NERVTAG: Immunity certification, 9 December 2020
Published 5 July 2021
Reason for bringing to SAGE
GO-Science made a request to answer the following questions:
How far does current evidence and understanding of infectiousness and immunity support - or not support - any of these types of certification (for example the current SAGE view is that “Immunity passports or equivalents are not currently advisable (due to uncertainties)”). Has this changed from previous advice through SAGE (we can reiterate previous advice if not).
What are the key uncertainties and unknowns that need to be taken into account (for example for vaccines – do we understand impact on transmission or if they confer sterilising immunity?). What do we know about variation in duration/strength of natural immunity following infection.
Summary
- 1. Following natural infection with SARS-CoV-2:
- a) short lived protection against symptomatic SARS-CoV-2 infection is high, estimated at 99% (95% CI 93.0% to 100%) (high confidence)
- b) protection against symptomatic SARS-CoV-2 infection lasts at least 3 months (high confidence) and possibly 6 months (moderate confidence) or more
- c) protection against asymptomatic SARS-CoV-2 infection is moderate, with protection against asymptomatic SARS-CoV-2 estimated at 57% (95% CI 49% to 69%) (high confidence)
- d) protection against infectiousness may be higher than protection against asymptomatic reinfection if reinfection results in lower viral loads than a primary infection; whilst this is likely to be the case, there are no available supporting data at this time
- 2. The issuance of an immunity certificate for a period of three months after RT-PCR confirmed SARS-CoV-2 infection is reasonable based on current evidence. However, it must be recognised that:
- a) a proportion of people will not develop immunity, so a certificate is not a full-proof guarantee of immunity against infection or disease (high confidence)
- b) an immunity certificate should not replace other measures to protect high-risk individuals for example health and social care workers should continue to wear appropriate PPE and continue to participate in testing regimens
3. At this stage, the issuance of an immunity certificate based upon either a sole positive lateral-flow antigen detection test or a sole antibody test is not advised due to the uncertain performance and interpretation of these tests.
4. Following vaccination against SARS-CoV-2 with the Pfizer or BioNTech or Moderna mRNA vaccines, a high proportion (more than 90%) of people develop immunity which is protective against disease (high confidence). Vaccination with the Oxford or AstraZeneca vaccine appears to be associated with slightly lower levels of protection against disease (62% for the standard dose and 90% for a low dose primed regimen). Efficacy is therefore vaccine specific and all vaccines cannot be assumed to be equal. The duration of protection following vaccination is not yet known.
5. A small number of individuals will not develop protection from disease after vaccination (high confidence). The proportion is likely to be less than 5% depending on the vaccine used (moderate confidence).
6. Following vaccination with the Oxford or AstraZeneca vaccine, the level of protection against subclinical reinfection (as opposed to disease) is reported as 59% (95% CI 1 to 83%) for the low dose/standard dose regimen and 4% (95% CI -72 to 46%) for the standard dose or standard dose regimen. Efficacy of protection against subclinical infections for the mRNA vaccines is not yet reported.
7. Issuance of immunity certification after vaccination requires further data and consideration, and might be taken forward by JCVI, with or without NERVTAG input.
Background
1. Sterilising immunity means that a person is protected against both infection and illness. Therefore, as well as being themselves protected from illness they cannot be a source of infection for others.
2. Non-sterilising immunity means that a person can still get infected but not become ill. Therefore, although themselves protected from illness, they may still be able to become infected, shed virus and be a source of infection for others.
3. This paper does not address behavioural, ethical, legal or operational issues related to immunity certification.
Immune responses following natural SARS-CoV-2 infection
4. One to 2 weeks following documented SARS-CoV-2 infection, more than 90% of people, including the elderly, have SARS-CoV-2 antibodies detectable in their serum. [footnote 1][footnote 5] Antibody levels tend to be higher in people who have suffered more severe disease, but antibodies do develop following asymptomatic SARS-CoV-2 infection or mild COVID-19. This is similar to MERS- coronavirus, where severity of infection is linked to antibody longevity.[footnote 6]
5. Antibodies begin to appear within 5 to 6 days of symptoms and are detectable for at least 6 months. [footnote 2], [footnote 4], [footnote 7].
6. Following documented SARS-CoV-2 infection, cell mediated immunity also develops and is detectable for at least 6 months.[footnote 8], [footnote 9]
7. Antibody waning following infection with seasonal coronaviruses can result in reinfections. [footnote 10] Reinfections with seasonal coronaviruses are associated with sustained circulation suggesting that onwards transmission occurs following reinfection, with the duration of immunity modelled to be about 45 weeks.[footnote 11]
Animal studies on SARS-CoV-2 immunity
8. In animal models, the presence of neutralising antibodies as a result of prior infection is associated with protection from disease and infection (sterilising immunity). [footnote 12], [footnote 13], [footnote 14]
9. Studies in hamsters and macaques indicate that passively administered antibodies are sufficient to suppress viral replication in both the upper and lower respiratory tract. [footnote 15],[footnote 16]
10. Depletion of CD8 T cells shows that cellular immunity can contribute to protection against SARS-CoV-2 re-challenge in convalescent macaques with waning antibody titers. [footnote 16]
Observational data on immunity following natural SARS-CoV-2 infection
11. A rapid literature review was undertaken on 5 November 2020 to identify cohort studies that may give information about the protective effect of baseline antibodies to SARS-CoV-2. These data were used to calculate relative risk and protective efficacy for each study (Protective efficacy = 100*(1-RR) and 95% CI). The analyses assumed equal follow up time for those with and without baseline antibodies. A variety of antibody assays were used in these studies, mostly ELISA against spike glycoprotein and /or Abbot nucleocapsid antibody assay. The fishing boat study looked at neutralising antibodies (number 5).
12. Seven studies were included:
- PHE study of 2 nursing home outbreaks. [Note 1]
- Data from the SIREN healthcare worker study [Note 2]
- Published data from a follow up study of healthcare workers at UCLH. [Note 3] [footnote 17].
- Preprint data from a cohort study of healthcare workers in Oxford [Note 4] [footnote 18]
- Data from an outbreak on a fishing boat [Note 5] [footnote 19]
- Data from a follow up study of key workers including healthcare workers, fire workers and police. [Note 6]
- Provisional Data from a community cohort in the USA [Note 7]
Notes:
- Protection against SARS-CoV-2 Infection – PHE London Care Home cohort studies Nov 2020 Paper for NERVTAG 27/11/2020.
- SIREN Provisional results from NERVTAG paper – Should recovered COVID-19 cases who remain well and are re-exposed be exempt from re-isolation for 90 days from their initial illness onset? 27/11/20, Information subsequently redacted.
- Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. www.thelancet.com, Vol 396 July 25, 2020.
- Antibodies to SARS-CoV-2 are associated with protection against reinfection. www.medrxiv.org/content/10.1101/2020.11.18.20234369v1.full.pdf
- Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J Clin Microbiol. 2020 Oct 21;58(11):e02107-20. doi: 10.1128/JCM.02107-20. Print 2020 Oct 21.
- SARS-CoV-2 responsive T cell numbers are associated with protection from COVID-19: A prospective cohort study in keyworkers. medRxiv preprint doi
- Personal Communication Adam Kucharski – US Cohort Study Preliminary findings.
- The overall estimate of the relative risk across all studies was 0.22 (0.16-0.30) corresponding to a protective efficacy of 78% (95% CI 70% to 84%) – figure 1. The study results can be categorised into three types of result (See table 1 and figures in appendix).
- a) Result only on symptomatic reinfection [table 1: 2a,4a,] The Oxford and SIREN HCW studies were able to report on the protective effect against classic symptoms of COVID-19. The Oxford study found no such events in those with antibodies and the SIREN study found only one such event. The combined protective effectiveness against infection with classical symptoms was 99% (95% CI 93.0% to 100%)
- b) Results against asymptomatic infection [table 1: 2b,4b,] The Oxford and SIREN HCW studies were able to report on the protective effect against asymptomatic infection (or infection without classical COVID-19 symptoms of fever OR cough OR loss of sense of smell/taste). The combined protective effectiveness against asymptomatic infection was 57% (95% CI 49% to 69%)
- c) Results against asymptomatic or symptomatic infection combined in studies that swabbed routinely regardless of symptoms. [table 1: 1-5] The combined protective effectiveness estimate was 78% (95% CI 70% to 84%),
13. Data on RT-PCR cycle threshold (ct) values from reinfected individuals in these studies are not yet available. Prior experience with seasonal coronavirus rechallenge in humans and animal studies would anticipate that reinfection (with the same virus strain) would be associated with lower levels of virus replication and excretion than a primary infection.
14. Despite the widespread and continued circulation of the virus in many countries, the worldwide number of confirmed reinfections is very low.
15. Human challenge experiments performed at the Common Cold unit showed that individuals who became reinfected on challenge with a seasonal coronavirus one year after their first challenge shed less virus and for shorter duration, implying transmission risk may be lower on reinfection.[footnote 16]
- 16. Conclusions
- a) short lived protection against symptomatic SARS-CoV-2 infection is high, estimated at 99% (95% CI 93.0%-100%) (high confidence)
- b) protection against symptomatic SARS-CoV-2 infection lasts at least 3 months (high confidence) and possibly 6 months (moderate confidence) or more
- c) protection against asymptomatic SARS-CoV-2 infection is moderate, with protection against asymptomatic SARS-CoV-2 estimated at 57% (95% CI 49%-69%) (high confidence)
Table 1: Summary of observational studies investigating protective effect of baseline antibodies on risk of subsequent infection.
| No. | Study | Duration follow up | PCR positive in those with antibodies | PCR Positive in those without antibodies | Relative Risk | p-value | Protective Efficacy (95% CI) | Comment |
|---|---|---|---|---|---|---|---|---|
| 1 | Nursing home | 6 months | 1/88 1.13% (0.28%-6.17%) | 22/73 30.14% (19.94%-42.00%) | 0.037 (0.005-0.267) | <0.0001 | 96.3% (73.3%-99.5%) | Testing regardless of symptoms |
| 2 | SIREN classical symptoms (Cough, Fever, Ageusia, anosmia) | 6 months | 1/6765 0.015% (0.0004%-0.082%) | 197/14344 1.37% (1.19%-1.58%) | 0.011 (0.002-0.077) | <0.001 | 98.92% (92.3% -99.8%) | Testing symptomatic |
| 2 | SIREN HCW asymptomatic or atypical symptoms only (GI, headache, myalgia, arthralgia, tiredness, headache) | 6 months | 37/6765 0.547% (0.385%-0.753%) | 176/14344 1.22% (0.11%-1.42%) | 0.446 (0.313-0.635) | <0.001 | 55.42% (36.5-68.7) | Testing regardless of symptoms |
| 2 | SIREN HCW Combined | 6 months | 38/6765 0.561% (0.40%-0.77%)-) | 319/14344 2.22% (1.99%-2.48%) | 0.253 (0.181- 0.353) | <0.001 | 74.74% (64.7-81,9%) | Combined |
| 3 | UCLH | 1 month | 1/33 3.03% (0.08%-15.76%) | 14/112 12.50% (7.01%-20.08%) | 0.242 (0.033-1.776) | 0.163 | 75.76% (-77.58%-96.7%) | Testing regardless of symptoms |
| 4 | Oxford HCW Symptomatic (Cough, fever anosmia/ageusia) | 6 months | 0/1246 0.00% (0.00%-0.30%) | 89/11052 0.81% (0.64%-0.99%) | 0.00 (0-3.30) | 0.015 | 100% (incalculable) | Symptomatic (0.46 cases per 10,000 days vs zero per 10,000 days) |
| 4b | Oxford HCW Asymptomatic | 6 months | 3/1246 0.24% (0.05%-0.70%) | 76/11052 0.68% (0.54%-0.86%) | 0.350 (0.111-1.108) | 0.061 | 64.99% (-10.80-88.94) | Asymptomatic (0.4 per 10,000 days vs 0.21 per 10,000 days) |
| 4c | Oxford HCW combined | 6 months | 3/1246 0.24% (0.05%-0.70%) | 165/11052 1.49% (1.28%-1.74%) | 0.161 (0.052-0.504) | 0.0003 | 83.87% (49.56%- 94.84%) | Combined Adjusted rate ratio 0.24 (0.08- 0.76, p=0.015) |
| 5 | Fishing boat | 1 month | 0/3 0.00% (0.00%-70.76%) | 104/119 87.39% (80.06%-92.77%) | 0.00 (incalculable) | 0.0028 | 100% (incalculable) | Testing Regardless of symptoms |
| 6 | Hospital/Fire/Police | 100 days | 0/32 0.00% (0.00%-10.88%) | 13/1913 0.68% (0.36%-1.16%) | 0.00 (incalculable) | 0.63 | 100% (incalculable) | Symptomatic testing |
| 7 | US Cohort Texas and California | Not known | 0/314 0.0% (0.00%-1.17%) | 33/4097 0.8% (0.56-1.12%) | 0.00 (incalculable) | 0.11 | 100% (incalculable) | Provisional data - Testing on demand – mainly symptomatic or as a contact |
Immunity following SARS-CoV-2 vaccination
17. Six weeks after the second vaccine dose, human clinical trial data are reporting protective efficacy against disease of up to 95%. Emerging data are suggesting high levels of protection after one dose.
18. To our knowledge only the Oxford or AstraZeneca vaccine trials have undertaken routine swabbing to detect asymptomatic infection. They report a protective efficacy against infection of 59% (95% CI 1 to 83%) for the low dose/standard dose regimen and 4% (95% CI -72 to 46%), but with very wide confidence intervals.
19. Animal (non-human primate, NHP) models show that some vaccines protect against disease but not all confer sterilizing immunity.
20. NHPs given the highest dose of Moderna vaccine, equivalent to that used in humans, did show sterilizing immunity upon challenge.[footnote 20] There is also evidence from NHP studies that the Pfizer or BioNtech vaccine induced sterilizing immunity upon challenge, with viral RNA detected in nasal swabs on Day 1 after challenge and not in swabs obtained on Day 3 or subsequently.[footnote 21]
21. There is also good evidence that the Jansen Ad26 vaccine provides sterilising immunity in NHPs.[footnote 22]
22. In animals studies the ChAdOx vaccine protected against disease but did not produce sterilizing immunity. The amount of viral RNA measured in the nose of vaccinated animals who became infected upon challenge was not different from naïve (non-vaccinated) animals. [footnote 23] However, the duration and level of viral replication in the lower respiratory tract was attenuated. A possible explanation is that the vaccine induced a strong IgG response that affects virus replication in the lung but not a mucosal IgA response that would control viral load in the upper respiratory tract. Also, the ChAdOx study involved a very high-dose challenge (TCID50 2.6 x 106 ) to both upper and lower respiratory tract.
23. At time of writing, no data are available on the duration of protective immunity induced by any SARS-CoV-2 vaccines and only limited data on the durability of measured immune responses in peripheral blood.
- 24. Conclusions:
- a) SARS-CoV-2 vaccines can provide a high level of protection against disease (high confidence)
- b) SARS-CoV-2 vaccines may provide protection against infection, but data are lacking
- c) The duration of immunity provided by SARS-CoV-2 vaccines is not yet known
Which tests to certify ‘immunity’
25. Testing options for issuing time-limited certificates following documented infection include a positive RT-PCR test, lateral flow device test, or antibody test, or any combination of these. The potential limitations of these approaches are discussed.
26. RT-PCR is the most sensitive and specific available test for acute SARS-CoV-2 infection. Since more than 95% of individuals who have been infected by SARS-CoV-2 mount an immune response, one could assume there is a high probability that a person who has a laboratory confirmed RT-PCR positive result will have some immunity.
27. Confirmation of acute infection (antigen) by lateral flow devices (LFDs) is less specific and less sensitive than RT-PCR. This is of particular concern when used in asymptomatic testing as the positive predictive value (and hence the proportion of those testing positive who are false positives) is highly dependent on both specificity and the prevalence of infection in those tested. Thus, use of lateral flow results from mass population asymptomatic screening to issue immunity certificates would lead to many being issued certificates when they are not immune.
Table 2: False positive rate with LFD in low prevalence settings
The proportion of lateral flow positive tests that are false positives at different infection prevalence levels and published sensitivity and specificity.
| Test | Prevalence 10% (equivalent to testing symptomatic people) | Prevalence 1% (equivalent to tier 3 areas) | Prevalence 0.5% (equivalent to tier 2 areas) | Prevalence 0.1% (equivalent to summer rate) |
|---|---|---|---|---|
| Innova Lateral Flow Sensitivity 71% specificity 99.68% Source | 3.6% | 29% | 45% | 81% |
28. Presence of antibody in serum. There is a good correlation between neutralising antibodies to SARS-CoV-2 and protection against infection. There is also good correlation between anti- receptor-binding-domain (RBD) antibodies and plasma virus neutralisation activity. However, there is as yet no standardisation of RBD antibody assays and no validated RBD antibody concentration that correlates with protection. In addition, a single positive antibody test does not provide information on the timing of infection and therefore the duration of protection cannot be inferred.
29. A combination of a RT-PCR positive test and subsequently the presence of antibody in serum would give greater assurance that the individual is immune to reinfection.
30. If there is a need to differentiate infected from vaccinated individuals, then the choice of antibody assay is important.
31. Currently there are many different types of tests that can be used to detect SARS-CoV-2 infection. Variation within these tests makes comparison between individuals in different part of the country problematic (high confidence).
32. For antibody testing an approach that would give a quantitative value necessary to maintain and re-certify an individual (post-infection or vaccination) on a rolling basis is ELISA (high confidence). This would probably be based on the receptor binding domain (RBD) of the spike glycoprotein which has good correlation with neutralising antibody. Cohort studies and standardised panels would be required (PHE or NIBSC) to establish confidence intervals for ‘protection’ using this metric.
33. Measurement of antibody, for example ELISA for RBD, will only establish the level of IgG anti-RBD in the plasma at the time of the test. If IgG levels wane, it is still possible that memory RBD plasma cells are present and upon re-infection could rapidly proliferate. The concertation of RBD antibodies that correlates with protection is not yet established. Also, the kinetics of RBD antibody decay are not fully understood. This makes it difficult to determine what RBD antibody concentration is considered protective and for what period of time that protection holds.
34. T-cells may also contribute to protection and the RBD assay won’t detect these. Assays for use at scale for these other immune parameters are not available. Therefore, the certificate might be ‘removed’ from a person even though they are still protected. However, this cautious approach may be prudent since in all human coronaviruses (seasonal and severe) there is strong evidence of reinfection in some individuals due to both waning immunity and likely strain variation.
- 35. Conclusions:
- a) issuance of a time-limited immunity certificate following RT-PCR confirmed SARS-CoV-2 infection is reasonable based on the current evidence
- b) due to the lower sensitivity and specificity of LFDs, it is not advised to issue time-limited immunity certificates based only on a positive LFD result
- c) due to the current lack of robust correlates of protection and the variability of antibody assays, it is not currently advised to issue time-limited immunity certificates based only on a positive antibody test
- d) it is recommended that one standardised RT-PCR and antibody test be adopted for diagnosing SARS-CoV-2 and for issuing immune certification, respectively; these underpinning, validated and standardised diagnostics would support immune certification in an ongoing vaccine environment
- e) a standardised RT-PCR test correlated with viral load is required to determine and provide measurement on ‘infectiousness’; this is critical to establish in re-infected (or vaccinated individuals) whether virus is present, how much and whether the person is likely to transmit
Other challenges with immunity certification
36. A proportion of people ‘certified’ as immune based on infection, vaccination or antibodies may be able to become silently reinfected and transmit infection. This possibility would need to be considered when assessing the risk-benefit balance of certification in certain groups for example people caring for high-risk individuals.
37. SARS-CoV-2 undergoes changes in the receptor binding domain of the spike glycoprotein that could lead to evasion of immunity that developed in response to infection with an earlier virus variant. Therefore, if SARS-CoV-2 variants emerge that evade existing immunity, immunity certificates due to prior infections could no longer be valid.
Potential ‘use cases’ for immunity certification
38. To our knowledge at present, the USA, and three European countries, including Ireland, recommend explicitly that recovered COVID-19 individuals who have been re-exposed to SARS-CoV-2, do not need to quarantine.
39. There are many options for who is ‘certified’ and what ‘certification’ would allow. Due to the incomplete protection against infection following natural infection, immunity certificates should not be a substitute for other measures to protect high-risk individuals (for example elderly patients and social care residents). As such, measures such as the use of personal protective equipment, symptoms reporting, and regular testing regimens should still apply to individuals holding immunity certificates.
Recommendations for additional work
- Ct values from RT-PCR positive results in the cohort studies are needed to assess if reinfection results in lower levels of virus replication, and therefore infectivity, than a primary infection
- Standardised RT-PCR and antibody assays are needed to increase confidence that positive results are associated with protection
- The effect of vaccination on sterilising immunity and virus shedding is a key unknown; studies should be planned now to capture this data as the vaccines are rolled out across UK whilst the virus still circulates
Appendix: figures
Figure 1. Main analysis - protective efficacy against PCR confirmed infection (with or without symptoms) all studies
Forest Plot Including all unique studies – When there are zero events in any cell meta-analyses commands add 0.5 events to treatment and control groups to enable calculation of RR.
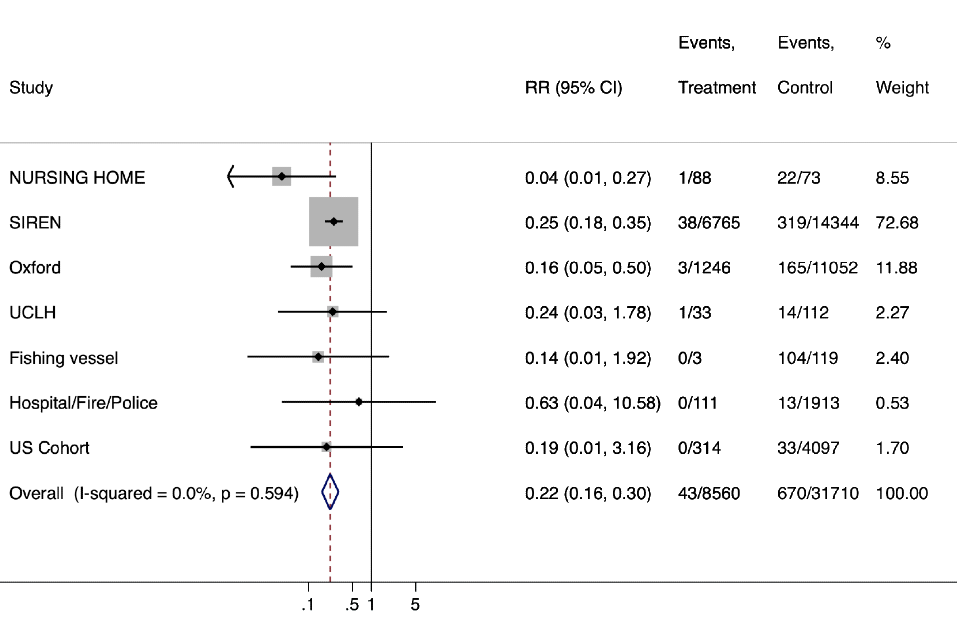
Graph shows the overall estimate of the relative risk across all studies was 0.22 (95%CI 0.16-0.30) corresponding to a protective efficacy of 78% (95%CI 70% - 84%).
| Study | RR (95% CI) | Events, Treatment | Events, Control | % Weight |
|---|---|---|---|---|
| NURSING HOME | 0.04 (0.01, 0.27) | 1/88 | 22/73 | 8.55 |
| SIREN | 0.25 (0.18, 0.35) | 38/6765 | 319/14344 | 72.68 |
| Oxford | 0.16 (0.05, 0.50) | 3/1246 | 165/11052 | 11.88 |
| UCLH | 0.24 (0.03, 1.78) | 1/33 | 14/112 | 2.27 |
| Fishing vessel | 0.14 (0.01, 1.92) | 0/3 | 104/119 | 2.40 |
| Hospital/Fire/Police | 0.63 (0.04, 10.58) | 0/111 | 13/1913 | .053 |
| US Cohort | 0.19 (0.01, 3.16) | 0/314 | 33/4097 | 1.70 |
| Overall (I-squared = 0.0%, p = 0.594) | 0.22 (0.16, 0.30) | 43/8560 | 670/31710 | 100.00 |
Figure 2. Analysis of protective effect against classic COVID-19 symptoms in HCW - up to 6 months follow up.

Graph shows the overall relative risk of infection was 0.01 (95%CI 0.00-0.07) corresponding to a combined protective effectiveness against infection with classical symptoms of 99% (95% CI 93.0%-100%).
| Study | RR (95% CI) | Events, Treatment | Events, Control | % Weight |
|---|---|---|---|---|
| SIREN | 0.01 (0.00, 0.08) | 1/6765 | 197/14541 | 78.95 |
| Oxford | 0.03 (0.00, 0.43) | 0/1246 | 165/11128 | 21.05 |
| Overall (I-squared = 0.0%, p = 0.601) | 0.01 (0.00, 0.07) | 1/8011 | 362/25669 | 100.00 |
Note: to calculate RR for the Oxford study the meta analysis adds 0.5 events to each arm leading to RR slightly above zero despite zero events in those with antibodies.
Figure 3. Analysis of protective effect against asymptomatic infection (or without classic COVID-19 symptoms) in HCW - up to 6 months follow up.
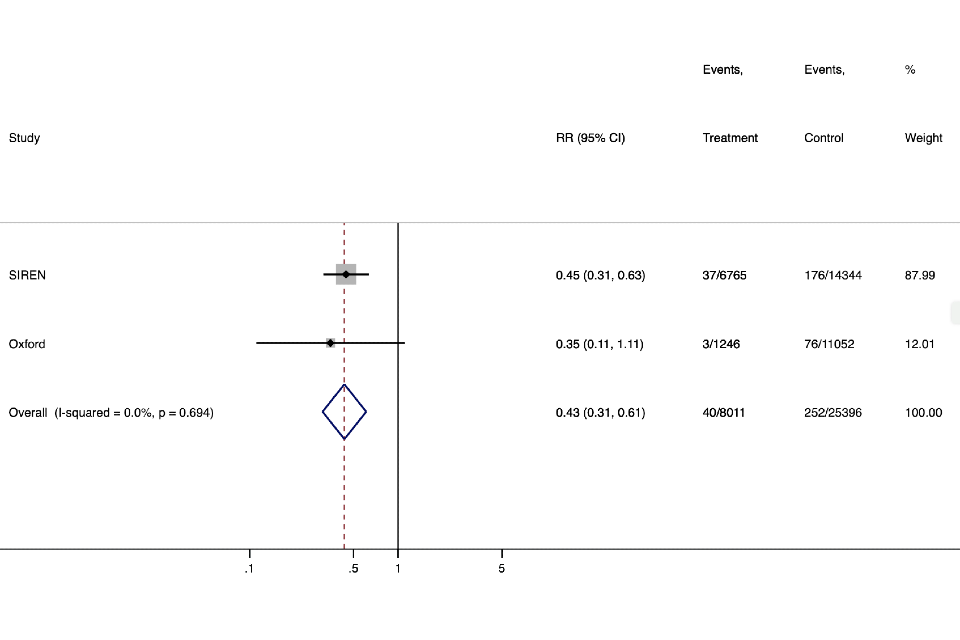
Graph shows the overall relative risk of infection was 0.43 (95%CI 0.31-0.61) corresponding to a protective effectiveness against asymptomatic infection of 57% (95% CI 49%-69%).
| Study | RR (95% CI) | Events, Treatment | Events, Control | % Weight |
|---|---|---|---|---|
| SIREN | 0.45 (0.31, 0.63) | 37/6765 | 176/14344 | 87.99 |
| Oxford | 0.35 (0.11, 1.11) | 3/1246 | 76/11052 | 12.01 |
| Overall (I-squared = 0.0%, p = 0.694) | 0.43 (0.31, 0.61) | 40/8011 | 252/25396 | 100.00 |
Figure 4. Meta-analysis of combined results of studies that swabbed routinely regardless of symptoms

Graph shows the overall relative risk of infection was 0.22 (16%CI 0.00-0.30) corresponding to a combined protective effectiveness against infection of 78% (95%CI 70% - 84%).
| Study | RR (95% CI) | Events, Treatment | Events, Control | % Weight |
|---|---|---|---|---|
| NURSING HOME | 0.04 (0.01, 0.27) | 1/88 | 22/73 | 8.74 |
| SIREN | 0.25 (0.18, 0.35) | 38/6765 | 319/14344 | 74.33 |
| Oxford | 0.16 (0.05, 0.50) | 3/1246 | 165/11052 | 12.16 |
| UCLH | 0.24 (0.03, 1.78) | 1/33 | 14/112 | 2.32 |
| Fishing vessel | 0.14 (0.01, 1.92) | 0/3 | 104/119 | 2.45 |
| Overall (I-squared = 2.5%, p = 0.392) | 0.22 (0.16, 0.30) | 43/8135 | 624/25700 | 100.00 |
References
-
Harvala H, Robb M, Watkins N, et al. Convalescent plasma therapy for the treatment of patients with COVID-19: Assessment of methods available for antibody detection and their correlation with neutralising antibody levels. medRxiv 2020: 2020.05.20.20091694. ↩
-
Ladhani SN, Jeffery-Smith A, Patel M, et al. High prevalence of SARS-CoV-2 antibodies in care homes affected by COVID-19: Prospective cohort study, England. EClinicalMedicine 2020; 28: 100597. ↩
-
Alshukairi AN, Khalid I, Ahmed WA, et al. Antibody Response and Disease Severity in Healthcare Worker MERS Survivors. Emerg Infect Dis 2016; 22(6). ↩
-
Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for greater than 6 months after infection. bioRxiv 2020: 2020.11.15.383323. ↩
-
Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral Immune Response to SARS-CoV-2 in Iceland. N Engl J Med 2020; 383(18): 1724-34. ↩
-
Grandjean L, Saso A, Ortiz AT, et al. Long-Term Persistence of Spike Antibody and Predictive Modeling of Antibody Dynamics Following Infection with SARS-CoV-2. medRxiv 2020:2020.11.20.20235697. ↩
-
Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020; 183(1): 158-68 e14. ↩
-
Zuo J, Dowell A, Pearce H, et al. Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection. bioRxiv 2020: 2020.11.01.362319. ↩
-
Edridge AWD, Kaczorowska J, Hoste ACR, et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med 2020; 26(11): 1691-3. ↩
-
Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science 2020; 368(6493): 860-8. ↩
-
Deng W, Bao L, Liu J, et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science 2020; 369(6505): 818-23. ↩
-
Chandrashekar A, Liu J, Martinot AJ, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020; 369(6505): 812-7. ↩
-
Bao L, Deng W, Gao H, et al. Lack of Reinfection in Rhesus Macaques Infected with SARS-CoV-2. bioRxiv 2020: 2020.03.13.990226. ↩
-
Imai M, Iwatsuki-Horimoto K, Hatta M, et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc Natl Acad Sci U S A 2020; 117(28): 16587-95. ↩
-
McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature 2020. ↩ ↩2 ↩3
-
Houlihan CF, Vora N, Byrne T, et al. Pandemic peak SARS-CoV-2 infection and seroconversion rates in London frontline health-care workers. Lancet 2020; 396(10246): e6-e7. ↩
-
Lumley SF, O’Donnell D, Stoesser NE, et al. Antibodies to SARS-CoV-2 are associated with protection against reinfection. medRxiv 2020: 2020.11.18.20234369. ↩
-
Addetia A, Crawford KHD, Dingens A, et al. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J Clin Microbiol 2020; 58(11). ↩
-
Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med 2020; 383(16): 1544-55. ↩
-
Vogel AB, Kanevsky I, Che Y, et al. A prefusion SARS-CoV-2 spike RNA vaccine is highly immunogenic and prevents lung infection in non-human primates. bioRxiv 2020: 2020.09.08.280818. ↩
-
Mercado NB, Zahn R, Wegmann F, et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020; 586(7830): 583-8. ↩
-
van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020; 586(7830): 578-82. ↩
