Genomics and personalised medicine: how partnership with the UK can transform healthcare
Published 9 March 2016
1. Introduction
The 100,000 Genomes Project, led by Genomics England, is sequencing the genomes of 100,000 NHS patients and combining this with NHS data.
This ground breaking work highlights the world leading position the UK holds in genomics. We are already at an advanced stage of a systematic long-term plan for integrating genomic and personalised medicine into the day-to-day delivery of healthcare.
The National Health Service (NHS) will be the world’s first healthcare system to launch a genomics medicine service.
International interest in the UK’s approach to genomics and personalised medicine is growing. There is huge potential for this expertise to be shared with governments, healthcare providers and commercial companies.
This prospectus explains what genomics and personalised medicine are, how they can be applied, and why the UK is at the forefront of this field.
The UK is investing heavily to set up the necessary infrastructure and levels of service integration to deliver population-wide benefits from genomics.
We are in the process of creating an unparalleled end-to-end service, integrating every step of the genomic pathway to maximise patient benefit.
You can draw on this knowledge and experience to invest in the facilities and services needed to optimise these benefits.
Genomics is complex field, but finding suitable commercial partners in the UK need not be. The simplest way to access this expertise is through Healthcare UK, UK government’s specialists in international healthcare partnership working.
2. Genomics - transforming medicine in the 21st century
Genomics, the study of an organism’s complete set of genetic instructions, is revolutionising medicine.
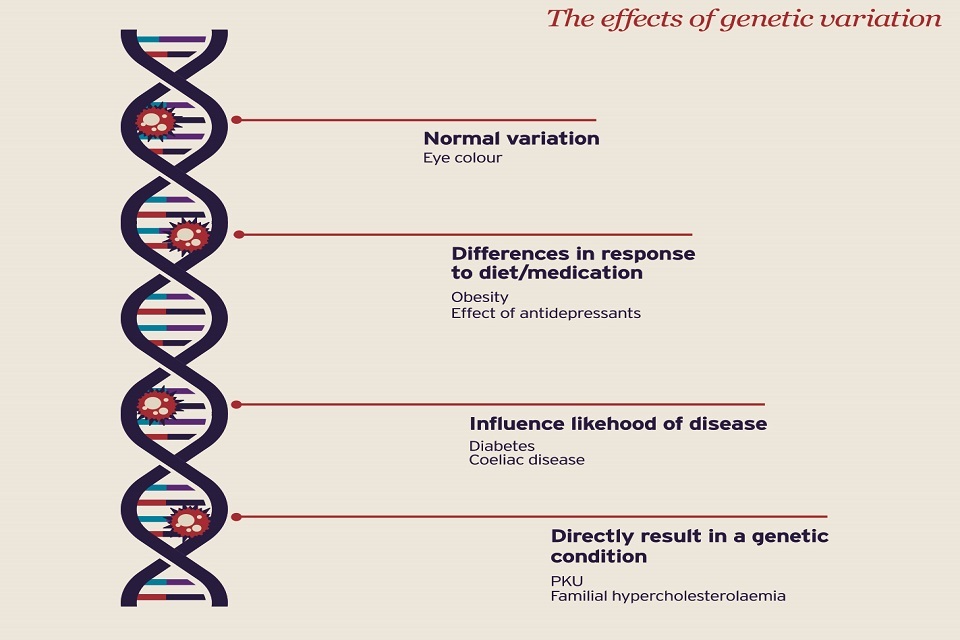
Diagram of the DNA helix showing the different effects genetic variations have
The ability to sequence the genetic code of a large sample of the population is revealing how small variations in our DNA can change our vulnerability to different diseases, and thus how medicine can be personalised for better prevention, diagnosis and treatment.
As the microscope and x-rays revolutionised medicine in the 19th and 20th centuries, so knowledge of the human genome will dramatically change medicine in the 21st century.
Sir Bruce Keogh Medical Director NHS England
3. What are genomics and personalised medicine?
Genomics has transformed our understanding of disease and our ability to deliver care in a way that is specific and personal to each individual patient.
By establishing the sequence of an individual’s genetic material it is possible to identify sequences or mutations which are specific to that person. Not only can these sequences identify the cause or stage of a disease, or the risk of future disease, they can also help us to predict the likely benefits or side effects in response to a particular medication.
Genomics therefore opens up the shift towards personalised treatment: medicines and other treatments can be prescribed not just for their general effect on a disease, but for the way they interact with a specific patient according to their genetic makeup.
This is a huge step forward as it also allows us to examine the underlying causes of ill-health, letting us tackle conditions before they have even started, rather than just identifying and managing patients once ill-health has taken hold.
This is particularly important as health systems start to tackle the rising burden of non-communicable diseases. These are conditions with strong links to lifestyle such as diabetes, cancer and cardiovascular disease.
Pharmaceutical companies recognise that currently their major treatments may only be effective in 30 to 60% of the population for these conditions.
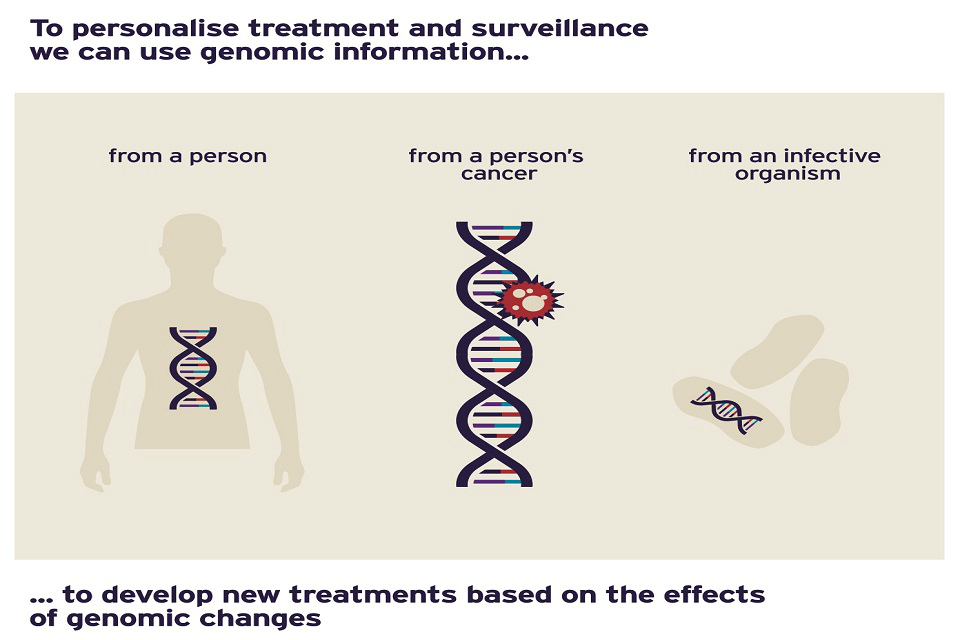
Genomic information can be used to personalise treatment based on the effects of genomic changes
4. The medical revolution
4.1 Neonatal diabetes
Genomic diagnosis is shaping precision management:
- mutations in over 25 different genes cause neonatal diabetes
- new genomic technology has found 5 new genetic subtypes which inform therapy options
| Mutation | Clinical outcome | Optional therapeutic strategy |
|---|---|---|
| KCNJ11 p.V59M | Permanent diabetes and development delay | Sulphonylurea therapy |
| EIF2AK3 p.E371* | Wolcott Rallinson Syndrome | Liver transplant |
| FOXP3 c.227deIT | IPEX syndrome | Bone marrow transplant |
| GATA6 c.1448-1455del | Syndromic pancreatic agenesis | Insulin and exocrine supplements |
| STAT3 p.T716M | Multi-organ autoimmune disease | STAT3 inhibitor |
4.2 Advanced melanoma
Melanoma is a common and often fatal skin cancer where the cancer cells have frequent genetic mutations.
Approximately 40 to 60% of cutaneous melanomas carry mutations which activate a gene called BRAF which then leads to further tumour growth.
The vast majority (80 to 90%) of these BRAF-activating mutations are the result of a small change in one tiny section of DNA.
The biological therapy drug vermurafenib interferes with the function of the BRAF gene and is therefore used for those patients with the relevant mutation where BRAF has been activated.
4.3 Safe use of azathioprine
Azathioprine is used to treat many diseases in rheumatology and dermatology. The drug is converted to its active metabolites by several enzymes, the most critical of which is thiopurine methyltransferase (TPMT).
Low levels of TPMT activity results in the overproduction of azathioprine metabolites that are toxic to bone marrow, leading to bone marrow suppression and potentially life-threatening side effects.
TPMT activity is affected by variations of the genetic sequence of the TPMT gene.
Individuals carrying certain mutations may therefore be at higher risk of adverse side effects from azathioprine treatment.
Genotyping of the TPMT gene allows the patients at higher risk of azathioprine drug-related toxicity to be identified and given an appropriate dose
5. Why partner with the UK?
Working with the UK enables a partner country to deliver the latest medical advances to its citizens by linking directly to state-of-the art advances in the fastest moving area of medical science.
There has never been a better time to develop genomics and personalised medicine services in partnership with the UK.
We have already established the infrastructure and facilities that form the foundation for these services. With a clear strategy in place to increase the use of genomic information in day-to-day clinical medicine, a stream of new programmes will be introduced in the UK over the coming decade.
Few countries are so well prepared and many risk falling behind.
You can draw on the wealth of experience that has taken us to this advanced stage and reap the rewards from the transfer of ground-breaking procedures as they are developed. There are major advantages for countries who undertake this transition in partnership with the UK.
It is extremely complex to create and develop the systems, processes and infrastructure for testing, analysing, interpreting and handling the information within the human genome.
Here are just some of the reasons why international healthcare organisations are looking to the UK to take forward the next stage of their genomics programmes.
5.1 The UK – home of life science discoveries
The UK has a rich heritage of life science discovery that has transformed scientific knowledge and continues to unlock clinical and commercial opportunities.
Many of the major scientific discoveries relating to genomics have been made in the UK, from the identification of the structure of DNA by Watson and Crick to the sequencing of the human genome at the Sanger Centre in Cambridge.
We continue this tradition through some of the best universities and research institutions in the world.
5.2 The National Health Service
The NHS is the world’s largest integrated health system. It has earned an international reputation for excellence for meeting the complex healthcare demands of a diverse population.
The NHS:
- treats 1 million patients every 36 hours
- delivers 300 million family doctor consultations per year
A rich mix of state-owned and private organisations provide care free at the point of need in a culture that puts patients first. Our international partners benefit from working with a genuinely world-class system.
The US based Commonwealth Fund found the NHS to be the best healthcare system studied in 11 advanced economies, including Switzerland, Germany, Sweden, the Netherlands and Australia.
5.3 A strategy for success
Translating genomic breakthroughs into direct benefit to patients requires a change in clinical care pathways. This creates the need for extensive training of clinical staff and the development of new systems and arrangements for care, as well as new diagnostics, sophisticated analytics and information technology.
Partnership with the UK can help international healthcare providers plan effectively for these changes. We have a clear strategy for using our existing infrastructure to develop and introduce genomics and personalised medicine into every day clinical care on a national scale.
5.4 Building on firm foundations
The infrastructure for our ambitious long-term development programme is in place.
The NHS has been running a network of genetics services for many years, where our major hospitals work closely with universities and research institutions. These networks have joined together within regions to form Genomic Medical Centres, collaborating to take genomics and personalised medicine to the next stage and support the 100,000 Genomes Project.
5.5 The 100,000 genomes project
This landmark project, led by Genomics England Ltd, will generate a step-change in our understanding of the link between genetic mutations and rare inherited conditions, cancer and infectious diseases.
The project will sequence 100,000 genomes from around 70,000 NHS patients with these conditions. The combination of genomic sequence data with medical records is creating a ground-breaking resource that will translate directly into better patient care in both the UK and our partner countries.
5.6 The benefits of scale
Population size is an important factor when it comes to gathering the breadth of data needed to build genomic knowledge and experience.
Although so-called ‘rare disease’ is surprisingly common. More than 6,000 individual conditions have already been identified. Each condition may only affect a handful of people in a small population.
Only a health service serving a population of many tens of millions will see a large enough group of similar patients to enable advice on targeted treatment. Partnering with the UK brings these benefits of scale. Your patients will benefit from the combined knowledge of a genomics programme that will be applied on a national basis.
5.7 Growth through innovation
Many key technologies have been developed in the UK. The scale of the 100,000 Genomes Project has created an environment for greater innovation and, in particular, had required linking of different technologies.
Some of the latest technologies in use in the UK are:
- DNA sequencing by synthesis technology (SBS) the newest generation of instruments which can generate over 1 terabase (Tb) of data per run.
- direct, electronic analysis of single molecules using nanopore science
- software for accurate, rapid and scalable interpretation of the entire DNA sequence of an individual.
5.8 Collaboration works…
We can increase the speed at which we use our growing understanding of the complex links between our genetic code and disease through collaboration between genomic centres across the world.
These partnerships can bring together information from different populations to create new medical knowledge that benefits everyone.
5.9 ….. and the UK is good at it
UK organisations have a long tradition of working in partnership to build healthcare capacity and capability across the globe.
Many countries continue to use the UK model of medical education, for example.
A large number of doctors from other countries either trained in the UK or accessed training and examination in their own countries through our Medical Royal Colleges. This enables patients around the world to benefit from UK standards of medical care.
Many universities and a growing number of hospitals have already formed international partnerships.
6. Building a service you can trust
Our genetic information is highly personal and potentially very powerful. It offers insights into our past, future and the future of our children and descendants. It is also complex to interpret, and has the potential for misuse or to cause unnecessary alarm if poorly managed.
Governments and healthcare organisations overseas that are developing a genomics programme will need to consider the sort of steps the UK is taking to address the ethical and security issues, as well as focusing on the medical benefits. These include:
- ensuring the supply of skilled professionals to interpret the information and recommend appropriate action
- training doctors to keep them up to speed with this rapidly evolving field and working to the highest ethical standards
- developing the necessary processes and protocols so that people have complete trust in all those with access to their genetic information
- building robust IT systems and processes that professionals and the public have total confidence in
- establishing tight regulation and close monitoring of the services by independent experts
7. Creating a genomics service
Introducing genomic and personalised medicine is a complex operation. Many activities and processes need to be integrated and this requires multiple organisations to work seamlessly together. The rigour and regulation of the testing procedure is vital, of course, but from a clinical perspective the ultimate focus for a new programme should be on improved care for patients.
The following section sets out the essential elements involved in creating a genomics and personalised medicine programme. The UK’s current and future approach is also described alongside each stage. To understand the UK’s approach, however, you need first to understand the role of a network of centres that lies at the heart of our programme: Genomic Medicine Centres.
7.1 Genomic Medicine Centres
The Government has created a national network of integrated Genomic Medicine Centres (GMCs), which have been built around the specialist care of rare diseases and cancer.
They work closely with Genomics England, and Health Education England.
GMCs in England
- North East and North Cumbria NHS GMC
- Yorkshire and Humber NHS GMC
- East of England NHS GMC
- North Thames NHS GMC
- West London NHS GMC
- South London NHS GMC
- Wessex NHS GMC
- South West NHS GMC
- West of England NHS GMC
- Oxford NHS GMC
- West Midlands NHS GMC
- North West Coast NHS GMC
- Greater Manchester NHS GMC
The GMCs bring together the existing NHS network of genetics services which include more than 200 consultant geneticists as well as existing laboratory services. The GMCs work with existing specialist genetic testing, across multiple hospitals to see patients from across the country.They work closely with counsellors who provide explanation and support to patients and groups of specialists with specific areas of expertise, including onco-genetics and cardiac genetics.
The GMCs include cancer centres which have the full range of diagnostic and specialist services and are at the hub of regional cancer networks.
Crucial to their success, the GMCs work closely with academic and industry partners in each English region and are a focus for innovation and research.
Their first priorities are to identify and enrol patients suitable for the 100,000 Genomes Project and drive forward the national training agenda.
GMCs typically serve a population of up to 5 million and provide the regional focus for a growing range of local services in every part of the country. Similar arrangements are under development for Scotland, Wales and Northern Ireland.
8. Steps to creating a genomics service and the UK’s approach
8.1 1 Developing new diagnostics, analytics, information and health records
1.1 Set up laboratory testing for genetic abnormalities or disease risk including sample handling and storage, genotyping, use of arrays, whole genome sequencing, bio banks etc.
UK approach: The NHS genetics services, academic research institutions and laboratories are well established and now networked regionally within GMCs.To access specialist facilities and services of an appropriate scale, GMCs link to the National Biosample Centre (which provides sample storage) and to Genomics England. This provides whole genome sequencing and analytics on a national scale and co-ordinates the linked research and management of new discoveries. Accreditation and quality assurance of organisations in the supply chain ensures that rigorous handling and laboratory processes are in place to produce reliable and informative genome sequences that serve as a basis for future treatment.
1.2 Develop cancer genetic molecular diagnostics.
UK approach: Within each GMC, the designated cancer centre is developing molecular diagnostic laboratories for cancer tissue testing and sub-characterisation of tumours on the basis of genomics. This is opening the way for new approaches to treatment by oncologists and cancer surgeons.
1.3 Develop analytics, bioinformatics and interpretation of genomic and clinical information.
UK approach: Sequencing the whole genome from patients rapidly produces terabytes of genetic sequence data which needs to be compared between groups of people with and without disease. The national drive to develop systems to do this, and to train bioinformatics staff and analysts, is carried out by Genomics England, a group of universities and specialist commercial partners.
1.4 Develop information technology to support systems and processes such as electronic patient records, bioinformatics, analytics, data handing and security.
UK approach: The NHS is investing in the information technology needed to link genomics data with day–to-day clinical care. There is a strong focus on robust data handling and data security - confidentiality of patient data is a cornerstone of the UK approach.
8.2 2 Developing new clinical pathways
2.1 Providing clinical care of patients across the care pathway from pre-testing through to careful treatment and management strategies for individuals and their families.
UK approach: For very rare diseases, the NHS is setting up a growing number of national networks. These consist of two or three experts to whom all the relevant patients are referred, or who coordinate the care delivered by local trained staff.
2.2 Ensuring patients feel informed through advice, counselling, and psychological support.
UK approach: For more common diseases with a genetic basis (such as cystic fibrosis), there are regional experts in organisations linked to each of the GMCs who take on this leadership role and run dedicated clinics.
2.3 Care for patients with or at high risk of disease, or lifelong actual or remote care, with necessary consultations and procedures undertaken as appropriate.
UK approach: The pathways of care for a growing number of such disorders are supported by guidance from the National Institute for Health and Care Excellence (NICE), Royal Colleges and specialist professional organisations.
2.4 Access to oncology clinical care for patients with cancer, based on genomic data and utilising personalised medicine.
UK approach: Cancer centres and their associated cancer networks link into GMCs, allowing oncologists, surgeons, physicians radiologists and the pathologists to review the precise genetic composition of tumours.
2.5 Set up systems to enable genotype-informed safe prescribing.
UK approach: Clinicians are able to design the appropriate personalised treatment for each individual patient. This enables rapid translation of new discoveries into patient benefit in every cancer service in the country.
2.6 Reproductive services made available, including prenatal genetic diagnosis and embryo selection in clinics which are rigorously regulated and inspected with transparent outcome data.
UK approach: Each GMC has a fertility service able to offer pre-gestational testing and in-vitro fertilisation (IVF) with embryo selection. This enables couples who carry the gene for a serious inherited disease to choose whether to prevent the mutation being transmitted to their children. It is vital that all fertility services offer the best outcomes. The services are audited and accredited by the Human Fertilisation and Embryology Authority, a national regulatory organisation.
8.3 3. Developing research and development capability
3.1 Opportunities for all patients to participate in and gain benefit from clinical trials.
UK approach: Within each GMC there are hospitals that are active in clinical trials, including some with dedicated facilities and specially trained staff. The NHS recognises that patients benefit from participation in trials and aims to maximise the number of people offered the opportunity to participate in them. This policy is a key component in ensuring rapid translation to clinical benefit.
3.2 Set up research and development partnerships in pharmacogenomics and development of personalised medicine.
UK approach: Each GMC is linked into a number of universities. These are engaged in fundamental genomics research, developments to improve bioinformatics and analytics and the creation of new approaches to testing, monitoring and drug development. Many of these university departments already work in partnership with industry and pharmaceutical companies.
8.4 4. Setting up a comprehensive education and training programme
4.1 Creating a programme to educate and train doctors and other healthcare professionals in regulated and quality assured courses, including in-service training, plus wider education and public engagement.
UK approach: Training is an integral part of the UK’s long term plan to advance the use of genomic information into mainstream clinical medicine. The Health Education England Genomics Education Programme comprises a range of qualifications, courses and resources for doctors and healthcare workers. Post-graduate courses have also been commissioned, delivered by universities linked to the GMCs. There is also a wider programme to educate the public about genomics and its implications, including through the use of social media.
8.5 5. Strong links to commercial organisations
5.1 Develop links with pharmaceutical companies to provide early access to new, precision medication.
UK approach: The NHS has strong links with the pharmaceutical industry. Partnerships are focused on the rapid development of new precision medicines which will prevent or treat disease as early as possible. This is in keeping with the NHS’s aim to use resources for keeping people healthy rather than treating them for advanced disease.
5.2 Creation of a wealth-generating, growing medical technology sector producing devices, assays, reagents and early stage medicines.
UK approach: The UK’s objective to increase the number of spin-out and wealth-generating medical technology companies is encouraging new innovations that international partners can benefit from. Innovation adoption is stimulated by joint working between local partners that include GMCs, universities, science parks, hospitals and primary care healthcare facilities. The Precision Medicine Catapult helps companies convert prototypes to large scale commercial production.
5.3 Engagement with a range of other businesses able to support the necessary infrastructure.
UK approach: There is growing expertise among the providers of commercial services in the UK that are essential to the development of the genomics programme infrastructure. We have world-leading legal practices with expertise in patents and intellectual property, skilled management consultancies and firms with relevant facility design expertise. London, the world’s leading financial centre, provides access to investors and sources of capital. Confidence in the growth of genomics in the UK is strong, and the sector and welcomes foreign direct investment.
9. UK capabilities
Overseas governments and healthcare providers can partner with UK providers for all of the stages involved in developing a genomics and personalised medicine programme.
The type and level of support available from UK providers is truly flexible in nature. It could take the form of a consortium of UK providers with the combined expertise to build a country’s capacity and capability to set up an entire programme.
Alternatively, you may want just to access UK expertise for a specific element of your programme.
The components of the broad package of support available to you include:
9.1 Capacity and capability building
There is the expertise in the UK to support any requirement for new or improved infrastructure, facilities and processes.
Examples include:
- strategy development, service design and business planning
- setting up and training of clinical genetics teams, including counsellors
- creation and management of facilities for sample collection, storing and testing
- creation of modern molecular diagnostics laboratory for cancer, combined with staff training, high standards of governance and quality assurance
- development of the analytics capability to interpret the data, with training of bioinformatics experts and analysts
- support in creating an IT infrastructure. Options include:
- creating joint training programmes,
- opportunities to partner in design, development and procurement,
- links with local IT suppliers and related UK commercial organisations
9.2 Education and training
There are opportunities to partner with the extensive education and training programmes developed by UK universities for undergraduates, postgraduate scientists and doctors.
All the courses are quality controlled and meet standards determined by Health Education England. Partnership could involve educating and training staff in the UK, or support for developing your own programmes.
9.3 Research and development
Creating clinical trial units that link to UK centres gives local patients access to the latest international clinical trials. The patients will benefit and the partner country will develop its capability in new areas of medical research.
9.4 Ongoing support
UK organisations can become long-term partners to support the continuous improvement of genomic and personalise medicine services.
Examples include:
- training and accreditation of medical staff in interpreting test results and help with developing appropriate clinical pathways
- support from the UK network of experts for each of the 6000 plus inherited diseases
- support and training from the network of specialists in the UK cancer centres, including access to tumour-specific expertise that can personalise medicine in response to an individual’s diagnostic information
- training and system development for genotype-informed safe prescribing
- partnering in the creation of fertility services which meet best international standards, are accredited by the UK and provide access to the latest pre-gestational diagnostic testing and embryo selection.
9.5 Access to commercial organisations
There are exciting opportunities to build links with pharmaceutical companies, including those doing genomic studies as part of their programme of new drug development.
Partnership opportunities also exist with UK medical technology companies that develop products for testing, patient monitoring or precision medicines.
You can also access expertise in every aspect of the services that support the development of critical infrastructure, including specialist legal firms, management consultancies, companies with expertise in facility development and investors and financial institutions who support the development of new business.
10. Partnership approaches
We are now using personalised medicine in clinical practice and are accelerating to the stage where this approach benefits all patients. International partners to the UK’s genomics programme can anticipate a decade of rapid and ground-breaking progress.
A stream of linked but discrete development programmes is in the pipeline and the know-how and skills for these different elements will be transferred to our partners as they are developed.
Within the core principles of partnership and capability building, overseas governments and healthcare organisations can engage with UK expertise at different levels.
Three possible models include:
10.1 The Genomic Medicine Centre
In this model, the NHS would replicate the UK’s approach by creating a Genomic Medicine Centre in the partner’s country.
This centre would undertake all the activities and services present in the UK centres. This in-country GMC would be a focus for training, with local experts, clinicians and patients all having access to the clinical expertise available in the UK.
This centre could also act as the focus for clinical trials, research and development and local academic links.
In this 2 way relationship, technical and service developments in the UK would be transferred to the overseas GMC and vice versa. The in-country GMC therefore benefits from a depth of resource that is way beyond that available to any individual organisation.
The creation of an international network of sister centres linked to those in the UK will provide access to a deep pool of research and development and commercial expertise. Innovation sharing will be the fastest route to bring new clinical benefits to populations in different countries.
10.2 Institutional partnering
Where a country has already established a genomics organisation and the main elements of the programme are in place, partnership with a UK organisation can be the best way to access expertise in particular areas.
This relationship can act as a conduit for links with other centres and a route to tap into new developments as they happen across the wider UK network.
10.3 Individual service-specific support
Where services are already well developed, a government, hospital or commercial organisation may still need help in a specific area. With so many individual organisations contributing to the UK’s thriving national programme, the exact type of expertise required will be available.
A close partnership with one UK organisation focussing on a particular element may be the most suitable arrangement.
11. Making partnership happen
There is a powerful argument for creating a linked network of genomic medicine centres around the world so that patients, wherever they are, benefit from the latest personalised therapies.
The UK is at the forefront of this field and is therefore the logical choice as a partner to other countries seeking to accelerate their own genomics programmes. We expect to make huge progress in the coming years, aided by the commitment from the UK Government to maintain the UK’s status as a world leader in ground-breaking research into cancer and rare diseases.
Healthcare UK is your route to access the expertise within the UK’s genomics sector.
Whatever stage you are at in developing a programme for genomics and personalised medicine, we can bring together the right organisations to meet your needs.
This brochure is one of a set of 7 that explains the benefits you gain by partnering with UK healthcare organisations. The full set comprises:
- education and training
- healthcare infrastructure services
- digital health
- clinical services
- health systems development
- genomics and personalised medicine
- improving the quality and safety of patient care
To find out how you can draw on UK expertise to extend, improve and transform healthcare provision in your country email Healthcare UK
12. Healthcare UK
The UK is internationally renowned for delivering excellent healthcare. Our National Health Service (NHS) is the world’s largest integrated health system. It has provided high-quality services for nearly 70 years, supported by academia and innovative commercial healthcare companies. This partnership creates breadth and depth of expertise that no other country can match.
Healthcare UK is your route to access this expertise. Whatever type of health facility, service or training programme you are planning, we can bring together the right UK organisations to meet your needs.
Our position in government gives us an excellent platform to support and promote international collaborations, working with the Department for International Trade’s (DIT) international network of offices in 107 markets around the world. As a joint initiative between the UK government’s Department of Health (DH), the NHS and DIT we connect UK expertise to business opportunities, drawing on our broad network across the NHS, the private sector and academia.
13. Disclaimer
Whereas every effort has been made to ensure that the information in this document is accurate, neither DIT, nor the Department of Health accept liability for any errors, omissions or misleading statements, and no warranty is given or responsibility accepted as to the standing of any individual, firm, company or other organisation mentioned.
© Crown copyright 2016
You may re-use this information free of charge in any format or medium, strictly in accordance with the terms of the Open Government Licence.
For further details, view the licence or e-mail us.
Where we have identified any third party copyright information in the material that you wish to use, you will need to obtain permission from the copyright holder(s) concerned.
Any enquiries regarding this material should be emailed to us or telephone +44 (0)20 7215 5000.
Published February 2016 by UK Trade and Investment
This was published originally by UK Trade and Investment which has since moved to the Department for International Trade (DIT).
UKTI/2015/92
