Data collection and quality assurance of administrative data
Published 25 August 2021
Applies to England
Introduction
This document provides an overview of the data collection processes and an investigation into the administrative data sources used by the National Cancer Registration and Analysis Service (NCRAS) as part of Public Health England (PHE) to produce the cancer registration and cancer survival statistics for England.
The UK Statistics Authority, in accordance with the Statistics and Registration Service Act 2007 and signifying compliance with the Code of Practice for Statistics, has designated the following statistics as National Statistics:
- Cancer registration statistics, England
- Adult cancer survival within Cancer survival in England
- Geographic patterns of cancer survival within Cancer survival in England
- Index of cancer survival for Clinical Commissioning Groups in England
- Adult cancer survival by stage at diagnosis for England within Cancer survival in England
- Cancer survival for children in England within Cancer survival in England
Cancer statistics have been assessed as being of ‘medium public interest and value’ since they receive more than limited media interest but are not highly politically sensitive. However, data collection is more complex. Coming from the National Health Service (NHS), PHE and the Office for National Statistics (ONS); potential areas of risks are monitored and checks that are detailed below are developed to reduce the level of risk, resulting in a medium level of data quality concern.
Applying these ratings of ‘medium public interest’ and ‘medium level of data quality concern’ to the risk matrix produced by the Office for Statistical Regulation for evaluating quality assurance and audit arrangements, the cancer statistics are considered to be of medium risk.
An informal discussion of the contents and uses of these publications is available in a user guide as well as more technical Quality and Methodology Information (QMI) reports on cancer registrations, the cancer survival bulletins.
The cancer registration data are also used in every cancer survival publication. For every cancer survival publication, a check on the vital status of each cancer patient is made through NHS Digital’s Personal Demographics Service (PDS). National population data, that combine ONS mortality data and ONS mid-year population estimates into life tables, are used in every cancer survival publication for adults. There is QMI published in the mortality QMI and life tables QMI and a briefing document on the PDS is available on request from NHS Digital.
Data collection
Cancer registration is the systematic collection of data about cancer and tumour diseases. In England, this data collection is managed by NCRAS, which is a part of the National Disease Registry Service (NDRS) of PHE. Each year, over 300,000 patients are newly diagnosed with cancer in England. The data collected includes patient details, as well as detailed data about the type of cancer, how advanced it is and the treatment the patient receives. PHE’s NCRAS records new cancer registrations covering the entire population of England and holds cancer registration data from the former regional cancer registries, who had registered tumour data since the 1960s. NCRAS hold data on cancer registrations for England from 1971 onwards.
Data are submitted to NCRAS from a range of healthcare providers and other services (for example, histopathology and haematology services, radiotherapy departments, screening services and general practitioners) (Figure 1). NCRAS then uses these multiple sources to build a comprehensive picture of cancer incidence in England, as well as other detailed analysis and interpretations covering the entire cancer pathway on all patients in England.
As the data come from different sources, the quality and accuracy of the data submitted may vary. NCRAS collates and validates the data for each registration. Careful checks are carried out to ensure that registrations are accurate. These include checking that each diagnosis is only registered once, that the cancer site, sex and associated histology are consistent, and that the sequence of dates are correct: for instance, diagnosis should not occur after death. These checks follow those published by the International Agency for Research on Cancer (IARC).
Registrations that pass all checks have a quality status of one. If any non-critical checks fail, the registration has a quality status of 2. If registration fails a critical validation check, for example an invalid NHS number, it has a quality status of 3. Registrations with a quality status of 3 are not used in any Official or National Statistics published by NCRAS.
For more than 10 years, the proportion of registrations that have a quality status of 3 has been 0.1% or less. NCRAS investigates all registrations that fail any of the checks and tries to correct them. In the data reference tables of the Cancer registration statistics for England publication, a table is included to report the quality of status of registrations.
For more information, please see the Data Resource Profile for the National Cancer Registration Dataset.
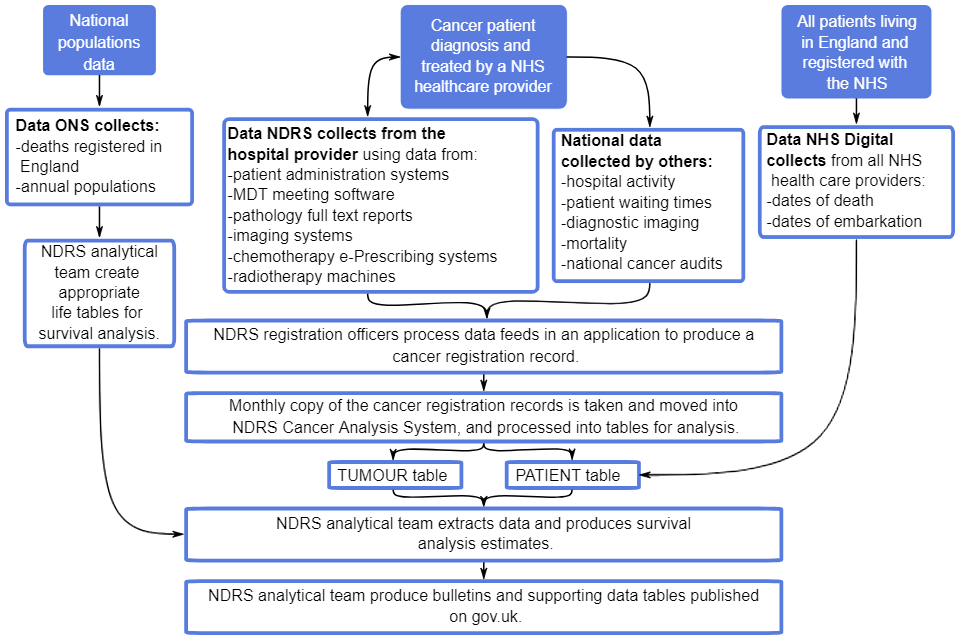
Figure 1 shows a summary of the administrative data flows that are captured and utilised in the statistical outputs on cancer registrations and survival.
The figure demonstrates how a cancer patient is diagnosed and treated by NHS healthcare providers. Data about their treatment is collected by NDRS from the hospitals:
- patient administration systems
- MDT meetings software
- pathology reports
- imaging systems
- chemotherapy e-Prescribing systems
- radiotherapy machines
This is joined with other national administrative healthcare data sets, such as:
- Hospital Episode Statistics (that measures hospital activity)
- Cancer Waiting Times
- Diagnostic Imaging Dataset
- ONS Mortality data
- national clinical audits into cancer
- NHS Digital Personal Demographics Service
The combined data is processed by NDRS cancer registration officers to produce a cancer registration record.
Monthly copies of the cancer registration records are taken and moved into NDRS Cancer Analysis System and processed into tables for analysis.
There are 2 main tables: a tumour table and a patient table. These tables are combined to produce a survival extract that is used for analysis. Alongside this, data collected by ONS of death registrations and annual populations is used to produce lifetables in order to produce the survival analysis.
The analysis is then written up as bulletins with supporting tables and published on GOV.UK.
Cancer registration data is used in all cancer publications. National population data is used in the cancer registration publication and all cancer survival publication for adults. For all cancer survival publications, the PDS is used to confirm the vital status of each cancer patient.
Figure 1: Summary administrative data flows for patient-level data
Accuracy and quality of data and estimates
In every data source, there are potential sources of bias and error that could materially impact on the estimates produced. Table 1 gives a list of the potential sources of bias and errors that could occur in each of the sources of administrative data and the steps taken to mitigate these risks.
Table 1: Potential sources of bias and error in the administrative data and mitigating steps taken to minimise the risks to data quality
| Potential source of bias and error | Safeguards taken to minimise the risks to data quality |
|---|---|
| The PDS of NHS Digital may not be notified in a timely manner of patients having embarked England or having died. | Each survival publication that uses the vital status returns from the PDS measures up to the end of a calendar year. |
| To reduce the likelihood of a delayed notification of death or embarkation, the patient files are sent to the PDS at least three weeks into the new calendar year. | |
| Corrupted identifiers may prevent the PDS data and the cancer registration data from linking. | Internal validation procedures in NHS Digital and PHE are applied to identifiers and the number of patients returned unmatched are monitored. |
| Cancer patients in the cancer registry are also routinely matched to other third-party datasets, including historical ONS cancer incidence records to test the validity of historical identifiers. | |
| Cancer registrations are known not to be complete until 5 years following the diagnosis date in up to 2% of cancers. | The completeness of cancer registrations is monitored for both the level of completeness and for any patterns in the types of cancer that are not registered in a timely manner. |
| Survival statistics can only consider people who have sought medical help with their cancer and so have a diagnosis before death. | Trends in the levels of patients whose cancer is only found on their death are monitored. |
| Cancer registration data items that define the cancer of a patient may contain errors, for example, uncertain dates that define the age at diagnosis of patients. | There are extensive internal quality assurance (QA) procedures applied in the registration processes. |
| A further set of survival QA tests are applied to each cohort of cancer patients that are being considered for survival analysis – see QA principles, standards and checks applied by data suppliers for a list of these checks. | |
| Coding errors in extracting and linking the data sources; coding errors in producing the survival estimates. | The Structured Query Language (SQL) and statistical code are written and executed independently by two teams to ensure the data and outputs agree. Further comparisons to check for consistency with previous estimates are made. |
| Life tables used in adult cancer survival publications reflect registered deaths of people in England and Wales; the populations used are estimates and may need adjusting if net migration patterns alter significantly from predictions. | Patients who were diagnosed in England but move and die abroad are censored from cancer survival calculations to prevent biasing of the estimates. |
| When population estimates require revision, a revised back series of survival estimates is produced for users. |
Data from NHS Digital
Survival estimates may be inflated by patients whose death or embarkation from England is not notified in a sufficiently timely manner to PDS (late notification); or by patients with corrupted identifiers that prevent cancer registration data from matching to PDS.
Each individual instance of late notification or corrupted identifiers will have a very small impact on the cancer survival estimates produced; monitoring of the returns from PDS over successive years shows that there are very few late registrations of deaths or leaving England. The cancer survival estimates reported at each geographical breakdown include a sufficient volume of diagnoses that these increases will not be statistically significant and will be undetectable at the level of reported precision of the estimates. A briefing document on the PDS is available on request from NHS Digital.
Data from ONS
ONS receives daily extracts of death registrations from registrars. These then pass through a series of automatic validation processes that highlight any inconsistencies. Internal consistency checks are then conducted to eliminate any errors made during the recording of deaths, and to ensure the annual dataset is complete. Any concerns relating to cause of death are referred to a medical advisor or medical epidemiologist. The User guide to mortality statistics provides more detail on all these checks and more details on methods can be found in the mortality statistics for England QMI.
For population estimates, ONS have produced uncertainty estimates to give users additional information of the quality of these estimates. Measures of statistical uncertainty are available for the years mid-2012 to mid-2016. It should be noted that the uncertainty measures currently available were calculated using the methodology used for the mid-year estimates prior to the March 2018 revisions to population estimates for mid-2012 to mid-2016. For more information on population estimates please refer to population estimates QMI.
Data registered by NCRAS
Completeness
Cancer registrations are over 98% complete when used in the cancer statistics bulletins. In common with cancer registries in other countries, cancer incidence in England can take up to 5 years after the end of a given calendar year to reach 100% completeness and stability. This is due to the following reasons:
- New cancer cases will be registered: this can include new ‘late’ registrations, where a case is registered after statistics have been published for a given year.
- Cancer records may need amending: this happens if more accurate diagnostic information becomes available.
- More accurate diagnostic information can mean cancelling a cancer registration. This does not happen frequently.
NCRAS may record ‘late registrations’ when preparing for the next reporting year. We assess the ‘late registrations’ as if they were for the next reporting year. If they pass the quality tests, they are included in a refreshed dataset. It is therefore possible to detect small difference in the counts of cancer registrations. These changes are unlikely to have a meaningful impact on the cancer incidence or survival estimates.
A less common reason for changes to historical data is a patient exercising their right to opt-out of the cancer registration datasets.
The potential accuracy gained from waiting for 100% completeness (another 4 years) is less than the need for timely reporting of cancer statistics.
The delay in arriving at a confirmed cancer registration is assessed in the cancer registrations bulletin and shows similar levels, slightly decreasing over time, in each reported diagnosis year. The assessment of the ‘completeness’ of a registration year can be done by comparing the number of registrations in that year, with the average number from the previous 3 years. The difference in numbers is an estimate of how many late registrations may occur. Analysis of the cancer registrations that are untimely does not reveal biases in the cancers, age at diagnosis (and death), sex or residential area of patients; this lack of bias means it is unlikely that published estimates are significantly impacted.
Errors and inconsistencies
Potential errors or inconsistencies in the cancer registration data can have implications for the incidence and survival estimates produced. These errors or inconsistencies can:
- cause bias in the estimates produced that alter the age distributions observed and consequently, the rates of cancer registrations reported
- mean that the time a patient with cancer is recorded as living following a diagnosis is too long or too short which will inflate survival estimates or decrease survival estimates respectively
The procedures in registering cancer diagnoses discussed in the Data Resource Profile address how these errors are mitigated. These procedures are the result of international agreements in how to code and record cancer, with coding systems and guidance being issued by the International Agency for Research on Cancer (the cancer agency of the World Health Organisation) and bodies specialising in classifying aspects of a cancer diagnosis (for example, the Union for International Cancer Control’s cancer staging system). To promote consistent reporting of cancer registration data to NCRAS, NCRAS has authored a Cancer Outcomes and Services Dataset (COSD) which has been the standard for reporting of cancer in the NHS since January 2013.
The cancer survival estimates are designed to reflect the effectiveness of the healthcare system in treating diagnosed patients. However, there are people who die with cancer that is detected only after death (called Death Certificate Only registrations or DCOs) and so may never have needed or sought treatment for cancer. Including these people will artificially depress survival estimates and are therefore not included in the survival estimates. For people with undiagnosed cancer at death, they may be reflected in the cancer registration bulletin if their cancer is detectable after death in a post-mortem. The rates of people in this position are monitored and are reported as part of the UK and Ireland Association of Cancer Registries Key Performance Indicators.
The survival estimates are more sensitive than rates of registration because of the type of statistical techniques applied in producing them. The cancer registration data can be correct and consistent but the age of the patient at diagnosis can still fall outside the range for which the life tables are reliable. There is an enhanced set of quality controls on the data which are applied to reduce the bias of the survival estimates, these are available from Control of data quality for population-based cancer survival analysis. Each individual error will have a low impact on the survival estimates produced. The checks on the data are designed to prevent many individual errors from being included, which may manifest in an error with a larger impact.
Furthermore, even small changes to life tables have a very large and significant impact on all the estimates produced because of the statistical techniques used. Sometimes, the number of people registered as having died at a given age and location in a year may be incorrectly recorded or the population estimates may need significant revision. If these errors cause the mortality rates in the life table to increase, then the survival estimates produced will also increase; conversely, if errors cause the mortality rates in the life tables to fall, then the survival estimates produced will also fall. These effects will be larger for 5-year survival estimates than 1-year survival estimates and larger for cancers with good prognosis than cancers with poor prognosis. When the data underlying the life tables need revising, the team produces a revised back series of survival estimates to allow users to assess trends on a consistent basis.
Coding errors can have a significant impact on the accuracy and quality of the estimates produced. The NCRAS team that extract and link the data used in the production of the cancer bulletins are trained in Structured Query Language (SQL), and follow documented extraction procedures. The extraction team works closely with their colleagues that develop the databases used and the cancer registration protocols so that changes to internal and external data recording is reflected in the data extracts produced. Two extracts are independently coded and produced; the value of each data item for each cancer diagnosis must match before this step is signed off.
The NCRAS team is also trained in applying and developing survival analysis techniques. Two sets of independently written code are run to produce the estimates in the bulletins and follow documented procedures; each estimate produced from the 2 sets of runs must match before the estimates can be considered for consistency checking against previous publications and international comparisons.
After applying consistency checks (for example, rarer cancers have a lower number of diagnoses than common cancers, cancers with generally poor prognosis have lower survival estimates than ones with good prognosis), the estimates are then shared with senior members of the NCRAS team for independent scrutiny against international and previous estimates, before agreeing the new estimates produced are fit for publication.
Communication with data supply partners
The provisions of Section 251 of the NHS Act 2006 provide the legal basis to PHE for collecting patient-level data on cancer patients for specified purposes, without individual consent. This is reviewed annually by the Confidentiality Advisory Group of the Health Research Authority. Strict technical and contractual controls are put in place to prevent unauthorised access and use of the data, with staff undergoing regular training on data protection and information governance.
Secure file transfer systems are used to send and receive patient-level data between the NHS and PHE. Within PHE, all patient-level data are stored on secure servers with role-based access.
The NCRAS team has responded to concerns raised about the statistical outputs created and have issued corrections to both cancer registrations and cancer survival outputs. They have also self-identified concerns in the appropriateness of older data sources before publication, which drove the development and publication of life tables by the ONS to support the calculation of cancer survival estimates in adults. As of 2020, the life tables are produced by the NCRAS analysis team and are available to the public on the CancerData website.
The data items shared by NCRAS to the NHS PDS to facilitate the checking of patients’ vital status are:
- NHS number
- date of birth
- postcode of residence
The data items returned by the NHS PDS are:
- local patient identifier
- trace result NHS number
- date of death
- old NHS number
- new NHS number
- returned current posting
- date field last modified
- returned date of current posting or date of death, if deceased
The data used by the PDS are updated by trained NHS staff who work in healthcare settings. The database (the Spine) that stores the data applies validation checks when records are attempted to be updated by a user.
The fields extracted by the NCRAS analysis team from the Cancer Analysis System (CAS) for producing cancer registration or cancer survival estimates are:
- NHS number
- tumour number
- sex
- date of birth
- date of diagnosis
- date of death, if deceased
- vital status
- vital status date
- International Statistical Classification of Diseases and Related Health Problems version 10 (ICD-10) topographical code
- ICD-O-2 morphological code
- ICD-O-2 behaviour code
- ICD-O-3 topographical code
- ICD-O-3 morphological code
- ICD-O-3 behaviour code
- DCO flag
- stage at diagnosis
- deprivation quintile
- country of residence code
- region of residence code
- NHS England Region of residence code
- Cancer Alliance of residence code
- Sustainability and Transformation Partnership of residence code
- Clinical Commissioning Group of residence code
The geographical codes are obtained from linking postcodes from the cancer registration tables to the National Statistics Postcode Lookup.
The mortality data transferred from the ONS to NCRAS comprise:
- year of death
- year of registration
- postcode
- sex
- age
- quinary age group
- ICD10
- Lower Super Output Area (LSOA) 2011 code
The population data transferred from the ONS to NCRAS comprise:
- year
- LSOA 2011 code
- age
- quinary age group
- population count
Quality assurance principles, standards and checks applied by data suppliers
The system NHS Digital uses for its PDS contains data entry validations that are enforced every time a user of their system updates a record. The returns provided to users of the PDS contains coding information to indicate where (and why) matches could not be found, so that users can check the data they hold and enable a dialogue to resolve any concerns that the users may have.
NCRAS monitors the returns of cases to investigate and correct the records of cancer registrations where the PDS has indicated there may be a problem; almost all of these are from cancer registrations that occurred more than 20 years ago and, consequently, would be not included for reporting upon in the statistical bulletins.
Cancer registrations are assessed by 2 cancer registration officers and any inconsistencies resolved. Every quarter, further data quality checks at the level of each record are applied, as well as checking trends and population-level metrics before the data registered during the quarter are released for statistical analysis. These higher-level checks are conducted by the cancer registration data quality team within NCRAS.
NCRAS has an information governance policy and training schedule that all its employees must follow. The cancer registration team has an enhanced level of training to complete because they are working with sensitive, patient-level data. NCRAS operates a data governance team to establish and monitor the use of safe methods of working with sensitive data and has a team dedicated to ensuring that applicants using NCRAS’s data do so with a legitimate legal basis, current information governance training and ethical approvals.
The cancer registration team in NCRAS also has data quality groups that meet to maintain or improve the quality of cancer registration data. A joint registration officer, data quality and analysis meeting take place to discuss unusual data patterns. This is to establish if there are epidemiological reasons for the pattern, or if some data need reviewing, as well as prioritising future developments of the cancer registration data tables.
If the quality of some parts of the cancer registration data are found to be falling, NCRAS has another data quality group of data liaison officers. The role of NCRAS’s data liaison officers is to go out to data providers and support providers in the use of their systems, so the data that NCRAS needs to make a cancer registration are available and of high quality.
This combined auditing and quality improvement work has ensured that serious errors (for example, an invalid date) in the main data items that make up a cancer registration have been found in fewer than 0.1% of cancer registrations for more than 10 years. To further ensure the survival estimates use cancer registration data that are self-consistent, a set of enhanced quality assurance tests are applied to survival analysis cohorts (these are discussed under producer’s quality assurance investigations and documentation).
All the statistical outputs on cancer published by NCRAS have been run by 2 analysts independently, the first step of which is to ensure the raw data extracts agree. Further checks, including sensitivity testing and studying potential outliers, are made at appropriate points during the analytical process before the output is finalised.
Producer’s software
The estimates produced for these publications have been produced by NCRAS. These national and experimental statistics for survival implement the UK and Ireland Association of Cancer Registries (UKIACR) ratified standard operating procedure Guidelines on Population Based Cancer Survival Analysis.
Structured Query Language (SQL) is used to extract data from NCRAS’ Cancer Analysis System and all statistical analyses are carried out using Stata and R. To promote transparency in the cancer survival estimates, annotated copies of the SQL and Stata code can be provided, free of charge, on request.
Please email any requests to: NCRASenquiries@phe.gov.uk
Producer’s quality assurance investigations and documentation
The extraction of data for the cancer registration bulletin is simple. The extraction follows a Standard Operating Procedure (SOP) written for the cancer tables, which ensures that no duplicate or unfinalised registrations are included. After the SQL script has been independently checked, the extract is then loaded into a statistical package to check the data meets the criteria of UKIACR.
At the point of creation of the tabulations, the current estimates are compared by the NCRAS team to previously published cancer registration bulletins. The NCRAS team carries out further checks for unusual patterns in the data, unusual combinations of cancer type and sex and check the new data against trends from earlier data.
The data required for survival analysis is more complex and requires a longer time series. A longer time series of data is required to ensure that the first tumour of each type is used for each patient in survival analysis. More complex data are required because of the enhanced quality assurance checks needing to be applied before survival analyses can be undertaken. These data quality checks ensure that patients and their cancer(s) are uniquely identified and that the data about the patient and their cancer(s) are self-consistent.
The following UKIACR criteria are used to identify the patients that are eligible to be included in the analysis (and the final number of eligible patients is provided as part of the publication release):
- patients should have a unique identifier; this is to make sure cancers for one patient are not assigned to another patient
- patients should have a complete date of birth, so their age can be calculated at various time points
- adults should be aged between 15 and 99 years at diagnosis
- children should be aged between 0 and 14 years at diagnosis
- patients should have a known sex; this is a data quality check and to match to life tables
- patients should have a complete date of cancer diagnosis
- patients who have died should have a complete registered date of death
- patients should have a known date of being recorded as alive or dead; data quality check and to calculate their survival time after diagnosis
- patients should be a resident of England and have a valid postcode for their usual place of residence at the time of diagnosis; match patients to life tables
- cancers should be (potentially) lethal, newly diagnosed in the studied cohort and a primary cancer (one that hasn’t spread from another part of the body); this is so the date of original diagnosis is known
- cancers of the blood (for example lymphomas, leukaemia and myelomas) should not occur in a solid cancer; data quality check
- patients are included even if they have further new cancer diagnoses later in the period of interest; this ensures that a patient is only included once in each group of patients and survival time is counted from the earliest diagnosis of the cancer of interest in each period of interest
- patients are excluded if they have had a primary cancer in the same site diagnosed before the period of interest; if a patient has 2 or more cancers of the same type, it is not clear whether survival time from that type of cancer should be measured from the first or later diagnosis
- patients are included where the earliest diagnosis of a cancer of interest occurred within the period of interest even if they have a primary cancer of another site diagnosed at any time; this treats the patients where the cancer registry is unaware of previous cancer diagnoses in the same way as where this medical history is known
- cancers where the only confirmed record of the cancer is on the patients’ death certificate are excluded; as cancer survival attempts to assess the effectiveness of the health system in treating patients with cancer, patients in which cancer is only found after death cannot contribute to this assessment
- the sequence of dates should be consistent; data quality check, for example a patient should not be diagnosed before they are born
Other decisions applied include:
- where a patient dies on the date of diagnosis and have more records than those on a death certificate, then these patients should be included in the survival analyses but should have one day added to the recorded date of death to prevent Stata’s stset command from excluding those patients
- when 2 or more tumours of the same type are diagnosed on the same day for a patient, the one with the worst prognosis is chosen for inclusion; this ensures that a patient is only included once in each group of patients
- coding the cancers with reference to ICD-10 to select similar groups of cancers; the details of the coding applied are included in each bulletin
Applying these checks mitigates against some of the potential sources of error in the data.
Having two analysts independently extract and produce the statistical outputs identifies uncertainty in how methodologies should be implemented; these differences are used as a tool for learning in the teams. Once there are agreed estimates, sensitivity testing (for example varying the composition of a cohort or the censor date) is undertaken to ensure the results are stable. Further comparisons are made to previously published output and results of appropriate international studies.
By consistently applying these quality assurance measures and continually monitoring the data received and the estimates produced, the NCRAS team can assess the quality of the administrative data received. The data governance and quality assurance teams that NCRAS have in place and the relationships they have with their data providers means that the likely degree of risk to the quality of the administrative data is low.
Timeliness and punctuality of publications
Starting from the 2009 registration year, work has been done to improve the timeliness of these publications. Historically, NCRAS had to provide cancer registration data to ONS within 18 months of the end of the calendar year. For the 2009 registration year, we provided the data within 15 months. Since the 2010 registration year, this has reduced to 12 months. Although the data now remains within NCRAS, we apply the same data cleaning standards as those from ONS.
As the data has become timelier, the publications have become timelier. To improve timeliness, we may publish cancer registration statistics using 2 releases, a preliminary release and a final release. ONS published the first release for 2014 registrations in February 2016. This is 14 months after the end of the registration year. The full release, complete with regional output, follows within a further 6 months. For the 2018 registration year, NCRAS published the first release in January 2020. This is 13 months after the end of the registration year.
It takes 9 months or more to gather all the information required to confirm a cancer diagnosis. For this reason, there is a time lag between publication and registration year.
The publication dates of all cancer statistical bulletins are pre-announced on the GOV.UK website at least one month in advance. We will publish annual publications at consistent times each year, plus or minus one month. The GOV.UK website provides a 12 month notice of release dates.
Exceptionally, we may need to make changes to the pre-announced release schedule. If that happens, we will notify a revised release date on GOV.UK, and explain the reasons why this change is needed. These are requirements of the Code of Practice for Official Statistics.
Legislation
The Statistics and Registration Service Act 2007 permits the Registrar General to provide to the UK Statistics Authority, to carry out any of its functions, both information that is kept under the Births and Deaths Registration Act 1953 and any other information received by the Registrar General in relation to any birth or death.
The Health Service (Control of Patient Information) Regulations 2002 Statutory Instrument No. 1438, Regulation 2, permits confidential patient information relating to patients referred for the diagnosis or treatment of cancer to be processed for the following purposes:
- The surveillance and analysis of health and disease.
- The monitoring and audit of health and health-related care provision and outcomes where such provision has been made.
- The planning and administration of the provision made for health and health-related care medical research approved by research ethics committees for the provision of information about individuals who have suffered from a particular disease or condition where; that information supports an analysis of the risk of developing that disease or condition, and it is required for the counselling and support of a person who is concerned about the risk of developing that disease or condition.
This regulation was made under Section 60 of the Health and Social Care Act 2001 and continues to have effect under Section 251 of the NHS Act 2006.
PHE processes and stores cancer registration data in accordance with the requirements of:
- the Data Protection Act 1998
- the Code of Practice for Statistics
- the International Association of Cancer Registries (IACR) and their Guidelines on Confidentiality in Cancer Registries (1992)
- the European Network of Cancer Registries (ENCR) Guidelines on Confidentiality and Ethics (2002)
- the NHS Act 2006
- the Statistics and Registration Service Act 2007
- the Health Service (Control of Patient Information) Regulations 2002
- Information: To share or not to share? The Information Governance Review March 2013
- the Freedom of Information Act 2000
- Section 251 of the NHS Act 2006 (originally enacted under Section 60 of the Health and Social Care Act 2001).
- Confidentiality: NHS Code of Practice 2003
- NHS Records Management Code of Practice
- Health and Social Care Act 2012
- the NHS Information Security Management Code of Practice 2007
- the Computer Misuse Act 1990
- the Human Rights Act 1998
