Safety Bulletin 7: Safety concern over imported liferafts containing medical kits
Published 27 April 2016
The MHRA has learned that liferafts may have been supplied to ships, fishing vessels, small commercial vessels and pleasure vessels containing:
Unlicensed imported medicines (see below image).
- Medical devices that do not carry a CE mark.
- The medical kits found were of Chinese origin.
Although not found in this incident, seasickness pills (which are stored within the liferaft but separate to the medical kit, and in the case of pleasure vessels can be supplied in isolation) must still be authorised for supply in the UK and handled in accordance with UK medicines legislative requirements.
Members of the public should note that these medicines are only likely to be made available and consumed in the rare event of a liferaft being deployed in an emergency. Anyone who has taken medicines originating from an inflatable liferaft and who is concerned about what they have taken should contact their healthcare professional for advice.
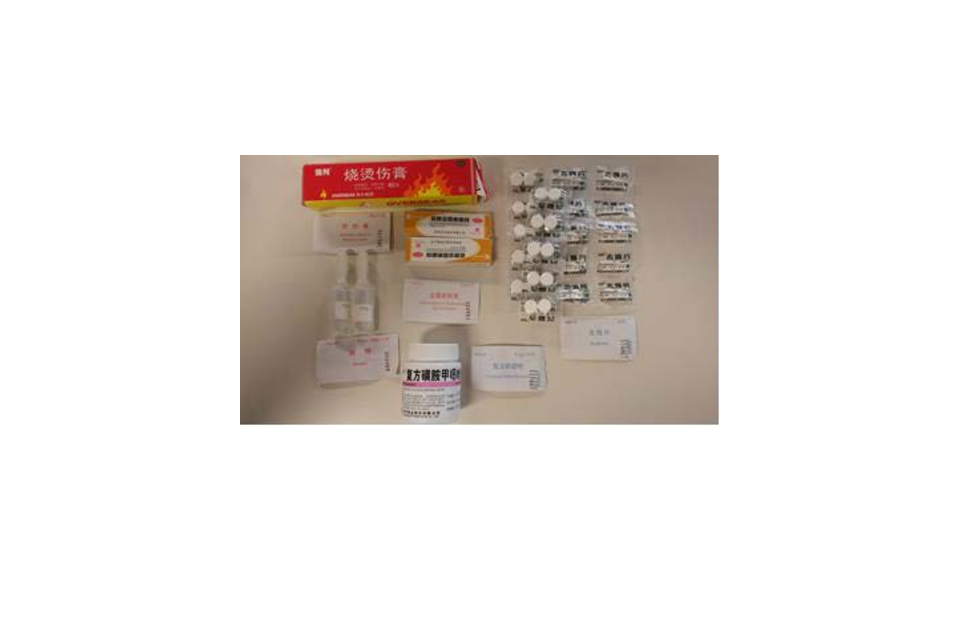
Fig 1: Contents of a non-compliant medical kit found by MHRA inspectors
Summary of issue
In the medical kit shown in Fig 1, the medicines have not been authorised for supply in the UK. Only a brief English description of each medicine is included which does not give any details of their contents or contraindications. In the example, the painkiller medicine tested was found to contain a controlled substance. Other substances in the painkiller were also found to present a risk to human health. The medical kit also contained medical devices that do not carry a CE mark. Work is under way to determine the potential scope of the issue but only medicines imported into the UK in liferafts are of immediate concern at this time. This includes seasickness pills.
It has also emerged that some UK-based suppliers of inflatable liferafts to the commercial and pleasure sectors may be unwittingly operating without MHRA or Home Office authorisation to import and/or supply the medicines contained within the liferaft.
In addition, it is believed that some unauthorised suppliers for medical kits containing prescription-only, pharmacy-only and unlicensed medicines are advertising their kits on the internet.
Actions to take
In order to determine the extent of the public health risk, there is an urgent requirement to identify any unlicensed medicines and medical devices without a CE Mark, within inflatable liferafts supplied in the UK.
Liferaft Suppliers and Distributors
If you supply, hire or service liferafts imported into the UK:
- Any medical kits contained in inflatable liferafts you handle must only contain authorised medicine and CE marked medical devices for supply in the UK.
- You must ensure that all your handling of medicines is conducted in accordance with UK medicines legislative requirements.
- Suppliers who intend to supply medical kits containing medicines by way of wholesale within the UK market (whether or not within an inflatable liferaft) must obtain a Wholesale Distribution Authorisation from MHRA. This includes chandleries and any marine company servicing or supplying liferafts carrying medicines in medical kits.
- If you are a supplier of imported liferafts, you must open at least one liferaft (in accordance with manufacturer’s instructions) from each non-UK liferaft supplier you deal with. Upon opening the liferaft, you must check that the medical kit only contains medicine and medical devices authorised for the UK. Equally, you must ensure that medicines supplied separately, are also authorised for sale in the UK.
- If you find a non-compliant kit, you must contact any customers affected to arrange for their medical kit to be replaced with a medical kit authorised for use in the UK.
- Repacking of opened liferafts must be done by a manufacturer-approved liferaft station.
- If you are supplying inflatable liferafts for other EEA countries, the contents of the medical kit should be authorised for that country.
Advice for mariners and vessel-owners
- If you are under any doubt as to the compliance of the medical kits or medicines within your liferaft, contact the liferaft supplier or service station that serviced, sold or rented the liferaft to you for advice.
- If you have any immediate concerns about the medicines or devices in your liferaft medical kit, please contact the MHRA.
- Continue to be vigilant for unauthorised imported medical kits when you are buying or replacing liferafts for your use or use by people on your vessel.
Legal requirements
Before a medicine can be marketed or sold in the UK, a number of licences are required. The product itself must have a licence called a ‘marketing authorisation’ (formerly a ‘product licence’) unless an exemption applies. This is printed on the packaging of the medicine together with the name of the manufacturer. In addition, the companies that are involved in any stages of the manufacture, import or distribution of the product need to have the relevant licence for the activity in question (Manufacturer’s and/or Wholesale Dealer’s Licences).
Possessing and supplying a controlled substance requires authorisation from the UK Home Office.
Import or supply without appropriate licence or authorisation is a breach of UK medicines regulations and a criminal offence.
It is against the law to advertise prescription-only medicines direct to the public. Any nonauthorised person selling a medical kit directly to a member of the public must ensure it only contains general sales list (GSL) medicines.
Online sellers of medicines to the public must display the EU common logo issued by MHRA and comply with UK medicines legislation. More information on registering for a distance selling logo can be found on gov.uk.

CE mark logo
A CE mark is a logo that is placed on medical devices. A medical device cannot be marketed without carrying a CE mark of conformity. A CE mark is applied by the manufacturer and means that the device meets the relevant legal requirements and, when used as intended, works properly and is acceptably safe. If a medical device has been sold in the UK and does not display a CE mark, the safety and quality of that device cannot be assured and it may be hazardous to the end user.
For certain UK commercial vessels, inflatable liferafts their medical kit and other contents must be approved. Inflatable liferafts for pleasure vessels need not contain medicines but those that do must comply with MHRA regulations.
Medical kits contained in inflatable liferafts supplied to another EEA State must comply with the national regulations for that State.
Further information
MHRA press release on unauthorised medical kits in liferafts
For information about the regulation of medicines in the UK: Medicines & Healthcare Products Regulatory Agency website
MHRA,
151 Buckingham Palace Rd,
London SW1W 9SZ
Tel: +44 (0)20 3080 6000
To apply for a MHRA licence: Apply for manufacturer or wholesaler of medicines licences
Register of authorised online sellers of medicines
For information on GSL medicines: NHS website
For information about the regulation and standards of liferafts, and medical kits required in liferafts:
Marine Technology Branch,
Maritime & Coastguard Agency
105 Commercial Road
Southampton
SO15 1EG
Tel: +44 (0)2380 329100 Email: marine.technology@mcga.gov.uk
For further guidance on the distribution of medicines in the marine sector: MGN 524.
