Guideline IV Signal management, including benefit-risk reports
Updated 7 April 2025
Applies to England, Scotland and Wales
1. Signal management process
1.1 Signal management process overview
Signal management is performed to detect potential safety signals and investigate the possibility of a previously unidentified potential risk, a change in the status of a known risk, or provide reassurance about the absence of a risk of a product or active substance.
A veterinary adverse event signal is information that arises from one or more sources which may suggest a new potentially causal association, or a new aspect of a known association, between an adverse event or set of related events and one or more veterinary medicinal products (VMPs) or active substances. The signal may involve a previously unknown event or could involve an event reported with a higher frequency than what is expected in a population. It may warrant further investigation and, when necessary, action. A signal does not always mean that a VMP has caused the suspected adverse event. Assessment of the signal is required to determine whether or not there is a causal relationship.
Signals may also be identified in relation to lack of efficacy or development of resistance.
Signals may originate from sources such as spontaneous reports, clinical, non-clinical or epidemiological studies, and published literature. They may be identified where:
-
a sudden increase in the number of adverse events in a short period is observed
-
an increase in the frequency of a particular clinical sign or Veterinary Dictionary for Drug Related Affairs (VeDDRA) Preferred Term (PT) is recorded, compared with the expected frequency for that sign
-
an increasing trend of a particular clinical sign or VeDDRA Preferred Term is noted over time
-
new, previously unidentified, clinical signs or VeDDRA PTs are identified
-
a potential impact on, or risk to public or animal health, or welfare is suspected
New information that may be relevant to the signal management process may include the severity, time to onset, duration or outcome of an adverse event, the country in which the event occurred, or clinical information such as gender, age, breed, cause of death or off-label product use.
Signals can relate to an active substance, a particular VMP, or a group of related active substances or VMPs. They may also relate to a specific strength or formulation of a VMP.
Multiple spontaneous adverse event reports are usually required for the threshold to be met for a signal. If a single report contains detailed information regarding an adverse event of significant seriousness or severity and/or involved multiple animals, it may be considered to meet the threshold, but this would only be applicable in very rare and specific circumstances where there is considered to be a significant risk to human, animal, or environmental health or welfare.
1.2 Signal management process requirements
Both the VMD and MAHs are responsible for detecting and managing signals.
The obligations of the Marketing Authorisation Holder (MAH) for signals associated with their VMPs for which Marketing Authorisations (MAs) are held are defined in the VMR 2013 (as amended) Schedule 1 Part 8 paragraph 56 and 59.
The VMR 2013 (as amended) defines the terms benefit-risk balance and signal management process which are referenced in this guideline and can also be found in the Glossary.
The signal management process is a process for performing active surveillance of pharmacovigilance data for VMPs and active substances, assessing that data, and determining whether there is any change to the benefit-risk balance of those products or active substances.
The process should include signal detection and analysis, validation, prioritisation, assessment and confirmation, and subsequent recommendation of proposals for action if required. The order in which these steps are taken may depend on the data available to the MAH. A risk-based approach should be utilised.
The benefit-risk balance is an evaluation of the positive effects of a VMP in relation to risks to human or animal health (relating to the quality, safety, or efficacy of the product), risks of undesirable effects on the environment, or any risk relating to the development of resistance.
MAHs should continuously monitor the benefit-risk balance of their VMPs via a documented signal management process, whether the reports derive from the UK or any other country. This allows for any emerging issues that may affect the benefit-risk balance to be promptly detected.
Both documentation detailing internal signal management processes and evidence that these processes have been followed should be promptly provided to the VMD upon request, whether in relation to an inspection or at any other time.
At a minimum the process should be able to identify:
-
a sudden and unexpected increase in the number of adverse events
-
an unexpected increase in the frequency of a known clinical sign
-
a new clinical sign
-
reports in scientific literature of any of the above
The MAH must record and submit the results of this signal management process on an annual basis to the VMD via a Benefit-risk report (BRR) and Signal notifications (see sections 2, 3 and 4 of this guideline).
If there is identification of a validated signal suggesting a new risk or change of the benefit-risk balance of the product, or if close monitoring or a post-marketing surveillance study is proposed, the MAH must notify the VMD promptly and within 30 calendar days of it being identified (further details on this timeline can be found in section 3.1.1 of this guideline). A new risk or change to the benefit-risk balance might involve circumstances where a change in the product literature, communication to vets or the public, or suspension or withdrawal were required, for instance. Close monitoring can be defined an increase in the frequency (and in some cases depth) of analysis of further data received to help determine whether there is a causal association or not and may also include additional signal detection methods (such as requesting specific information at follow-up to increase the quality of cases). If an urgent safety signal is identified, the MAH should notify the VMD without delay and no later than the next working day for signals that require restrictions to be implemented or 3 days where no restrictions are deemed necessary (see section 2 of this Guideline). Where the process identifies the necessity for a variation in an authorisation, the MAH must also promptly submit an application for such a variation.
Any additional validated signals that do not suggest a new risk, change to the benefit-risk balance or that do not require further investigation or monitoring following assessment, should be submitted as part of the BRSR.
The MAH is obliged to inform the VMD of any valid signal identified by any regulatory authority in any country where the product is marketed which might influence the evaluation of the benefit-risk balance of the product concerned (see sections 2, 3 and 4 of this Guideline).
1.3 Signal detection and analysis
MAHs should aim to utilise all relevant post-marketing pharmacovigilance data which they could reasonably be expected to be aware of, including:
-
data collected within a pharmacovigilance database, such as:
- spontaneous reports obtained via direct reporting to the MAH from all reporters, including those received via sales representatives or other company employees,
- spontaneous reports obtained as part of an enquiry or product defect concern,
- spontaneous reports that the MAH is aware of reported on social media or other media sources,
- spontaneous and solicited reports received from other MAHs, manufacturers, or regulatory authorities,
- reports from routine searches of published literature.
-
post-marketing trials, post-marketing surveillance studies or other observational studies
-
new toxicology and international safety data updates
-
sales data
Increasing the number and variability of data sources, and additional focused data analysis of certain product groups or patient demographics may additionally benefit the signal detection process.
Signals may be detected via qualitative review of individual spontaneous adverse event reports or using semi-quantitative and quantitative statistical analysis methods. A combination of both methods is generally preferable, although the method(s) used may depend on the volume of reports received by, and the size of, the MAH.
When a large number of adverse event reports are received or multiple data sources are integrated, it is recommended to use statistical methods and data mining algorithms based on 2X2 contingency tables producing disproportionality analysis.
Essentially, this provides a measure of the risk (for a particular product/active substance/product group) of an event being reported versus other events, compared to a reference risk (that observed for all products/active substances in the database or for all other products in that product group).
Frequently used measures of association include proportional reporting ratio (PRR), reporting odds ratio (ROR), relative reporting ratio (RRR), and information coefficient (IC). The individual approach of an MAH is considered best selected depending on the database available.
MAHs should continuously monitor their products as part of their signal management process, in a risk-based manner, for instance a monthly review of the number of new cases received and the number of animals reacted could be performed to ensure early detection of potential signals. MAHs should be able to provide evidence of continuous monitoring on request or at time of inspection. Sales fluctuations and seasonal patterns should be taken into consideration when analysing these numbers.
1.4 Signal prioritisation
Signals should be prioritised for assessment according to their likelihood of having a significant impact on individual, population, environmental, or public health. Prioritisation should take into consideration the benefit-risk balance of a product or its active substance and those most likely to lead to risk minimisation measures or regulatory actions, particularly those that might need to occur in a timely fashion.
Signal prioritisation should be carried out throughout the signal management process. The below requirements are expected to be applied as standard, although should not be considered inflexible as appropriate judgement should always be applied to the entire signal management process.
Prioritisation is not required where all signals identified are fully assessed.
Prioritisation requirements are split into two stages (1 and 2). Stage 1 prioritisation should be performed first, and Stage 2 prioritisation should be further performed on the signals that have been prioritised in Stage 1. Stage 1 acts as an initial sift of the signals, and Stage 2 a further sift of those signals already sifted at Stage 1.
Additional monitoring is required for products in certain high-risk groupings.
The prioritisation stages can be carried out at any phase of the usual signal management process, or across multiple phases. For example, if an MAH already routinely performs disproportionality analysis, applies case threshold exclusion and identifies and prioritises Medically Important Terms at the detection phase of their internal signal management process and then excludes terms adequately reflected in the product literature at their validation phase, this will fulfil the stage 1 criteria.
Stage 1 prioritisation
Differing prioritisation criteria should be applied depending on product.
For broadly-marketed products, where a large and adequately diverse database containing the full dataset for the MAHs products (MAH database or other multinational database) is used for signal identification:
-
All Medically Important Term (MIT) signals as listed in section 1.4.3 should be prioritised for assessment unless adequately reflected in the product literature. They should always be prioritised even in the absence of any statistical disproportionality measure, although disproportionality analysis (thresholds ROR>=2, ROR(-)>=1) can be used to determine them amongst other methods. MITs are VeDDRA PTs that are considered significant medical concepts, and some MITs are species-specific. As a general rule, a minimum of 3 cases would be required to prioritise MIT signals determined using disproportionality analysis
-
Non-MIT signals should be determined using disproportionality analysis (thresholds ROR>=2, ROR(-)>=1) and prioritised unless the term is adequately reflected in the product literature
-
As a general rule, a minimum of 5 cases would be required to prioritise non-MIT signals determined using disproportionality analysis
-
Failure to reach these case threshold limits should not automatically preclude a signal from prioritisation. Additional signals should be prioritised as applicable, based on factors such as the frequency of the event, severity of the event, population risk (determined by the size of the exposed population), type of medicinal product/active substance, the length of time the product has been on the market, and the amount of known pharmacovigilance data over the lifecycle of the product.
-
MAHs are encouraged to avoid complete reliance on disproportionality analysis for identification and prioritisation of signals and should utilise additional methods including review of high-frequency events and routine individual case assessment review, as applicable to their system/products.
For products where adverse event data is solely within a national or small database, or where an MAH is able to conduct a thorough review of data through qualitative or non-disproportionality quantitative methods alone :
-
Disproportionality analysis is not considered a robust enough tool for use in smaller databases
-
All Medically Important Term (MIT) signals as listed in section 1.4.3 should be prioritised for assessment unless adequately reflected in the product literature. MITs are VeDDRA PTs that are considered significant medical concepts, and some MITs are species-specific.
-
All non-MIT signals should also be initially prioritised for assessment unless adequately reflected in the product literature.
Stage 2 prioritisation for non-MITs
From the signals prioritised in Stage 1, the following non-MIT signals should then be selected for prioritisation for assessment regardless of the type of database utilised:
- Lack of efficacy signals occurring for products indicated for sedation, anaesthesia, and euthanasia
- Signals already under Close Monitoring
- New signals (those that have not previously been identified as a signal).
Signals within these categories do not need to be prioritised as standard if they clearly involve either:
- No anatomical association (an administration site that is not applicable to the VMP e.g. an injection site signal for a spot-on product), unless there is repeated off-label or misuse of the product
- Lack of efficacy which applies to an unlisted indication.
1.4.1 Monitoring by product category (following Stage 1 and 2 prioritisation)
New products, particularly those containing novel active substances, and existing products where certain alterations have been made to the product literature, should undergo additional monitoring (frequency +/- depth). At minimum, after carrying out Stage 2 prioritisation, all remaining non-MIT signals for these products should be actively considered for reprioritisation every 3 months and MAHs should be able to justify the prioritisation decisions made on request or at time of inspection. This period of additional monitoring should be applied where at the time of analysis the product falls within one of the below product categories:
-
Newly authorised products with fewer than 2 full years of GB sales, excluding copycat (informed consent) products which have not undergone any variations affecting the areas listed in point 3 of this list since first authorised:
- For products that have already had active sales within another jurisdiction for a period of more than 2 full years at the time that GB sales first occur, this period of additional monitoring can be reduced to 1 year
-
Newly authorised products including novel active substances with fewer than 3 full years of GB sales:
- For products that have already had active sales within another jurisdiction for a period of more than 2 full years at the time that GB sales first occur, this period of additional monitoring can be reduced to 2 years
-
Existing products where any of the following have been added to or changed within the product literature within the previous year:
- Indication
- Species or population (only signals occurring in this species must be automatically prioritised)
- Route of administration
- Change to the recommended dosage (amount or frequency)
- Qualitative or quantitative composition
All signals assessed (following prioritisation and validation) through this additional monitoring should be reported via signal notifications or within the annual BRSR following the standard processes detailed within the guidance.
1.4.2 Additional considerations
For non-MIT signals which are not captured by Stage 1 or Stage 2, MAHs may determine the best method for any further prioritisation of signals for their specific products i.e. MAHs can exclude from automatic prioritisation non-MIT signals outside of the criteria (but are expected to maintain an overview and include additional valid signals for assessment if deemed necessary).
It is expected that for all products at minimum a periodic analysis of lack of efficacy trends should be performed and individual case analysis performed as required. The frequency of this analysis will depend on the individual product and should take into consideration factors such as seasonal patterns. MAHs should particularly monitor lack of efficacy trends resulting in death, and again perform individual case analysis as required. MAHs are also expected to adequately monitor reports involving medication and product use errors and off-label use, and environmental reports. Evidence of these periodic analyses will be reviewed as part of the inspection process or may be requested at any time.
MAHs are reminded that these specified criteria should solely be used for initial automatic prioritisation of signals for assessment and do not exclude prioritisation of other signals. For instance, signals should not be automatically excluded at prioritisation/validation steps if the terms already appear in the product literature, or the signal has been previously refuted. These signals may not necessitate full further assessment, however, may include important new aspects of known causal associations or require alteration of the product literature frequency category, and new data may alter a historical assessment. MAHs are expected to perform robust enough overall signal management to ensure that this information is considered, and evidence of this will be required as part of the inspection process or may be requested at any time.
Additional signals should always be prioritised as applicable, in a risk-based manner.
In certain situations, signals that may be linked to topical public concerns or media attention may need to be prioritised, if there may be a benefit to the release of prompt communications.
Prioritisation stage workflow
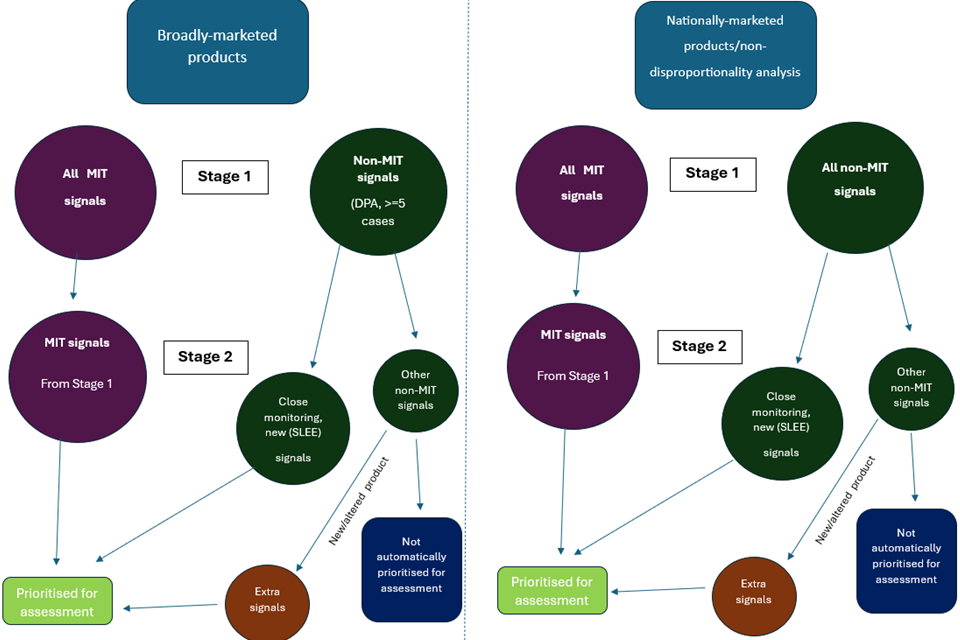
Prioritisation of signals for specific products overview

1.4.3 List of Medically Important Terms (MITs)
This section provides a list of MITs at the level of VeDDRA PT. This list will be regularly updated and therefore MAHs should always ensure that the latest version is being used. It is intended to be used as guidance for prioritisation and analysis of data during the signal management process, however, absence of an event from this list does not exclude that event from analysis.
| PTs | Species |
|---|---|
| All | Human |
| Abdominal pain | Horse |
| Abomasitis | Ruminant, Camelid |
| Abortion | All |
| Acute mastitis | Ruminant, Camelid, Horse |
| Aggression | All |
| Anaphylaxis | All |
| Anorexia | Horse |
| Apnoea | All |
| Ataxia | Horse |
| Bee systemic disorder NOS | Bee |
| Birth defect | All |
| Blindness | All |
| Bone marrow hypoplasia | All |
| Cardiac arrest | All |
| Cardiac insufficiency | All |
| Circulatory shock | All |
| Coagulopathy | All |
| Collapse NOS | All |
| Coma | All |
| Convulsion | All |
| Deafness | All |
| Death | All |
| Diabetes mellitus | All |
| Disseminated intravascular coagulation | All |
| Dyspnoea | All |
| Epileptic seizure | All |
| Fish asphyxia | Fish |
| Fish body deformity | Fish |
| Haemolytic anaemia | All |
| Haemorrhagic gastroenteritis | All |
| Heart block | All |
| Hepatic failure | All |
| Hypersensitivity reaction | All |
| Hypocalcaemic condition | Ruminant, Camelid |
| Hypomagnesaemic condition | Ruminant, Camelid |
| Impaired hearing | All |
| Impaired vision | All |
| Immune mediated thrombocytopenia | All |
| Increased coagulation time | All |
| Ketosis | Ruminant, Camelid |
| Laminitis | Horse |
| Loss of consciousness | All |
| Lying down | Horse, Ruminant, Pig, Camelid |
| Metastatic neoplasia | All |
| Metritis | Horse, Ruminant, Camelid |
| Moribund | All |
| Multi-organ failure NOS | All |
| Myoglobinuria | Horse |
| Paralysis | All |
| Paresis | All |
| Perinatal mortality | All |
| Recumbency | Horse, Ruminant, Pig, Camelid |
| Renal insufficiency | All |
| Reticulitis | Ruminant, Camelid |
| Stillbirth | All |
| Suspected infectious agent transmission | All |
| Thrombocytopenia | All |
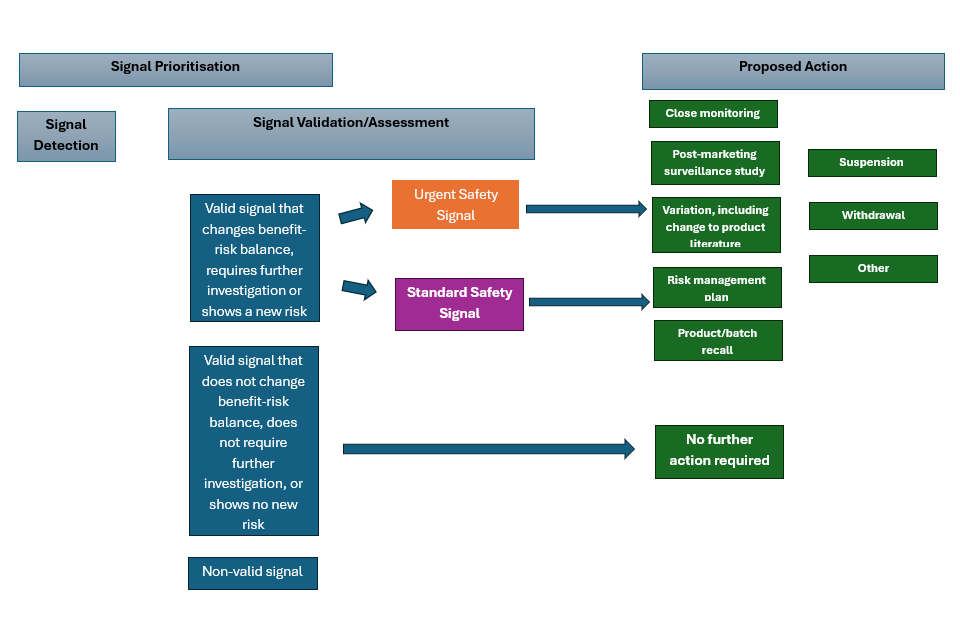
1.5 Signal validation
An initial validation step should be carried out to determine whether more detailed analysis is justified.
As far as possible, it should be ensured that the signal is not based on duplicate reports.
The event must have occurred after exposure to the product occurred.
Signals related to adverse event terms (or clinical signs that can be considered covered by the wording) already adequately reflected in the product information, should generally be counted as non-valid signals. However, if a signal highlights an additional aspect of a current association that may warrant alteration of the wording (for instance regarding the reversibility or expected duration of an event) or to the frequency category, it may be a valid signal. MAHs should be able to justify during an inspection, or on request, that the information is adequately reflected in the current product information when classing such signals as invalid.
All validated signals prioritised for further assessment must be reported to the VMD via a signal notification or as part of the annual Benefit-Risk Submission Report (BRSR).
1.6 Signal assessment and confirmation
Once a signal has been validated, further evaluation should be carried out to determine whether or not there is a possible causal association, or new aspect of a known association between the product and event, and whether the evidence is strong enough to consider further action.
Assessment should ideally involve review of all data available, for example from spontaneous reports, literature reports, pre-clinical trials and clinical studies, toxicology and epidemiological updates, international regulatory data, and sales data. All previously reported cases including the same events and products/active substances should be reviewed as applicable. In some cases, it is recommended to additionally review any previously reported cases involving similar events and similar products/active substances.
The evaluation should provide clinical context to the report and take into consideration other knowledge of an association (previous or concurrent reports, previous signals, previous analysis via Periodic Safety Update Reports (PSURs)/BRSRs for example).
How strong the evidence is may also depend on the total number of reports, the source of the reports (such as if they are from one or different sources, the quality of the source, and if the data is incomplete or vague), and the availability of supportive laboratory data. The potential for over-reporting should also be taken into consideration, for example due to increased media attention, or a known significant increase in sales volumes.
The following list contains considerations that may be made when assessing signals:
-
total number of cases (excluding duplicates)
-
increase in the number of reports (incidence)
-
clinical relevance (seriousness, severity, outcome, reversibility, relationship to signalment)
-
dose-adverse event relationship
-
drug-drug interaction
-
the consistency between reports (for instance in terms of time to onset, pattern of events, outcome, dosages involved)
-
plausibility of the pharmacological/biological mechanism linking the product and event, or a lack of alternative causes
-
dechallenge/rechallenge data
-
supportive relevant investigation/laboratory data
-
clinically similar events occurring in additional reports
-
other potentially impacting clinical variables such as concurrently administered products, medical history
-
potential for over-reporting, for example due to increased media attention, or a known increase in sales volumes
Following assessment of a signal, a conclusion should be made as to whether or not there is good enough evidence to suggest a potential causal association between the product/active substance and event, in order to determine whether action needs to be taken.
1.7 Recommendations for action
If a potential causal relationship between a product/active substance and event is considered unlikely or there is not strong enough evidence at that time, the benefit-risk balance is considered to remain the same and no action is required at that time. A record of assessed signals that have been determined not to meet the threshold for further action should be maintained for review during subsequent signal assessment and potential re-assessment upon obtaining further information.
If a potential causal relationship is considered possible, it should be determined whether further information is required to provide additional evidence, or whether the benefit-risk balance can be considered to have been altered.
If further information is required, one of the following recommendations for action should be proposed by the MAH, with an explanation of the reasoning behind the proposal:
-
close monitoring
-
post-marketing surveillance study
Close monitoring requires in-depth assessment of all new or updated adverse event reports pertaining to the signal. The VMD may also request additional information to be provided at any time. If this occurs then the reason for the requirement and the time by which, or the period during which, the requirement must be complied with will be discussed with the MAH.
If the benefit-risk balance is considered altered; the MAH should propose risk minimisation measures or other relevant actions as appropriate.
The benefit-risk balance may be improved either by increasing the benefits, for example including further explanation of how best to use the product, or by reducing the risks by risk minimisation measures, for example by contraindicating the use in animals particularly at risk, reducing dosage, or introducing precautions for use. When proposing measures to improve the benefit-risk balance of a VMP, the feasibility of those measures under normal conditions of use should be taken into account. If dose reduction is considered as a method of risk minimisation, the impact of dose reduction on efficacy should be carefully evaluated.
The following types of management actions may be necessary and may be initiated by the MAH or by the VMD:
-
variation of marketing authorisations (MAs) in respect of the indication, dosing recommendations, contraindications, warnings and precautions for use or information about adverse events or other sections of the product literature
-
direct provision of important safety information to veterinarians and other health-care professionals and animal owners, for example through letters, bulletins, via electronic media etc
-
urgent safety restrictions may be taken by MAHs in the event of risk to human or animal health or to the environment. If the MAH implements an urgent safety restriction, the MAH shall give the VMD prior or simultaneous notification. Urgent safety restrictions may also be initiated by the VMD
-
suspension or withdrawal of the MA of a VMP, in the event that, the overall benefit-risk balance is considered unfavourable and proposed risk minimisation measures are considered inadequate. Relevant stakeholders should be informed as appropriate
Such actions may be taken voluntarily by MAHs. However, it is recommended that any such intended measure be discussed at an early stage with the VMD.
If the resulting action requires a variation to an MA, the MAH must promptly submit an application for such a variation, alongside data to support the proposed variation. The VMD will assess, and accept or reject the variation, based on the benefit-risk balance.
The MAH should also inform the VMD of any prohibition or restriction imposed by the regulatory authority of any country in which the VMP is authorised within 30 calendar days of the receipt of such information, if no equivalent measure has been already taken in GB. This includes any pharmacovigilance-related changes to the product literature. The 30-day timeline starts from the date that the regulatory authority requested/imposed the action. This information should be presented to the VMD in the form of a signal notification (standard or urgent as applicable). Further details on standard and urgent safety signal submissions can be found in sections 2 and 3 of this guideline and in the Technical guidance for completion of Standard and Urgent Signal Notifications at Benefit-risk report (BRR) and signal notification submissions - GOV.UK.
2. Urgent safety signals
Urgent safety signals are those containing new information affecting the benefit-risk balance which require rapid implementation of risk minimisation/safety measures.
These signals may be identified from studies, spontaneous reports, published literature, or be related to regulatory actions proposed by other regulatory authorities.
Urgent safety restrictions may need to be taken in the event of a risk to human or animal health, or to the environment, and may take the form of an interim change to the product literature or distribution category, for example, therapeutic indications, dosage information, contraindications or warnings, a risk management plan, batch recall or suspension/withdrawal of a product. It may be necessary to provide safety information communications to relevant stakeholders.
If urgent safety restrictions are proposed by an MAH, the VMD must be informed no later than the next working day from when evidence comes to the attention of the MAH of the reasons for the action. The VMD should also be notified of any urgent safety restrictions imposed by other regulatory authorities immediately.
The VMD should otherwise be informed of a potential urgent safety signal not deemed to require restrictions following the conclusion of an initial internal signal management process within 3 days.
The initial notification of an urgent safety signal leading to an urgent safety restriction should include, within the ‘Evaluation and summary of findings’ field, brief clinical details and an initial assessment of the urgency and potential impact of the signal. It is appreciated that a full assessment cannot be performed within 1 working day, and that further investigation by the MAH/VMD may ultimately conclude that the initial assessment of the signal/impact means that no further action is required.
Any emerging information following this initial notification should be promptly provided to the VMD.
Following further assessment, for both urgent signals requiring restrictions and those deemed no to require restrictions, the MAH should have provided a detailed description of the signal, including information on the source, incidence, and clinical details, alongside an evaluation of the urgency and potential impact. Any proposed actions, or those that have already been taken should also have been clearly detailed.
The VMD will assess the information as provided and discuss the implementation of actions with the MAH.
Urgent safety measures including any related safety communications can be proposed and implemented by the MAH, however the VMD should be informed of these with prior or simultaneous notice. Urgent safety measures may also be proposed by the VMD at any time where there is a significant concern that the benefit-risk balance has altered.
3. Notifying VMD of a signal
3.1.1 Validated signals suggesting a change to the benefit-risk balance
The VMD should be notified without delay of all validated signals which following assessment, suggest a new risk, change to the benefit-risk balance, or require further investigation (including those signals put under close monitoring). Close monitoring can be defined an increase in the frequency (and in some cases depth) of analysis of further data received to help determine whether there is a causal association or not and may also include additional signal detection methods (such as requesting specific information at follow-up to increase the quality of cases).
This means VMD should be notified of these signals using the Signal notification option or Urgent signal notification option on the BRSR/Signal notification template (for further details see Technical guidance for completion of Standard and Urgent Signal Notifications which can be found on the Benefit-risk report (BRR) and signal notification submissions page. Signals must be submitted within 30 calendar days for standard signals, or no later than the next working day for urgent signals that require restrictions to be implemented or 3 days where no restrictions are deemed necessary.
The 30-day period for standard signals starts upon conclusion of initial internal signal management processes, or once a valid signal that suggests this change to the benefit-risk balance (or where it is clear further investigation/monitoring will be required for a period of time) is identified. For some MAHs the entire signal management process will have concluded at the point of submission. It is appreciated that there is variation across signal management processes carried out by MAHs however, and that it may take some time to complete signal analysis to this level. For the purposes of compliance, at the very least a concerted effort by the MAH to procure and assess as much information as possible within this time period must be proven. If the 30-day timeline is not expected to be met, a communication including justification for the delay must be provided to the VMD in order for a new timeline to be mutually agreed as appropriate.
Valid signals identified by any regulatory authority in any country where the product is authorised which might influence the evaluation of the benefit-risk balance of the product concerned should also be reported in this way. This includes any signals subsequently requiring close monitoring. The 30-day timeline starts from the date that the MAH was made aware of the identification of the signal by the regulatory authority. Signals involving event terms already adequately reflected on the SPC in the UK (following review of both the term and frequency category) do not require submission as signal notifications but should be included within the next BRSR.
Signals should be accompanied by a proposal by the MAH to either obtain further information (via close monitoring or a post-marketing surveillance study), perform a regulatory action (change to product literature via variation, risk management plan (RMP), product recall, suspension of product, withdrawal of product) or for there to be no further action taken at that time.
A separate signal notification document must be provided for each signal or set of related signals. The signal will be linked to the MA number or Product Group Code stated on the template (see section 4.3 of this Guideline).
A flowchart showing an overview of signal submissions can be found in section 3.1.2 of this guideline.
3.1.2 Validated signals that do not suggest a change to the benefit-risk balance
All validated signals, having been prioritised for further assessment which, following assessment, are deemed to not suggest a new risk or change to the benefit-risk balance, or to require close monitoring, should be submitted annually via the BRSR template on the ‘Signals and regulatory actions’ tab. These are signals that have been assessed by the MAH as requiring no further action. Applicable signals that have been assessed via other regulatory authorities should also be included within this section.
Signals that have previously been submitted as Signal notifications (urgent or standard) do not need to be re-submitted as a new signal notification unless new relevant information that may alter previously agreed actions has come to the attention of the MAH. Further details can be found in section 3.1.3 of this guideline.
Non-validated signals should be recorded by the MAH but should not be submitted to the VMD.
Multiple signals (adverse event terms) for the same MA number/Product Group Code can be reported within the same annual BRSR, but Signal notifications must only contain one signal (PT or set of related PTs) per notification.
Submission of signals overview
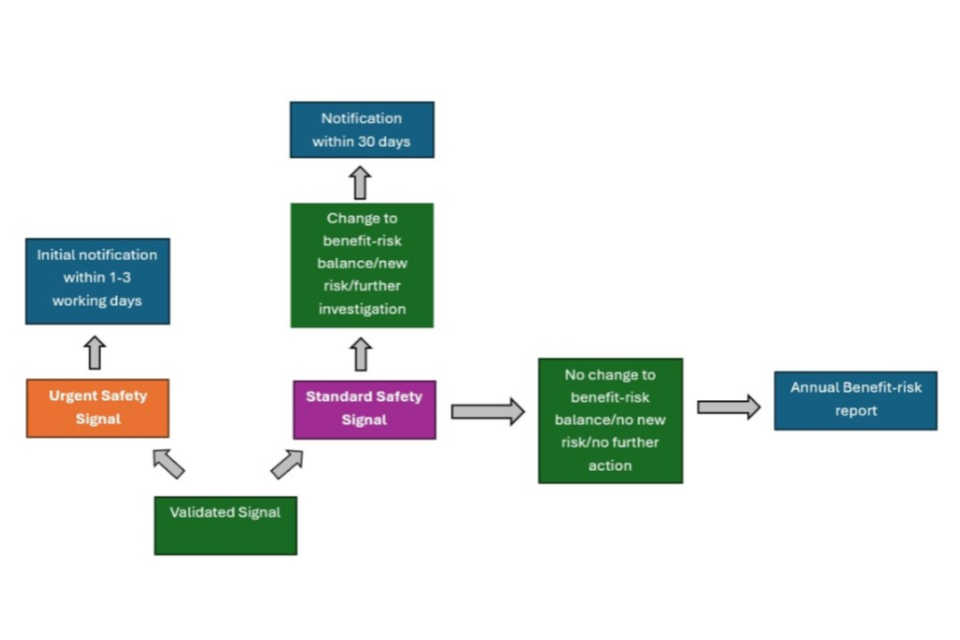
3.1.3 Submission of Signal notifications
Signal notifications should be submitted using the BRSR template. Further details on completion of this template can be found in the Technical guidance for completion of Standard and Urgent Signal Notifications on the Benefit-risk report (BRR) and signal notification submissions page.
Signals that have been reported as urgent or standard signal notifications within the reporting (cover) period do not routinely need to be re-submitted as a signal notification, or at the time of the BRSR, if no new relevant information that may alter previously agreed actions comes to the attention of the MAH. This applies to all signals including those involving Medically Important Terms (MITs).
If further information on these previously submitted signals is specifically required at the time of the BRSR (or any other time), for example if the outcome of a signal notification was an action for close monitoring and further information is expected to be submitted, this requirement will usually have been made clear in the VMD Signal Notification Assessment Report.
A ‘follow-up’ signal notification should be routinely submitted in the following scenarios, unless the VMD has already agreed with the MAH that the information should be received via a different method:
- An MAH wishes to alter a previously Proposed action (as an example, the previously Proposed action was Close Monitoring but now a batch recall is required)
- An MAH wishes to add a Final action (as an example, the previously proposed action was Close Monitoring and the MAH now deems no further action to be required, or a variation has been completed)
- There are any changes to the action required/further actions required by another regulatory authority
- If relevant additional information is identified, which the MAH deems significant enough to alter the prior assessment of a signal, even if it does not alter the action (as an example, Close monitoring was previously proposed with full re-assessment every 6 months, but further information is received which means the MAH will now fully re-assess every 3 months)
Follow-up signal notifications should be submitted within 1-3 days for urgent safety signals or 30 days for standard safety signals. These timelines apply to the current status of the signal (e.g. if a signal was previously classed as standard, and further information alters the classification to urgent, the follow-up signal notification should be submitted within 1-3 days of that further information being received). Follow-up signal notifications can be submitted at any time, including across reporting periods. Further information on follow-up signal notifications can be found within the Technical guidance for completion of Standard and Urgent Signal Notifications at Benefit-risk report (BRR) and signal notification submissions.
Multiple BRSR template documents can be submitted per VMDS submission as long as only one template is submitted per MA or Product Group Code (PGC) (further details on PGCs can be found in section 4.3 of this Guideline). If further information is requested, or the MAH wishes to submit further documentation, then this can be added to the submission as a separate document. The following document types can be accepted via VMDS secure messaging: .zip, .pdf, .doc, .docx, .xls, .xlsx, .ppt, .pptx, .jpeg, .jpg, .xml. Multiple files can total up to 10GB in size.
Signal documents should be submitted using the Veterinary Medicines Digital Service (VMDS), a secure messaging service. Any MAH not signed up to VMDS can register at Veterinary Medicines Digital Service.
Once signed in to the VMDS account, the MAH should select the relevant group. Non-urgent ‘standard’ signal notification submissions should be sent to PHV Signals, and urgent safety signals should be sent to PHV Urgent Safety Signals.
Queries related to urgent safety signal submission or requests for assistance with VMDS in relation to urgent safety signals should be sent by e-mail to adverse.events@vmd.gov.uk.
Queries related to all other (non-urgent) signal notification submissions should be directed either via VMDS to the PSUR Queries group or via e-mail to psur.queries@vmd.gov.uk.
Signal management process overview
4. Benefit-risk reports
A BRR is a set of important documents provided by the MAH to the VMD post-authorisation on an annual basis. The documents are intended to provide an update on the worldwide benefit-risk balance of a product, including any validated signals detected that have not previously been submitted to the VMD.
BRRs are required for all products authorised in the UK regardless of whether or not a product has been marketed. Electronic copies of the BRR templates found on the Benefit-risk report (BRR) and signal notification submissions page should be submitted in Excel format to the VMD using the Veterinary Medicines Digital Service (VMDS), a secure messaging service. Any MAH not signed up to VMDS can register at Veterinary Medicines Digital Service.
4.1 Benefit-risk report submissions
Following the placing of a VMP on the market in the UK, an MAH must submit all required components of a BRR over the course of every year during the period of validity of the authorisation. A benefit-risk statement alongside any related signals/regulatory actions must be submitted once annually, and sales data should be submitted either once annually or on a rolling basis across the year.
The Benefit-Risk Submission Report (BRSR) comprises the benefit-risk statement, and any related signals/regulatory actions. The Pharmacovigilance Sales Submission (PSS) covers sales data only.
BRSRs should be submitted regardless of whether the product was marketed, whether there were any signals detected throughout the year and whether the MAH deems there to be any change to the benefit-risk balance or not. They should cover the period of time since the data lock point (DLP) in the preceding BRSR (or for first submission of a BRSR, the final PSUR) and there should be no gaps in, or overlapping, of data.
The DLP should be no earlier than 2 months prior to the submission date.
It is recommended that the DLP date be on the last day of a month.
Worldwide authorisation status data must be up to date at the time of BRSR submission, if provided.
Any information received after the DLP and prior to BRSR submission which the MAH deems important can be submitted within the same BRSR if necessary. (This should be highlighted within the BRSR template by entering different dates in the Data Lock Point and Period of report to cells as detailed within the Technical guidance on completion of the Benefit-Risk Submission Report (BRSR) which can be found on the Benefit-risk report (BRR) and signal notification submissions page.
Signals that have been reported as urgent or standard signal notifications within the reporting (cover) period will be assessed by the VMD and actions will be agreed with the MAH. These signals do not routinely need to be re-submitted as another signal notification, or at the time of the BRSR, if no new relevant information that may alter previously agreed actions comes to the attention of the MAH. This applies to all signals including those involving Medically Important Terms (MITs).
If further information is required at the time of the BRSR (or any other time), for example if the outcome of a signal notification was an action for close monitoring and further information is expected to be submitted, this requirement will have been made clear in the VMD Signal Notification Assessment Report.
Valid signals not meeting the criteria for reporting via a signal notification (i.e. ‘No further action’ signals), must be included within the next BRSR due following identification of the valid signal, even if they have been included in a previous year’s BRSR. Submission dates for BRSRs should align with the grouped active substance recommended due dates of submitting annual statements available on the EMA website.
For new Anatomical Therapeutic Chemical Classification System for veterinary medicinal products (ATCvet) codes which are not included in the list, or if there are any queries regarding ATCvet codes, MAHs should contact psur.queries@vmd.gov.uk.
The BRSR should be submitted to the VMD no later than the applicable submission due date for the product unless agreed with the VMD in advance.
In exceptional circumstances, MAHs can request to change the submission dates (and DLP) for the annual BRSR by contacting psur.queries@vmd.gov.uk with a proposal for an alternative date, and an explanation of the reason for the request. Where possible, alternative dates should be the last day of a month.
BRSRs should also be submitted on request by the VMD on an ad hoc basis. The DLP and submission date for these ad hoc requests will be proposed by the VMD depending on the urgency of the issue and should be agreed with the MAH.
BRSRs should be submitted as one completed Excel document per MA number or Product Group Code (PGC) (see 4.3 of this Guideline for further details) as per the BRSR template. Sales data should be submitted using the separate Pharmacovigilance Sales Submission (PSS) (see section 4.5 of this Guideline for further details).
The templates must be submitted in the Excel format type .xlsx.
For BSRS naming conventions see the Technical guidance for completion of Benefit-Risk Submission Report (BRSR) which can be found on the Benefit-risk report (BRR) and signal notification submissions page.
BRSRs should be submitted using the Veterinary Medicines Digital Service (VMDS), a secure messaging service. Any MAH not signed up to VMDS can register at Veterinary Medicines Digital Service.
Once signed in to the VMDS account, the MAH should select the relevant group: BRR Submission.
Note that if a Benefit-Risk Submission Report is submitted at the same time as a Pharmacovigilance Sales Submission, the BRSR must be submitted to the group ‘BRR Submission’ and the PSS must be submitted to the group ‘Sales’.
Queries related to BRR submission should be directed either via VMDS to the PSUR Queries group or via e-mail to psur.queries@vmd.gov.uk.
4.2. Content of benefit-risk reports
All BRSRs should be completed in English; and all declarations and signals must take into account all adverse events arising in the UK and outside of the UK regardless of the route of authorisation.
It is strongly recommended that, before submitting the BRSR, the MAH should make sure that all adverse event reports have been submitted electronically where required and duplicate detection performed.
For pharmacovigilance data to be correctly attributed to the right product in the VMD databases MAHs must use either an up-to-date MA number or a Product Group Code (PGC) within a BRSR. Note that for sales submissions including EEA data and split GB/NI MAs, a PGC will be required. Further details on PGCs can be found in section 4.3 of this Guideline.
For sales data to be correctly attributed to the corresponding BRSR, MAHs must use either up-to-date MA numbers or PGCs within the column A of the Pharmacovigilance Sales Submission template (‘Product Group Code (PGC) or MA Number’).
Any non-serious UK cases not previously submitted to the VMD i.e. since last PSUR DLP, can either be submitted electronically or should be included in a line listing in the relevant sheet ‘Backlog line listings’ of the BRSR. In subsequent BRSRs this sheet does not need to be completed as all adverse event cases should be sent electronically within 30 calendar days of receipt by the MAH to the VMD.
Guidance on the required information for adverse event reports, and particular types of reports can be found in section 4 of Guideline III Adverse event reporting.
Full guidance on completion of the BRSR can be found in the Technical guidance for completion of the Benefit-Risk Submission Report (BRSR) on the Benefit-risk report (BRR) and signal notification submissions page.
4.3 Product Group Code (PGC)
A Product Group Code (PGC) links a set of individual Marketing Authorisations together into a single group. It should be used to link both UK MAs and, at minimum, their EEA equivalent products if the product is authorised in EEA.
A PGC must be used if one of the following apply:
-
Data for multiple MAs are to be submitted within the same BRSR (this may include different strengths of the same product, products with split GB/NI MA numbers, and ‘similar’ products)
-
A MAH has EEA authorised products
Products only authorised in GB with no equivalent third country products or products with UK-wide authorisation without split GB and NI MA numbers do not require a PGC, and the MA can be used. However, a PGC can still be used for these products, and this is preferred by the VMD.
The same product group should be intended to be used for all future submissions. The VMD must be contacted if the MAH wishes to add products (for example, a newly authorised product), remove products (for example, a product no longer authorised or moved under the responsibility of a different MAH) or alter products within this grouping.
A PGC must be either proposed by the MAH and accepted by the VMD or requested by the MAH at least one month prior to the first BRSR or PSS submission, and a BRSR or PSS should not be submitted until this Product Group Code has been agreed. Signal notification submissions must be made using a PGC if this has been agreed.
Any future changes to products grouped within the Product Group Code should also be agreed at least one month prior to the next BRSR (or PSS) submission due.
A MAH must either:
-
Propose:
a) A list of MA numbers and their associated brand name and authorisation country grouped by PGC
b) One or more PGCs following the below naming convention
c) The ATCvet code submission due date by which this PGC will be submitted
-
Provide (in order for the VMD to create a PGC):
a) A list of MA numbers and their associated brand name and authorisation country grouped by PGC
b) The ATCvet code submission due date by which this PGC will be submitted
-
Provide (if no PGC is required):
a) A list of MA numbers which will be submitted individually and their brand names
b) The ATCvet code submission due date by which each MA will be submitted
The group should generally contain only similar VMPs which:
-
Originate from the same MAH being responsible for pharmacovigilance of these VMPs
-
Have the same active ingredients (substances)
-
Have the same major excipients with the same or similar pharmaceutical function
-
In general, at least one common registered species.
On some occasions, the VMD may allow grouping of products that do not fulfil all of these criteria; however, this would be decided on an individual product basis.
In some cases, it will only include different strengths of the same product. Product groupings that are considered too large or diverse may be declined by the VMD and an alternative suggestion made.
The Product Group Code should generally take the following format:
MAHORGID-GROUPNAME
The MAHORGID is the 8-digit organisational ID (used as part of your worldwide case number (AERID)) used for adverse event case submission. The group name must be an overarching product name for all the MAs within that PGC.
For example, if the VMD were to have a Product Group Code for the invented products Benefitas 10 mg and Benefitas 20 mg, with a chosen overarching product name of Benefitas, the PGC would be VMDDEFRA-BENEFITAS.
If the MAGHORID does not reflect the MAH name clearly an alternative to the MAGHORID can be proposed, but this ID should be no longer than 10 characters in length.
The overarching product name might be better suited to a name referencing the active substance and formulation if there are multiple different brand names within the group.
Queries related to Product Group Codes should be directed either via VMDS to the PSUR Queries group or via e-mail to psur.queries@vmd.gov.uk.
4.4 Incidence calculations
Incidence % should be calculated for signals submitted within signal notifications or the BRSR, using the following formula:
Incidence % = (Number of animals reacted ÷ number of animals treated) x 100
Incidence should be provided as a percentage figure within the Evaluation and summary of findings cell of the template or provided within a separate document submitted alongside the BRSR. For further details, see the Technical guidance for completion of the Benefit-Risk Submission Report (BRSR) or the Technical guidance for completion of Standard and Urgent Signal Notifications which can be found on the Benefit-risk report (BRR) and signal notification submissions page. A brief explanation of how the number of animals reacted was determined should be provided (e.g. whether off label events and events unlikely to be product related (N causality events) were included).
No incidence calculations are required when no signals are being submitted within a BRSR.
When an adverse event signal is reported, either via a signal notification, or as part of the BRSR, the incidence per event term for each signal (including refuted signals within the BRSR) AND an overall adverse event incidence excluding suspected lack of efficacy should be provided.
When a lack of efficacy signal is reported, the incidence per lack of efficacy event term for each signal (again including refuted signals within the BRSR) AND overall lack of efficacy incidence should be provided.
As signals are reported per species, these incidences should be species specific.
Additional incidence information can be provided if deemed applicable to the signal, and if it is felt that the above guidance does not apply to the signal being reported, explanation of alternative calculations used should be provided.
For signals requiring no further action reported within a BRSR where the MAH deems the causal association to be extremely unlikely and an incidence calculation is deemed irrelevant to any decision making involved for that signal, an incidence does not need to be provided, however this should be briefly explained. An example would be a lack of efficacy signal that applies to an indication not listed for the product.
4.5 Sales data submissions
Sales submissions must be made if there have been any sales of the product(s) in the UK and/or in any EEA country.
If there have been zero sales, sales submissions are not required.
Sales data must be submitted using the standalone Pharmacovigilance Sales Submission (PSS) Excel template.
The template must be submitted in the Excel format type .xlsx.
For naming conventions, see the Technical guidance for completion of the Pharmacovigilance Sales Submission (PSS) which can be found on the Benefit-risk report (BRR) and signal notification submissions page.
PSSs should be submitted using the Veterinary Medicines Digital Service (VMDS), a secure messaging service. Any MAH not signed up to VMDS can register at Veterinary Medicines Digital Service - GOV.UK (www.gov.uk).
Once signed in to the VMDS account, the MAH should select the relevant group: Sales.
Note that if a Benefit-Risk Submission Report is submitted at the same time as a Pharmacovigilance Sales Submission, the BRSR must be submitted to the group ‘BRR Submission’ and the PSS must be submitted to the group ‘Sales’.
Additional documents (for example detailed dose factor justification that is too lengthy to fit within the PSS template) can be submitted alongside the PSS within the same VMDS submission. The following document types can be accepted via VMDS secure messaging: .zip, .pdf, .doc, .docx, .xls, .xlsx, .ppt, .pptx, .jpeg, .jpg, .xml. Multiple files can total up to 10GB in size.
PSSs involving products within a PGC should not be submitted until this PGC has been agreed with the VMD (at least one month prior to first submission). For further details see section 4.3 of this Guideline.
Sales submissions can be made at the same time as the Benefit-Risk Submission Report or split into multiple submissions throughout the year (submitted at any time).
UK and EEA sales data for relevant products must have been submitted at least up to the end of the previous calendar year by the time of the BRSR submission, although if more recent data are available their submission is preferable.
There must be no gaps or overlap in data.
Multiple products with different MAs or within different PGCs can be submitted at the same time within the same PSS.
PSSs may also be requested at any time by the VMD on an ad hoc basis.
Sales submissions should contain one or more of the following at each submission:
-
Sales data for the UK as a whole (not separated into GB/NI)
-
Sales data for EEA countries (listed individually)
Sales data for non-EEA countries does not need to be submitted as part of the annual BRR, however may be requested by the VMD at any time for submission within an agreed timeline.
Sales data for the UK should be provided for all pack sizes per product (per specific MA or for split GB/NI MAs, combined).
Sales data for EEA countries should be provided for all pack sizes per UK-equivalent product or for a nominal pack size that covers all individual pack sizes. See the Technical guidance for completion of the Pharmacovigilance Sales Submission (PSS) which can be found on the Benefit-risk report (BRR) and signal notification submissions page for detailed guidance on completion of the PSS.
Queries related to selection of the appropriate unit, pack size or dose factor, or any query regarding the sales submission process should be directed to sales@vmd.gov.uk or via VMDS secure messaging to the Sales group.
4.5.1 UK Sales data
UK sales data must be provided by individual pack size (one pack size per row).
Sales data for multiple MAs or PGCs can be submitted within the same PSS.
The breakdown of sales data submitted should be monthly. If accurate monthly figures for sales data are unknown, an estimate should be calculated from the shortest time period available to provide the most accurate data. For example, if both 3-monthly and 12-monthly sales data breakdowns are available, the 3-monthly data should be divided by 3 to achieve the estimated monthly sales, rather than using the 12-monthly sales data.
If there have been zero sales, sales submissions are not required.
The PGC/MA Number listed in column A of the PSS must be accurate to allow linking of sales data to the correct BRSR. The PGC must be entered unless none exists for the product. Only use the MA Number in this column if the product is GB-only authorised and no PGC has been created.
Further details on completion of the PSS can be found in the Technical guidance on completion of the Pharmacovigilance Sales Submission (PSS) which can be found on the Benefit-risk report (BRR) and signal notification submissions page.
4.5.2 EEA sales data
EEA sales data must be provided by individual pack size or nominal pack size (one pack size or nominal pack size per row). Sales data must be provided per country.
Sales data for multiple MAs or PGCs can be submitted within the same PSS.
The breakdown of sales data submitted should be per month. If accurate monthly figures for sales data are unknown, an estimate should be calculated from the shortest time period available to provide the most accurate data. For example, if both 3-monthly and 12-monthly sales data breakdowns are available, the 3-monthly data should be divided by 3 to achieve the estimated monthly sales, rather than using the 12-monthly sales data.
If there have been zero sales, sales submissions are not required.
Nominal pack sizes can be used for EEA data if sales data per specific pack size is not provided. These pack sizes may be different to any UK authorised pack, but the dose factor must relate to this nominal pack.
MAHs should choose one or the other of these options within a single submission and for ongoing submissions to allow for comparison of data over time.
The PGC listed in column A of the PSS must be accurate to allow linking of sales data to the correct BRSR.
Further details on nominal pack sizes and completion of the PSS can be found in the Technical guidance on completion of the Pharmacovigilance Sales Submission (PSS) which can be found on the Benefit-risk report (BRR) and signal notification submissions page.
4.5.3 Non-EEA sales data
Sales data for non-EEA countries does not need to be submitted as part of the annual BRR, however can be requested by the VMD at any time for submission within an agreed timeline.
4.6 BRR assessment
If the VMD has any comments or questions related to the BRR submission, the VMD will inform the MAH of these via a BRR assessment report. Responses to questions should be addressed by the MAH within 60 calendar days. Any changes or variations requested should be submitted within 6 months unless otherwise requested or agreed.
Changes may be requested for any section of the product literature.
Requests for changes to the product literature should be made in line with the appropriate GB QRD template.
