrated to case data, ran a wide range of parameter values, calibration planned | The model allows for imperfect case ascertainment The model allows for isolation in individuals who have their infection ascertained |
Populations modelled
All the models focused on sexual contact, as that has been evidenced to be the dominant route of transmission of MPXV in England. One of the models also included the possibility of types of transmission other than sexual contact (with a background rate of human-to-human transmission). Some models focused exclusively on GBMSM, whereas other models also included women and MSEW.
Networks constructed
One model approximated network structures by grouping together individuals with the same number of sexual contacts or those in the same geographical location. Another used a branching process, where the sexual contacts of a case are drawn from a distribution. A third modelled the formation and dissolution of partners using a stochastic pairs approximation. All the other models use an explicit network structure, with either a static (partners are constant), or dynamic network (allowing for partner formation and dissolution).
The networks incorporate heterogeneities in different ways: allowing assortativity based on geography, or so that individuals with high numbers of partners are more likely to form partnerships with other high-partnership individuals.
Data for networks
Networks generally focused on sexual partnerships in England, though some covered the wider UK. Commonly used survey data on sexual partners were: Britain’s National Survey of Sexual Attitudes and Lifestyles (Natsal), and unpublished data from the Reducing Inequality in Sexual Health during the COVID-19 pandemic (RiiSH-COVID) Survey of GBMSM. See Mercer and others (2016), Howarth and others (2021) and Brown and others (2022) for publications on sexual behaviour in GBMSM using these surveys. One model also used results from the European Men’s Internet Survey (2017). The surveys were used to calibrate the networks for a desired degree distribution.
The surveys only capture how many partners people have, not who those partners are, meaning expected assortativity of sexual partners by number of partners is not known. Natsal-3 only captures limited information to estimate assortativity with respect to age, or geography, which also is built into some models (participants give details about their three most recent same-sex partners only). Other evidence was required for other target features of networks created. One of the models which disaggregated partnerships into different types (long term, short term and one off) used US data from Jenness and others (2016) and Anderson and others (2021) to achieve this. Some modellers fit distributions (for example, power law) through observed partnership numbers from surveys, whereas other sampled directly from the observed partner distribution.
Transmission models
Mechanistic disease models were used to simulate MPXV spreading through the relevant population (for example, GBMSM for some models, or the whole population for others). The most common disease model structure used was a SEIRV model where individuals move from being Susceptible, to Exposed (but not infectious), to Infectious and then to Recovered (and immune from future infection). In some scenarios, vaccination was modelled, so susceptible individuals could gain immunity through vaccination without ever being infected.
One of the models was built on a simpler transmission framework, but still included susceptible depletion. None of the models included the possibility of waning immunity over the modelled period.
Some of the models that included vaccination assumed “all or nothing” vaccination, where individuals either receive total protection from vaccination or remain totally susceptible. Other models assumed “leaky vaccination”, where all individuals who are vaccinated have a chance of becoming infected, albeit at a reduced rate. Vaccinated individuals who experienced breakthrough infections were assumed to transmit infection equally to unvaccinated infectious individuals.
Some models allowed for imported cases to occur throughout the model period, so individuals could become infected without contacting an infected node within the model; importations were generally at low levels (around 2 per day). Fomite or zoonotic routes of transmission were not captured in any of the models.
Disease parameters and calibration
The main area where modellers pointed to evidence supporting input parameters was on the incubation period of MPXV, which has been estimated but is still uncertain (Miura and others, 2022; UKHSA, 2022). Other parameters were considered to be even more uncertain. This uncertainty means models were either calibrated to case data (with methods detailed below) or a broad range of combinations of parameters were used to account for considerable uncertainty. All the disease models presented were stochastic, so individuals in the models changed states probabilistically rather than deterministically.
One disease model was formulated as a partially observed Markov process and fit to English case data using maximum likelihood methods. Two other disease models were calibrated to English case data using Approximate Bayesian Computation – Sequential Monte Carlo. Three disease models had not been calibrated to case data yet at the time of the meeting.
Additional model features
Different models treated case ascertainment separately. Some models were calibrated (or compared) to case data with no parameter introduced for the inevitable difference between infections and confirmed cases. Other models did allow for the number of infections to be larger than the number of observed cases, with implications for cumulative proportion of the population estimated to have been infected and recovered. One of the models included isolation of ascertained cases preventing further infections from that case (where perfect compliance is a limiting simplifying assumption).
Appendix 2.3. Key conclusions to date
What are credible ranges of the final size of MPXV in GBMSM in England without vaccination (expected cumulative proportion of GBMSM infected)?
One model estimated the likely final size of the epidemic to be between 2,500 to 6,000 out of 50,000 nodes (5.0% to 12.0%, CrI 89%) in the network of sexually active GBMSM without vaccination, but this is subject to greater uncertainty than the credible interval implies (see Appendix 2.4, Known uncertainties). Another group hypothesised the final size could be greater than the other models implied. It could be 20% of GBMSM in the absence of mitigation or behaviour change, however it would take multiple years for these infections to occur. One model that was not calibrated to case data suggested it was likely that the final size in GBMSM would be higher in London than other parts of England, where the model is initialised with 20 infected individuals all in London. Finally, one model found that even without vaccination, the final size could be as low as between 0.4% to 3.0% of sexually active GBMSM in a given country, where the model was run for plausible rates of the secondary attack rate (proportion of an index case’s contacts infected).
What impact will vaccination have on MPXV incidence in England (different scenarios could involve targeting vaccination at groups with riskier behaviour)?
With the epidemic in exponential decline in England before vaccination was widely administered, models calibrated to case data saw incidence peak even without vaccination.
One model suggested vaccination administered to date in England could have reduced estimated new symptomatic infections by approximately 47% by the end of August. This assumes 50,000 vaccinations administered to GBMSM in England by the end of August 2022 and effectiveness of one dose is 65% against infection with 2-week delay. Eighty percent of these vaccines are administered to GBMSM with high-risk behaviour and 20% are administered at random. This scenario is shown in Figure 11.
Another model running prospective hypothetical vaccination scenarios suggested that administering vaccinations to 25% of GBMSM (targeted at GBMSM with higher numbers of partners) could reduce case incidence post vaccination by around 70%. This hypothetical and illustrative scenario assumed that all vaccination happens on one day and takes effect instantly and that vaccine effectiveness following one dose is 65% The effectiveness of the vaccination after one dose is highly uncertain and 65% was chosen by several modelling groups for consistency rather than because there is a strong evidence base.
Another model supported the case that continued vaccination will have a large impact on bringing case incidence in England to low levels more quickly, this group used 70% to 85% vaccine effectiveness.
Figure 11. Counterfactual scenario where no vaccines had been administered to date in England
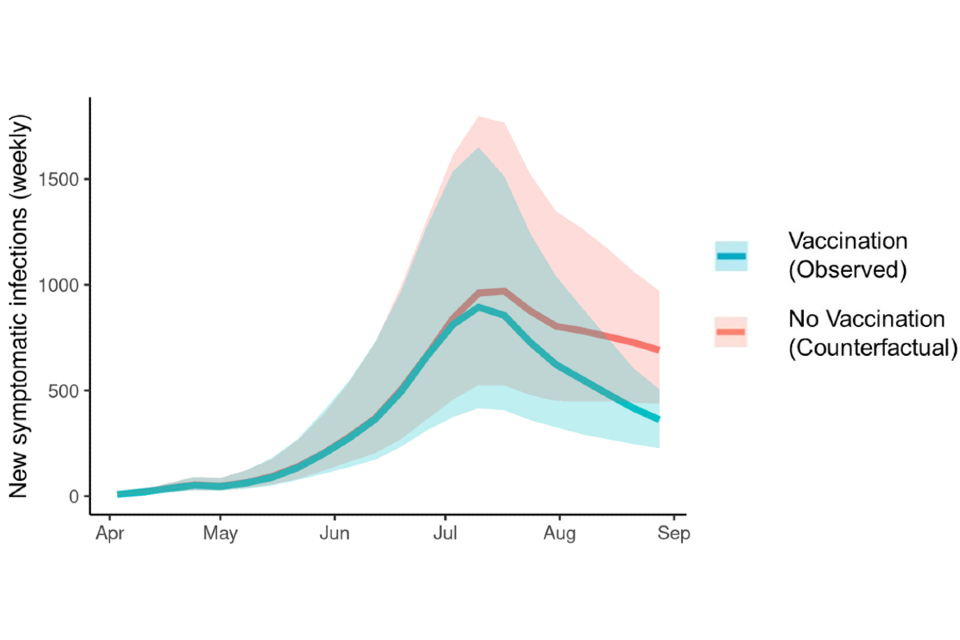
Supplementary data is not available for this figure.
Figure 12 shows the results of a simple analysis run by one of the groups. Different proportions of high contact nodes are removed from the network and the proportion of nodes still in the largest component (sub-network) is calculated. The network of GBMSM already starts to disassemble with as few as 5% of high contact nodes removed, meaning there is no longer one network that connects all nodes. It is almost totally disassembled when 20% of nodes are removed. This implies vaccinating high risk GBMSM could disproportionately reduce MPXV transmission across the network because changes in edges (partners) would be required to spread the virus between the sub-networks that remain. This network was informed by unpublished RiiSH-COVID data. We note that MPXV was already present across a wide portion of the transmission network when vaccination occurred so transmission would continue in multiple disconnected components.
Figure 12. The proportion of a network simulating GBMSM sexual contacts that remains within a largest component when high-degree nodes are iteratively deleted
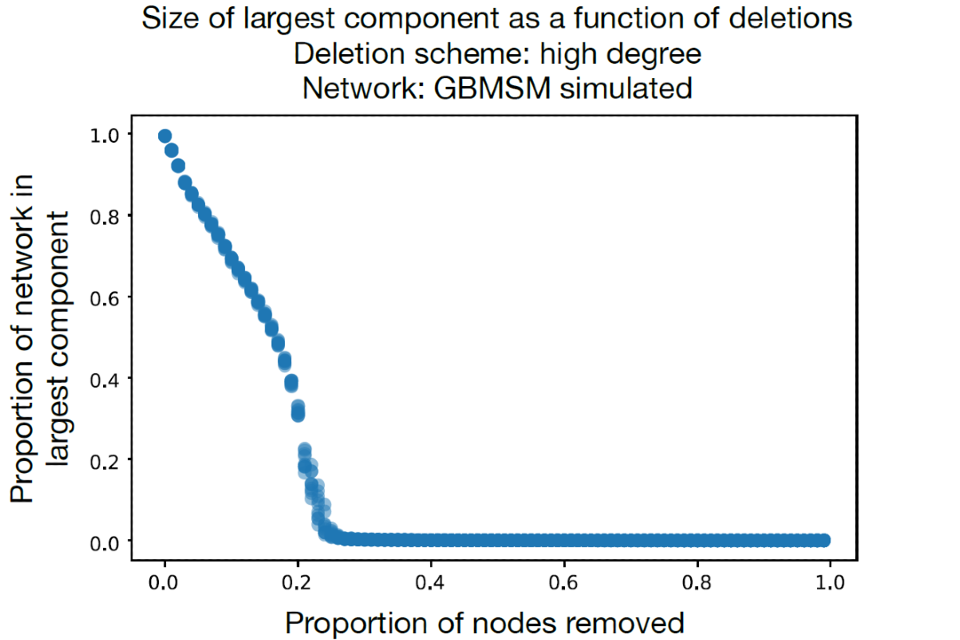
Supplementary data is not available for this figure.
What probability do people put on MPXV growing exponentially in the men who have sex exclusively with women (MSEW) or women part of the sexual network in England?
All modelling groups that attempted this question found that the probability of MPXV growing exponentially in sexual networks other than GBMSM was low. One model expected only about 1% of cases to be in MSEW and women, most of these cases came from men who have sex with men and women (MSMAW) infecting women, rather than chains of transmission between women and MSEW. This scenario assumes the secondary attack rate through sexual contact is the same for sex between men and heterosexual sex. Another model supported these results, suggesting that across a range of combinations of parameters sufficient to create exponential case growth in GBMSM models did not describe exponential growth in women and MSEW. Finally, one model suggests that the secondary attack rate in sexual partners would have to be over 75% to have Rt greater than 1 in the women and MSEW sexual network. Several participants commented that available survey data may not capture some groups, such as female sex workers, effectively.
A caveat to these findings is that the models are heavily dependent on available case data. When low numbers of cases in women have been observed in England, it is unsurprising that models consider high case growth unlikely. If there have been insufficient infections in women for outbreaks to take off (as spreading from GBMSM to women with high numbers of partners is a highly stochastic process), or there is major under-ascertainment of infections in women and MSEW, then these results may not hold. Though, this under ascertainment infections of women would have to be a wider phenomenon, as there is evidence that MPXV is concentrated in GBMSM in countries other than England.
What are the implications of adding non-sexual close contacts into network models (for example, household) in England? How consistent could this be with historic patterns of transmission?
This question was generally not answered by the modelling groups, partly because cases still appear to be concentrated in GBMSM, so transmission through sexual contact appears to be much more important than other types of contact. One model found that the posterior distribution of the non-sexual R0 had density almost exclusively below 1, even during the phase of the epidemic in England when case incidence grew relatively quickly. This suggests that it is highly unlikely MPXV could grow exponentially without the frequency of close contact observed in parts of the sexual network. This result is highly uncertain. Several modelling groups plan further development to answer this question in the future.
How long does modelling imply there will be domestic transmission of MPXV in England?
All models predicted only one peak in cases and then low levels of cases driven by importations and short chains of domestic transmission. As such, a resurgence in case incidence is not expected in England in the short term in the base case of the models used here. This means domestic transmission of MPXV is likely to be predominantly driven by uptake of vaccination, the rate of importation and the degree of sub-critical transmission leading to outbreaks in GBMSM with risk behaviours who remain susceptible. Longer term scenarios were not attempted. Various known and unknown factors may mean there is considerable deviation from the base case and that case incidence starts increasing again. See Appendix 2.4 for more information.
Estimates of R0 and Rt in England from models
One model, which has the shortest generation time estimates that R0 was approximately 1.5, with the latest Rt around 0.8. Another model estimates R0 was approximately 2.0 (1.5 to 2.5, CrI 89%), with the latest Rt approximately 0.6 (0.4 to 0.9, CrI 89%). Another model suggests R0 could have been considerably higher with R0 between 2.8 and 5.5 for a plausible range of values of the secondary attack rate. However, the R number falls very quickly in this latter model as individuals with high numbers of partners become infected and are no longer susceptible. Yet another model finds that the early Rt, as of early May 2022, was 6.3 (1.8 to 12.0, Crl 80%). As in other models, the R number falls away rapidly and in early September 2020 the Rt estimate was 0.6 (0.3 to 1.0, Crl 80%). Rt less than 1 implies case incidence is falling.
Reasons for MPXV case incidence peaking in England and other countries
One model could explain the peak in case incidence in England in July 2022 based on network structure and susceptible depletion alone. Though there was a question about whether the partner distribution of infections this implies is consistent with UKHSA data published in previous technical reports.
Three other models could explain the peak most plausibly through reductions in the transition parameter from Susceptible to Infected in July 2022 in England. The most plausible interpretation of this reduction in transmission is a reduction in sexual behaviour that was previously driving transmission; this could be reduced partnership numbers (as has been observed in survey data from the US CDC, 2022) or reduced probability of transmission per partner because infected people are less likely to have sexual contact now they know the symptoms of MPX.
Other factors that may be partially responsible for this reduction in transmission are:
- More overdispersion in sexual partnerships in GBMSM than implied by survey data, meaning those people infected to date had an even more disproportionate number of partners and impact on transmission than current network models estimate.
- Very high degree assortativity in sexual partnerships in GBMSM.
- Low case ascertainment meaning considerably more GBMSM have been infected and recovered than case data would imply.
- Overestimation of the GBMSM population in England, meaning a greater proportion of GBMSM have been infected and recovered.
- Reductions in imported cases which were increasing transmission.
However, factors 3 and 4 would also require other countries where cases have peaked, to have similarly low case ascertainment or overestimation of GBMSM population. Also, one model explicitly modelled 4 and found it did not lead to a rapid enough fall in Rt over the early period of the model to be a material driver of the fall in incidence.
Appendix 2.4. Known uncertainties
Before 2022, widespread human to human transmission of MPX in countries without a zoonotic reservoir had not been observed. As such, there is still considerable potential for our understanding of the transmission of this pathogen in the human population to be refined. These refinements may be reflected in future models and lead to different results.
Some known uncertainties are:
- uncertainty around numbers of partners and other sexual behaviour within GBMSM
- the potential risk of MPXV spreading in women and MSEW who have high numbers of partners
- whether survey data on sexual activity is a true representation of population level behaviour, or if there are biases and certain groups are under (or over) represented
- the exact structure of the sexual network of GBMSM in England, and the level of assortativity with respect to various characteristics in the network, and the extent of other network features like clustering
- the extent to which changes in sexual behaviour in GBMSM reduced transmission and whether behaviour will change again leading to a resurgence in cases
- how behaviour may have changed, for example whether there has been: a reduction in certain risky sexual behaviours, general reductions in partner numbers or changes in behaviour of infected people only
- the impact other relevant types of contacts (for example, household or social) may have on the conclusions reported above
- the extent of case ascertainment within GBMSM and other groups
- the exact impact of vaccination on individuals, duration of protection and how this influences transmission across the network
- the exact size of the GBMSM population in England
- uncertainty on numbers of importations and their contribution to domestic transmission
- the herd immunity threshold for GBMSM
Appendix 2.5. List of references
Anderson EJ and others (2021). ‘HIV and sexually transmitted infection epidemic potential of networks of men who have sex with men in 2 cities’
Brand S and others (2022). ‘The role of vaccination and public awareness in medium-term forecasts of monkeypox incidence in the United Kingdom.’
Brown JR and others (2022). ‘Changes in STI and HIV testing and testing need among men who have sex with men during the UK’s COVID-19 pandemic response.’
Endo A and others (2022). ‘Heavy-tailed sexual contact networks and the epidemiology of monkeypox outbreak in non-endemic regions, May 2022.’
European Centre for Disease Prevention and Control (2017). ‘EMIS-2017 The European men-who-have-sex-with-men internet survey.’
Howarth AR and others (2021). ‘“Stay at home …”: exploring the impact of the COVID-19 public health response on sexual behaviour and health service use among men who have sex with men: findings from a large online survey in the UK.’
Jenness SM and others (2016). ‘Impact of the Centers for Disease Control’s HIV preexposure prophylaxis guidelines for men who have sex with men in the United States.’
Krivitsky PN and Handcock MS (2014). ‘A separable model for dynamic networks.’
Krivitsky PN and Morris M (2017). ‘Inference for social network models from egocentrically sampled data, with application to understanding persistent racial disparities in HIV prevalence in the US.’
Mercer CH and others (2016). ‘The health and well-being of men who have sex with men (MSM) in Britain: Evidence from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3).’
Miller JC and Volz E (2013). ‘Model hierarchies in edge-based compartmental modeling for infectious disease spread.’
Miura F and others (2022). ‘Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022.’
UKHSA (2022). Investigation into monkeypox outbreak in England: technical briefing 2
Volz E and Meyers LA (2007). Susceptible-infected-recovered epidemics in dynamic contact networks.’
Whittles LK and others (2017). ‘A dynamic power-law sexual network model of gonorrhoea outbreaks.’
