Guidance for training and continuous professional development under the Animals (Scientific Procedures) Act 1986 (accessible)
Published 14 November 2024
December 2023
Summary
1. This guidance was laid before Parliament on 12 July 2023 and came into force on 24 October 2023. This version of the guidance, published on 20 December 2023, incorporates various minor amendments to increase the clarity of the guidance for users.
What this guidance covers
2. This guidance sets the governance framework for training and continuous professional development under the Animals (Scientific Procedures) Act 1986 (ASPA), the specific training requirements for licensing under ASPA, and the learning outcomes of the training required.
What this guidance replaces
3. This guidance replaces section 9 of the “Guidance on the operation of the Animals (Scientific Procedures) Act 1986”, published in March 2014. All previous references to section 9 in the 2014 guidance should be read as references to the appropriate section in this guidance.
Who this guidance is for
4. This guidance is for accreditation bodies, training providers, establishments and individuals conducting work, or seeking to conduct work, regulated by ASPA.
Introduction
5. The Animals (Scientific Procedures) Act 1986 (ASPA) requires that persons with certain responsibilities relating to the use of animals in science have appropriate knowledge and training. This is a requirement for each of the licences under ASPA: establishments; projects; and people (Sections 2C, 5C and 4, respectively).
6. This guidance seeks to maintain and continuously review high standards of training and Continuing Professional Development (CPD) that ensure individuals using animals in science have the necessary skills and competence to do so, in line with ASPA.
7. The system for training and continuous professional development aims to:
i. enable quality science;
ii. promote protections for animals; and
iii. be open and transparent.
8. To do so, the system must:
i. meet the legislative requirements and provide demonstrable assurances of compliance to the relevant Regulators[footnote 1];
ii. deliver standardised, continuously reviewed and updated high quality training; and,
iii. assure competence and continuous professional development.
9. This guidance sets out:
i. the roles and responsibilities of all parties (as shown in the diagram below);
ii. common agreed standards of accreditation;
iii. how the Home Office and Department of Health, Northern Ireland (DoHNI) will assure delivery against the framework; and
iv. the training requirement to meet the requirements of ASPA for licensing purposes.
10. This guidance applies to all mandatory training, with the exception of the system for approval of the Named Veterinary Surgeon (NVS) module by the Royal College of Veterinary Surgeons, which is not within scope of this document.
11. This guidance applies to all of the UK. Its implementation in England, Scotland and Wales is the responsibility of the Home Office; and implementation in Northern Ireland is the responsibility of the DoHNI.
Legal basis
12. This guidance document has been produced conforming to the procedure set out in Section 21 of ASPA. It replaces Section 9 of the Guidance on the Operation of ASPA (2014).
13. An applicant for a personal licence must demonstrate that they:
i. have “appropriate education and training for the purpose of applying the regulated procedures that the licence would qualify the person to apply”; and
ii. are “competent to apply those procedures in accordance with the conditions which are to be included in the licence and to handle and take care of laboratory animals” (ASPA, Section 4).
14. An applicant for a project licence must demonstrate that they have:
i. “received instruction in a scientific discipline relevant to the programme of work to be specified in the licence”;
ii. “specific knowledge relating to the species of animal that is to be subjected to regulated procedures as part of that programme of work”; and
iii. “appropriate education and training for the purpose of designing programmes of work involving the application of regulated procedures” (ASPA, Section 5C).
15. An applicant for an establishment licence must demonstrate the suitability of persons at that establishment responsible for the welfare, care and treatment of animals (ASPA, Section 2C).
Governance Framework
Roles and Responsibilities
16. The framework for training and continuous professional development consists of five key parties. They are the:
i. Government (Home Office and DoHNI);
ii. Accreditation bodies;
iii. Training providers;
iv. Establishments; and
v. Individuals conducting work, or seeking to conduct work, regulated by ASPA.
17. Figure 1 illustrates the relationships and key responsibilities of each party in the governance framework.
Figure 1
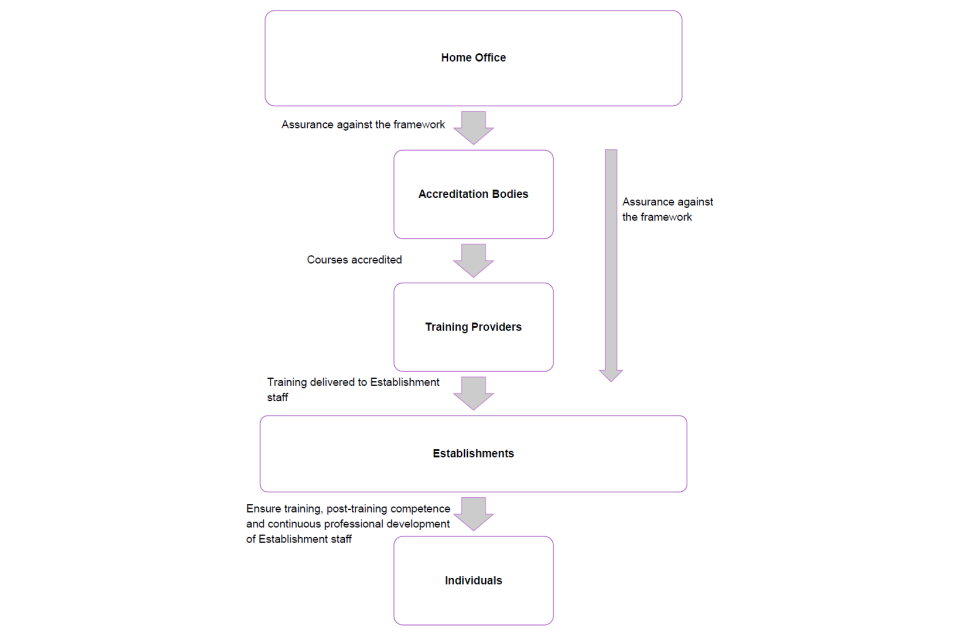
The Home Office provides assurance against the framework for accreditation bodies, training providers and establishments. Accreditation bodies accredit courses for training providers. Training providers deliver training to establishment staff. Establishments ensure training, post-training competence and continuous professional development of individuals.
Home Office and DoHNI
18. The Home Office is formally accountable to Parliament for delivery of ASPA in Great Britain.
19. Under ASPA, the Home Office and DoHNI are responsible for:
i. the policies and governance framework for training and CPD under ASPA;
ii. setting the training requirement for the issuance and maintenance of licences under ASPA;
iii. assurance of accreditation body and establishment delivery under this framework; and
iv. operational decisions to grant licences, where appropriate, based on evidence of relevant accredited training and consideration of exemptions.
20. The Home Office will regularly engage with stakeholders on strategic policy and operational issues relating to training and, along with the DoHNI, will regularly review this framework with input from stakeholders.
21. The Home Office will conduct regular assurance reviews of accreditation bodies to assure the standards set out in this framework are met. The Home Office may withdraw acceptance of courses as evidence of training received, in the event of substantial and/or persistent failure to meet the standards of this framework.
22. The Home Office Regulator and DoHNI inspectors, as part of their audit and inspection of establishments and licensees, will review training records, CPD records and the roles of the establishment licence holder and Named Training and Competency Officer (NTCO) in ensuring appropriate training, competence and continuing professional development.
Accreditation Bodies
23. Accreditation bodies are responsible for the quality assurance of training in the UK.
Accreditation principles
24. Accreditation bodies will abide by the following principles:
i. independence from the training providers;
ii. provide a proportionate and affordable service;
iii. work to sustainable and consistent standards across the accrediting bodies;
iv. work with openness and transparency;
v. hold the confidence of trainees, trainers, and the Home Office; and
vi. provide suitably experienced assessors.
Accreditation of training courses
25. Accreditation bodies will accredit courses from training providers against whether the training delivered meets all the relevant Learning Outcomes (Annex A). The period of accreditation may be for up to 5 years. Any significant changes to courses after accreditation should be reviewed and approved by the accreditation body.
26. Accreditation bodies will agree consistent standard criteria across equivalent training courses, to include assessment methods, numbers and types of questions, content and pass mark.
27. Before accrediting a training course, accreditation bodies will assess:
i. the training provider organisation and personnel as capable and suitably qualified;
ii. the content of the course as meeting the relevant learning outcomes and proactively promoting the latest scientific developments, new methods, and 3Rs developments;
iii. the quality of the training facilities, including online where relevant, and suitability of teacher-student ratio; and
iv. the measures in place to ensure the integrity of the course and to combat fraud.
28. In making these assessments, accreditation bodies will use evidence from:
i. written submissions from the training provider by way of an application form; and
ii. in-person and/or online visit and associated report of an assessor with detailed observations of aspects including the course materials and course venue.
29. Accreditation bodies will provide certification in a format that is compatible with the relevant Home Office / DoHNI licensing system.
30. Any trainers who are a member of an accreditation body must not be involved in the accreditation of their own course. Where members conduct both roles, they must report any conflict of interest to the accreditation body who will take appropriate action to mitigate any conflict of interest, and inform the Home Office.
Assurance post-accreditation, monitoring, and reporting
31. Accreditation bodies will implement ongoing monitoring to assure the quality of training provided through mechanisms which may include:
i. observation of training courses;
ii. conversations with training providers;
iii. liaison with training providers, including to share good practice and respond to queries;
iv. post-course feedback from trainees for all training courses; and
v. feedback from training providers.
32. Accreditation bodies will conduct a formal mid-point review of each training course they have accredited. The formal review should assess whether the training still meets the requirements to be accredited, applying the same assessments as for an initial accreditation. The formal review should take place between 24 and 36 months after initial accreditation.
33. Where an issue is identified, the accreditation bodies will meet with the training provider, and/or the relevant NTCO and/or Establishment Licence Holder, as appropriate, to gather factual information before taking remedial action. Accreditation bodies will have policies in place to address issues proportionately, ranging from minor transgressions to malpractice and fraud. Where there is an issue that may compromise the accreditation of a particular training course, accreditation bodies will notify the Home Office / DoHNI at the earliest opportunity.
Continuous review and best practice
34. Accreditation bodies will be proactive to ensure the training courses they accredit are continuously reviewed to reflect developments in good practice. They will do this through:
i. engagement on good practice between accreditation bodies, with relevant stakeholders, and establishments; and
ii. regular engagement between the accreditation bodies and relevant stakeholders to ensure all training courses reflect the latest evidence on the 3Rs.
Accountability to Home Office
35. Accreditation bodies will provide relevant information to the Home Office on request, and participate fully in assurance reviews.
Training Providers
36. Training providers will develop and deliver training courses that meet the training requirements set out in this guidance and the requirements of the relevant accreditation body.
37. Training providers will deliver interactive and innovative training that is updated with latest educational practices.
38. The training providers will ensure:
i. the training provider organisation and personnel are capable and suitably qualified;
ii. the content of the course meets the relevant learning outcomes and proactively promotes the latest scientific developments, new methods, and 3Rs developments;
iii. the quality of the training facilities, including online where relevant, and suitability of teacher-student ratio; and
iv. there are measures in place to ensure the integrity of the course and to combat fraud.
39. Training providers will be proactive in adopting good practice and sharing good practice with accreditation bodies of new and/or improved practices.
Establishments
40. Establishments will ensure access to appropriate initial training and ensure the competence and continuous professional development of an individual post- training. This will be delivered through:
i. record-keeping of training completed and review to ensure re-training where necessary;
ii. identification of new training needs, where appropriate;
iii. the provision of additional applied learning tailored to the specific establishment, procedure, and individual;
iv. regular and recorded assessment of competence (including by the project licence holder) for all individual licence holders.
Individual licence holder
41. Individual licence holders will take personal responsibility for their competence by proactively identifying learning and development needs, consulting with the relevant named persons in their establishment, and ensuring their training records and CPD records are accurate and up-to-date.
Training and Continuous Professional Development Requirement
Introduction
42. The current ASPA legislative requirements for persons using animals in science are set out in sections 2C, 4 and 5C, of ASPA, which requires that establishment, project and personal licences must only be issued where relevant persons have appropriate knowledge and training.
43. ASPA requires that staff are adequately educated and trained to perform any of the following roles:
i. personal licence applicants/holders;
ii. project licence applicants/holders;
iii. persons taking care of animals; or
iv. persons only killing animals by schedule 1 methods.
44. Table 1 shows the minimum training required for people undertaking each of these roles and the modules required. Table 2 sets out the modules required for specified named roles under ASPA. Each module has specific learning outcomes which must be covered at a minimum. These learning outcomes are set out in Annex A.
45. Exemptions may apply. Equivalent training received overseas may be accepted as meeting the training requirement.
Table 1: Formal training requirements for roles under ASPA
| Module number and content | Module Reference | Personal Licence holder (Cat A) | Personal Licence holder (Cat A&B) | Personal Licence holder (Cat A, B, C) | Project Licence holder | Persons taking care of animals | Persons only killing animals (by schedule 1 methods) |
|---|---|---|---|---|---|---|---|
| 1. Legislation | L | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| 2. Ethics, Animal Welfare & the 3Rs (level 1) | E1 | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| 3.1. Basic and Appropriate Biology – Species Specific (theory) | PILA (theory) (species specific) | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| 4. Animal Care, Health and Management – Species Specific | PILA (theory) (species specific) | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| 5. Recognition of Pain, Suffering and Distress – Specifies Specific | PILA (theory) (species specific) | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| 7. Minimally Invasive Procedures Without Anaesthesia – Species Specific | PILA (theory) (species specific) | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| 3.2. Basic and Appropriate Biology – Species Specific (practical) | PILA (skills) (species specific) | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| 8. Minimally Invasive Procedures Without Anaesthesia – Species Specific (skills) | PILA (skills) (species specific) | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| 20. Anaesthesia for Minor Procedures | PILB | Mandatory | Mandatory | As appropriate | |||
| 21. Anaesthesia, Advanced e.g. for Surgical Procedures | PILC | Mandatory | As appropriate | ||||
| 22. Principles of Surgery | PILC | Mandatory | As appropriate | ||||
| 6.1. Humane Methods of Killing (theory) | K (theory) | Mandatory | Mandatory | Mandatory | Mandatory | Mandatory | |
| 6.2. Humane Methods of Killing (skills) | K (skills) (species specific) | Mandatory | Mandatory | Mandatory | Mandatory | ||
| 9. Ethics, Animal Welfare and the 3Rs (level 2) | E2 | Mandatory | |||||
| 10. Experimental Design | PPL | Mandatory | |||||
| 11. Design and Management of Procedures and Projects | PPL | Mandatory | |||||
| 6.3. Stand-alone Module for Killing under schedule 1 methods | KD (species specific) | Mandatory |
Personal licence holder
46. Applicants for personal licences are required to complete training as follows.
i. For a category A personal licence, the applicant must successfully complete modules L, E1, PILA (theory) (species specific), PILA (skills) (species specific), K (theory) and K (skills) (species specific).
ii. For a category A+B personal licence, the applicant must also successfully complete module PILB.
iii. For a category A+B+C personal licence, the applicant must also successfully complete module PILC.
iv. To apply to use neuromuscular blocking agents (category D), additional training and experience is required. Refer to Appendix H of the Guidance on the Operation of ASPA for full details of the additional training requirements when applying to use neuromuscular blocking agents. All applicants for a category D licence must hold a category A, B and C personal licence.
v. A category E personal licence applies where an applicant who does not already hold a personal licence intends to attend an education or training course conducted under a single project licence. Applicants should consult their course provider for details of pre-course formal training requirements (if any).
vi. A category F licence applies to applicants who do not intend to undertake surgical procedures but do need to administer prolonged/balanced anaesthesia. Completion of the full module PILC may not be necessary. Instead, applicants will need to have undertaken an accredited module covering the learning outcomes for anaesthesia for surgical or prolonged procedures. Category F may also capture other miscellaneous applicants.
47. Applicants that have obtained their personal licence may work only under supervision until they have been assessed by their establishment as having the appropriate level of competence to conduct regulated procedures on their own (this is in addition to and separate from the competency assessment required under ASPA to grant a personal licence).The licensee’s project licence holder must arrange for a suitable person to train the licensee in the conduct of regulated procedures and the NTCO will ensure that the licensee’s personal training records are completed.
48. The licensee’s NTCO at their establishment will ensure regular review of the licensee’s competence and identification of any additional training needs. The frequency of the review will depend upon the technical nature of the procedures the licensee performs; how frequently the licensee undertakes those procedures; whether the licensee is proposing to embark on new procedures or use new types of animals; and the state of change in the field in which the licensee is working (e.g. new methods or agents for anaesthesia or analgesia). Reviews will be no more than five years apart and generally more frequent. In any case, if the licensee has not used their licence, or part of the authorities (species, categories) for five years they should undergo formal retraining before starting that type of work again.
49. Licensees working at another establishment should expect to have their level of competence reassessed at that establishment initially before starting work and at any time thereafter.
Project licence holder
50. Project licence applicants should hold an appropriate position of authority at the establishment and have the necessary scientific expertise and knowledge to direct and manage the programme of work. Project licence holders are responsible for the conduct of all regulated procedures performed by personal licensees working, and for the care of all animals being used, under their project licence.
51. Project licence applicants are required to complete accredited training:
i. L;
ii. E1;
iii. PILA, theory and skills;
iv. Module K (theory);
v. PPL;
vi. E2;
vii. PILB, if a programme of work involves basic anaesthesia; and
viii. PILC, if the project requires surgery and/or advanced anaesthesia.
52. For new project licence applicants, certificates should be dated within the last five years.
53. Project licence applicants who hold (or have recently held) a personal licence or project licence may be exempt from some or all of these formal training requirements.
i. A current or recently held (within the previous five years) personal licence will qualify the applicant for exemption from retaking modules L and E1 training and relevant applicable modules species specific PILA (theory and skills), PILB, PILC and K (theory).
ii. Applicants who have held a project licence within the previous five years may claim exemption from module L plus E1, PILA (theory and skills), PILB, PILC and K (theory) in relevant species and modules E2 plus PPL training.
iii. Applicants proposing to work with a new type of animal will be required to undergo relevant modules PILA (theory and skills) in that species.
iv. Exceptionally, exemption from PILA (skills) will be considered.
People caring for animals
54. Persons with responsibility for the care and welfare of animals must successfully complete modules L, E1, PILA (theory) (species specific), PILA (skills) (species specific), K (theory) and K (skills) (species specific).
55. Persons taking care of animals under the supervision of a qualified and experienced animal technologist can only work on their own when the supervisory animal technologist is content with their competence.
56. Training records, and records of assessments of competence, must be maintained as determined by the NTCO.
People killing animals
57. The establishment licence holder must ensure that anyone killing an animal is provided with appropriate training and supervision by an experienced person. Once a person is assessed as competent to kill a given species by a particular method, their details must be held in the register held by the establishment licence holder, or by the NTCO on their behalf. This applies to people killing by both Schedule 1 and non-Schedule 1 methods, including killing as part of an authorised programme of work. People killing animals by non-Schedule 1 methods as part of a programme of work will be required to hold personal licences undertaking training set out in Table 1. The following modules should be completed successfully: L, E1, PILA (theory) (species specific), PILA (skills) (species specific), K (theory) and K (skills) (species specific).
58. Persons killing animals will often be personal licensees or be competent to care for animals having undertaken training and achieved competence under that role.
59. Persons killing animals by Schedule 1 methods but not undertaking any regulated procedures nor having care responsibilities, and who have not undertaken training in legal, ethical, theoretical and practical aspects of killing animals, nor in the application of the 3Rs, must undertake standalone module KD to provide the knowledge necessary for their limited role.
Table 2: Training for other Named Persons
| Module number and content | Module Reference | PEL holder/NPRC | NVS | NACWO | NTCO | NIO |
|---|---|---|---|---|---|---|
| 1. Legislation | L | Recommended | Mandatory | Mandatory | Recommended | Recommended |
| 2. Ethics, Animal Welfare & the 3Rs (level 1) | E1 | Recommended | Mandatory | Mandatory | Recommended | Recommended |
| 3.1. Basic and Appropriate Biology – Species specific (theory) | PILA (theory) (species specific) | As appropriate | Mandatory | |||
| 4. Animal Care, Health and Management – Species Specific (theory) | PILA (theory) (species specific) | As appropriate | Mandatory | |||
| 5. Recognition of Pain, Suffering and Distress – Species Specific | PILA (theory) (species specific) | As appropriate | Mandatory | |||
| 7. Minimally Invasive Procedures Without Anaesthesia - Species Specific (skills) | PILA (theory) (species specific) | As appropriate | Mandatory | |||
| 3.2. Basic and Appropriate Biology – Species Specific (practical) | PILA (skills) (species specific) | As appropriate | Mandatory | |||
| 8. Minimally Invasive Procedures Without Anaesthesia – Species Specific (skills) | PILA (skills) (species specific) | As appropriate | Mandatory | |||
| 20. Anaesthesia for Minor Procedures | PILB | As appropriate | ||||
| 21. Anaesthesia, Advanced e.g. for Surgical Procedures | PILC | As appropriate | ||||
| 22. Principles of Surgery | PILC | As appropriate | ||||
| 6.1. Human Methods of Killing (theory) | K (theory) | Mandatory | Mandatory | |||
| 6.2. Humane Methods of Killing (skills) | K (skills) (species specific) | As appropriate | Recommended | |||
| 9. Ethics, Animal Welfare and the 3Rs (level 2) | E2 | Mandatory | Mandatory | Recommended | ||
| 10. Experimental Design | PPL | Recommended | Recommended | |||
| 11. Design and Management of Procedures and Projects | PPL | Recommended | Recommended | |||
| 23. Animals Husbandry, Care and Enrichment Practices | NACWO | Mandatory | ||||
| 24. Designated Veterinarian | NVS | Mandatory | ||||
| 50. Introduction to the local environment | LOCAL | Recommended | Recommended | Recommended | Recommended | Recommended |
Establishment licence holder/Named Person Responsible for Compliance
60. New establishment licence holders/Named Persons Responsible for Compliance (NPRCs) are expected to understand the relevant legal and ethical issues relating to the use of animals under the Act. They are recommended to undertake accredited training at least in modules L plus E1 to provide this understanding.
61. All establishment licence holders/NPRC’s should keep up to date with current legal and ethical and management issues relating to the scientific use of animals.
62. Information on current regulatory issues is supplied by the Home Office through periodic newsletters and circulars. The applicant will need to read these and it is their responsibility to ensure they are distributed to the NIO and others within their establishment as appropriate.
63. It is recommended that the applicant establishment licence holder/NPRC ensures the development of, and attends, a local module.
Named Veterinary Surgeons (NVSs)
64. Qualified veterinary surgeons (M or FRCVS or eligible for membership) are required to undertake one of the training courses for Named Veterinary Surgeons that has been approved by the Royal College of Veterinary Surgeons, either before taking up the role or, at a minimum, within the first year of their appointment. The NVS training should include the mandatory requirements indicated in Table 2. The applicant NVS needs to understand:
i. ASPA, as amended, and this Guidance;
ii. standard conditions on licences and their role in promoting compliance;
iii. ethical issues relating to the use of animals;
iv. relevant husbandry and care practices to promote compliance with Code of Practice requirements for all species for which they are responsible, or determining refinement where appropriate;
v. Schedule 1 methods of euthanasia for all species under their care;
vi. the 3Rs relevant to work at the establishment; and
vii. required record keeping systems relevant to NVS role.
65. It is recommended that the NVS undertakes the PPL module in order to understand the requirements for project licence holders and to facilitate providing informed advice to them.
66. The NVS should be familiar with the husbandry, biology, physiology and clinical issues relevant to the species for which they are responsible. Where this has not been covered during their veterinary training or subsequent experience, they will need to undertake additional training which may include, but is unlikely to be limited to, accredited training in PILA and K (skills).
67. The NVS should be aware of the local structure for management and responsibilities relating to animal use at their establishment, including how key roles and related tasks are fulfilled. This should be covered in a local module.
Named Animal Care and Welfare Officers (NACWOs)
68. A NACWO is responsible for overseeing the work of those taking care of animals. The training of a NACWO should give sufficient understanding of the biology and husbandry of the relevant species as well as a thorough understanding of the regulations governing the use of animals in research and their welfare. Specifically, the NACWO should have a good appreciation and knowledge of:
i. ASPA and associated Guidance and Code of Practice;
ii. standard conditions on licences and their role in promoting compliance;
iii. ethical issues relating to the use of animals;
iv. relevant husbandry and care practices, including methods of euthanasia, ensuring compliance with Code of Practice requirements;
v. the 3Rs relevant to work at the establishment;
vi. recognition of indictors of poor welfare, pain and other forms of suffering; and ways of alleviating;
vii. record keeping systems relevant to NACWO role; and
viii. the local structure for management and responsibilities relating to animal use at their establishment, including how key roles and related tasks are fulfilled. This should be covered in a local module.
69. The NACWO is required to complete accredited training normally before taking up the position. If this is not possible, then justification for the delay and the expected date of attendance at the course should be included in their application. The inspector will consider each case individually.
70. In practice, most NACWOs will hold a personal licence and will have undertaken relevant accredited training as well as be competent to humanely kill relevant protected animals. In many cases, it is likely that they will have completed higher level training in animal technology. If they have not, they must show that they have achieved the learning outcomes relevant to the higher level of Animal Husbandry, Care and Enrichment Practices.
71. It is recommended that the NACWO extends their training to understand the requirements for project licence holders and facilitate them providing informed advice in their roles by completing the PPL training module.
Named Training and Competency Officers (NTCOs)
72. NTCOs are expected to be familiar with the Act and its implementation. It is therefore recommended that, as a minimum, the NTCO takes accredited training in module L plus E1 to gain this understanding. In addition, the NTCO will need to be familiar with the regulated procedures and species being used at their establishment and with the Home Office licensing system, including how to submit licence applications, amendments and reviews. The NTCO will need to be aware of local processes, people and structures such as the Local module and AWERB.
73. The NTCO must have a thorough understanding of the formal training requirements for licence applicants and of the normally accepted exemptions from formal training and how to apply for them. The NTCO can get this information from this Home Office Guidance, as well as Advice Notes and guidance associated with licence applications and available on the Research and testing using animals page of the GOV.UK website. The NTCO will need to understand the importance of continuing education and training and the maintenance of appropriate records. The NTCO should ensure that they have access to the newsletters circulated regularly by the Home Office to establishment licence holders, Named Persons Responsible for Compliance, and others performing roles under ASPA.
74. The NTCO must also have a thorough understanding of the local measures for managing training at their establishment, including the arrangements for supervision, training and assessment of competence as covered by the Local module. The NTCO will need to know the types of work carried out and the species used at their establishment. A basic understanding of teaching principles may be advantageous.
75. The NTCO will need to liaise with NTCOs from other establishments to make sure that all licensees moving into their establishment, or proposing to work at their establishment, are authorised to use the necessary animals and categories of procedures by their personal licence and they are aware of their training and competence. The NTCO will need to determine the degree of training and supervision that they will need. The NTCO will need to ensure that they are trained in the local control measures at their establishment which may differ from those in other establishments.
Named Information Officers (NIOs)
76. The NIO should have a good understanding of legal and ethical aspects of use of animals for scientific purposes and be familiar with the concept, principles and potential applications of the 3Rs and we therefore recommend that they complete at least modules L and E1 and E2. The NIO should know where to find current information on the 3Rs and be proactive in finding and disseminating information relating to the 3Rs that is relevant to work at their establishment.
77. The NIO should have expertise in sourcing, retrieving and storing relevant information. They should be aware of different search tools and methods of search or be able to identify someone who has these skills. They need to have a good understanding of the local structure for management and responsibilities relating to animal use at their establishment to assist in forming networks and deciding how and to whom to distribute information. This should be covered in the Local module.
78. The NIO should ensure they have access to the newsletters circulated regularly by the Home Office to establishment licence holders, NPRC, and others performing roles under ASPA.
Local module
79. We recommend that each establishment should prepare a local module incorporating information on the local management structure relating to animal use, the names of people fulfilling the key roles, including those of named persons, and their related tasks. It should describe how each of the local key people (including the named roles) contributes to the welfare of animals, high quality science, and implementation of the 3Rs. Information on the roles and processes of the local AWERB and how the local culture of care is promoted should be included.
80. This will allow people fulfilling key roles to understand their own role within the local structure and how they personally contribute and work with others. We recommend that this module is completed by each of the named persons including licensees and should form part of the induction process for all those working with animals or in allied roles.
Continuing Professional Development (CPD)
81. For all people fulfilling a role under the Act, it is expected that sufficient continued professional development will be undertaken to ensure that knowledge and skills are maintained, to keep abreast of relevant developments (in particular in the 3Rs) and to fulfil professional requirements. Where there are changes to the species, models or types of work, named persons and licence holders should undertake relevant additional training. Records need to be kept in association with the NTCO.
Annex A – Module Learning Outcomes
1. Legislation (Module L)
This module provides a relevant level of understanding of the legal and regulatory framework within which projects involving animals are constructed and managed and of the legal responsibilities of the people involved, i.e. those carrying out procedures on animals; designing procedures and projects; taking care of animals; or killing animals, and may cover other relevant legislation.
Learning outcomes
Trainees should be able to:
i. Identify and describe the law and guidance which regulate the scientific use of animals and in particular the activities of those carrying out scientific procedures involving them.
ii. Identify and describe related animal welfare legislation.
iii. Describe the authorisation that is needed before acting as user, breeder or supplier of laboratory animals and especially the authorisation required for projects and individuals.
iv. List sources of information and support that are available (regarding national legislation).
v. Describe the role of the NACWO, NIO, NTCO and NVS mentioned, and their statutory duties and other responsibilities under ASPA.
vi. Describe the roles and responsibilities of the Animal Welfare and Ethical Review Bodies and the Animals in Science committee for the protection of animals used for scientific purposes.
vii. Indicate who is responsible for compliance at an establishment and how this responsibility may be exercised (e.g. through the AWERB).
viii. Describe when a procedure becomes regulated under ASPA (minimum threshold of pain, suffering, distress or lasting harm).
ix. Indicate who bears primary responsibility for the animals undergoing procedures.
x. List which species, including respective stages of development that are included in the scope of the ASPA.
xi. Indicate the circumstances in which animals under the scope of ASPA should be humanely killed or removed from the study to receive veterinary treatment.
xii. Describe the legislative controls over the killing of animals bred or used for scientific procedures.
2. Ethics, animal welfare and the 3Rs (level 1) (Module E1)
This module provides guidance and information to enable individuals working with animals to identify, understand and respond appropriately, to the ethical and welfare issues raised by the use of animals in scientific procedures generally and, where appropriate, within their own programme of work. It provides information to enable individuals to understand and to apply the basic principles of the 3Rs.
Learning Outcomes
Trainees should be able to:
i. Describe the differing views, within society, relating to the scientific uses of animals and recognise the need to respect these.
ii. Describe the responsibility of humans when working with research animals and recognise the importance of having a respectful and humane attitude towards working with animals in research.
iii. Identify ethical and animal welfare issues in their own work and be aware and able to reflect on the consequences of their own actions.
iv. Recognise that compliance with ethical principles may contribute to the long- term trust and acceptance in scientific research from the general public.
v. Describe how the law is based on an ethical framework which requires 1) weighing the harms and benefits of projects (the harm/benefit assessment) 2) applying the 3Rs to minimise the harm, maximise benefits and 3) promote good animal welfare practices.
vi. Describe and discuss the importance of the 3Rs as a guiding principle in the use of animals in scientific procedures.
vii. Explain the Five Domains and how these apply to laboratory species.
viii. Describe the concept of harms to animals including avoidable and unavoidable suffering, direct, contingent and cumulative suffering.
ix. Describe the severity classification system, and give examples of each category. Describe cumulative severity and the effect this may have on the severity classification.
x. Describe the regulations regarding re-use of animals.
xi. Describe the importance of good animal welfare including its effect on scientific outcomes as well as for societal and moral reasons.
xii. Describe the need for a culture of care and the individual’s role in contributing to this.
xiii. Describe relevant sources of information relating to ethics, animal welfare and the implementation of the 3Rs.
xiv. Be aware of different search tools and methods of search.
3. 1 Basic and appropriate biology – species specific (theory) (part of Module PILA (theory))
This module provides an introduction to the basic principles of animal behaviour, care, biology and husbandry. It incorporates information in relation to anatomy and physiological features, including reproduction, behaviour and routine animal husbandry and enrichment practices. It is not intended to provide more than the minimum background information which is needed for someone to be able to begin work under supervision.
Following this module, practical training under supervision should provide each individual with the expertise and skills needed for them to carry out their particular role. Practical training requirements will, inevitably, differ according to role.
Learning Outcomes
Trainees should be able to:
i. Describe basic anatomy, physiology, reproduction and behaviour of the relevant species.
ii. Recognize and describe life events that have the potential to cause suffering including sourcing, transport, housing, husbandry, handling and procedures (on a basic level).
iii. Indicate how good welfare can promote good science: e.g. explain how the failure to attend to biological and behavioural needs may affect the outcome of procedures.
iv. Indicate how husbandry and care may influence experimental outcome and the number of animals needed e.g. example where the place in the room influences the outcome, hence randomisation.
v. Describe the dietary requirements of the relevant animal species and explain how these can be met.
vi. Describe the importance of providing an enriched environment (appropriate to both the species and the science) including social housing and opportunities for exercise, resting and sleeping.
vii. When relevant to the species, recognise that there are different strains, and that these can have different characteristics which can affect both welfare and science.
viii. When relevant to the species, recognise that alterations to the genome can affect the phenotype in unexpected and subtle ways, and the importance of monitoring such animals very carefully.
ix. Maintain and interpret accurate, comprehensive records of animals held in the animal facility, including the wellbeing of the animals.
3.2 Basic and appropriate biology – species specific (practical) (part of Module PILA (skills))
Learning Outcomes
Trainees should be able to:
i. Be able to approach, handle/pick up and restrain an animal and return it to its cage/pen in a calm, confident and empathetic manner such that the animal is not distressed or caused harm.
4. Animal care, health and management – species specific (theory) (part of Module PILA (theory))
This module provides information on various aspects of animal health, care and management including, environmental controls, husbandry practices, diet, health status and disease. It also includes relevant basic learning outcomes relating to personal health and zoonoses.
Learning Outcomes
Trainees should be able to:
i. Describe suitable routines and husbandry practices for the maintenance, care and welfare for a range of animals used in research, to include small laboratory species and large animal species where appropriate.
ii. Describe suitable environmental and housing conditions for laboratory animals, how conditions are monitored and identify the consequences for the animal resulting from inappropriate environmental conditions.
iii. Recognise that changes to or disruption of circadian or photoperiod can effect animals.
iv. Describe the biological consequences of acclimatisation, habituation and training.
v. Describe how the animal facility is organized to maintain an appropriate health status for the animals and the scientific procedures.
vi. Describe how to provide water and an appropriate diet for laboratory animals including the sourcing, storage and presentation of suitable foodstuffs and water.
vii. List the methods, and demonstrate an understanding of appropriate, safe and humane handling, sexing and restraint of one or more named species for common scientific procedures.
viii. Name different methods for marking individual animals and state an advantages and disadvantage for each method.
ix. List potential disease risks in the animal facility, including specific predisposing factors which may be relevant. Name methods available for maintaining appropriate health status (including use of barriers, different containment levels use of sentinels as relevant to the species).
x. Describe appropriate breeding programmes.
xi. Describe how genetically altered animals can be used for scientific research and the importance of monitoring such animals very carefully.
xii. List the correct procedures for ensuring health, welfare and care of animals during their transport.
xiii. List potential human health hazards associated with contact with laboratory animals (including allergy, injury, infection, zoonosis) and how these can be prevented.
5. Recognition of pain, suffering and distress – species specific (part of Module PILA (theory))
This module prepares individuals to be able to identify normal condition and behaviour of experimental animals and enable them to differentiate between a normal animal and one which is showing signs of pain, suffering or distress which could be a result of factors including environment, husbandry or the effect of experimental protocols. It will also provide information regarding severity classifications, cumulative severity and the use of humane endpoints.
Learning Outcomes
Trainees should be able to:
i. Recognise normal or desirable behaviour and appearance of the individuals in the context of species, environment and physiological status.
ii. Recognise abnormal behaviour and signs of discomfort, pain, suffering, or distress, as well as signs of positive well-being and principles of how pain, suffering and distress can be managed.
iii. Discuss factors to be considered and methods available for assessing and recording the welfare of animals e.g. score sheets.
iv. Describe what a humane end point is. Identify criteria to be used to set humane endpoints. Define action to be taken when a humane endpoint is reached and consider possible options for refining methods to finish at an earlier endpoint.
v. Describe the severity classifications included in ASPA and give examples of each category; explain cumulative severity and the effect this may have on the severity classification.
vi. Describe the circumstances when anaesthesia or analgesia may be necessary to minimise pain, suffering, distress or lasting harm.
6. 1 Humane methods of killing (theory) (Module K (theory))
This module provides information on the principles of humane killing and the need to have someone available, at all times, who is able to kill an animal quickly and humanely if required. The module will include information and descriptions of the different methods available, details of the species for which these methods are suitable and information to help trainees compare the methods permitted and determine how to select the most appropriate method.
Learning Outcomes
Trainees should be able to:
i. Describe the principles of humane killing (e.g. what constitutes ‘a good death’).
ii. Describe the different methods by which the relevant animals are allowed to be killed, the influence different methods can have on scientific outcomes, and how to select the most appropriate method.
iii. Explain why someone competent to kill animals should be available at all times (whether care staff or person carrying out procedures).
6.2 Humane methods of killing (skills) (Module K (skills))
This module provides practical training to reflect the information and principles delivered in module covering Human Methods of Killing (theory) and will involve practical training in the appropriate methods for the species and suitable methods of confirming death.
Learning Outcomes
Trainees should be able to:
i. Proficiently and humanely carry out euthanasia using appropriate techniques on relevant species of laboratory animals.
ii. Demonstrate how death is confirmed and how cadavers should be processed or otherwise disposed of.
6.3 Humane methods of killing – stand-alone module for those who only perform killing of animals by schedule 1 methods (Module KD)
This module has been designed for those who only perform the role of killing of animals by schedule 1 methods and is a pre-requisite for this role which can be delivered in place of a number of other modules for anyone who will only be involved in the humane killing of animals. This module combines Learning Outcomes from the modules relating to legislation, ethics and the 3Rs with practical animal handling, safe working practices and the theory and practical elements of the humane killing modules.
Learning Outcomes
Trainees should be able to:
Legislation, 3Rs and ethics (i.e. subset of Modules L and E1)
i. Describe the regulatory framework for the scientific use of animals and in particular controls relating to the conduct of humane killing and confirming death – including role of named persons and the Animal Welfare Ethical Review Body.
ii. Recognize differing societal views about the scientific use and humane killing of animals.
iii. Have an understanding of the ethical principles underlying the use of animals and of their own role in contributing to the ‘culture of care’.
iv. Relate ways in which the 3Rs can be applied to the humane killing of animals.
Species specific handling
v. Demonstrate appropriate techniques for the safe and competent handling of relevant species. Be able to approach, handle/pick up and restrain an animal and return it to its cage/pen in a calm, confident and empathetic manner such that the animal is not distressed or caused harm. Explain the importance of transporting animals correctly and safely.
vi. Describe the normal and abnormal behaviour and the behavioural requirements of relevant species and be able to recognise and discuss strategies for minimising and responding to occurrences of pain, suffering and distress.
vii. Describe in outline the basic biological and husbandry needs of relevant species.
Safe working practices
viii. Discuss the importance of correct storage and handling of chemical agents used for humane killing and maintaining hygiene in the workplace.
ix. Describe the correct procedures to deal with accidental exposure or spillage.
x. Describe the basic hygiene rules and relate these to the workplace.
xi. Relate the importance of correct disposal of different categories of waste (clinical waste, hazardous waste and normal waste) and describe appropriate strategies.
xii. Explain how engineering solutions combined with personal protection equipment can minimise exposure to laboratory animal allergens preventing sensitisation.
xiii. Identify clinical symptoms commonly associated with allergy to laboratory animals.
xiv. Describe what is meant by zoonosis, and explain why contact with different species (in particular non-human primates) constitutes a potential human health hazard.
Species specific humane killing
xv. Describe the principles of humane killing (e.g. what constitutes ‘a good death’).
xvi. Describe the different methods by which the relevant animals are allowed to be killed, the influence different methods can have on scientific outcomes, if relevant, and how to select the most appropriate method.
xvii. Explain why someone competent to kill animals should be available at all times (whether care staff or person carrying out procedures).
xviii. Proficiently and humanely carry out euthanasia using appropriate of techniques on relevant species of laboratory animal.
xix. Demonstrate how death is confirmed and how cadavers should be processed or otherwise disposed of.
7. Minimally invasive procedures without anaesthesia – species specific (theory) (part of Module PILA (theory))
This module provides an introduction to the theory relating to minor procedures. It provides information about appropriate methods of handling and restraint and describes appropriate techniques for injection, dosing and sampling relevant to the species. It should provide information sufficient for individuals to understand what will be required of them before they go on to trained in the practical aspects of these skills whilst under supervision.
Learning Outcomes
Trainees should be able to:
i. Describe appropriate methods and principles to be followed when handling animals (including methods of manual restraint and use of restricted environments).
ii. Describe the biological impact of procedures and restraint on physiology.
iii. Describe refinement opportunities for procedures and restraint e.g. through training (using positive re-enforcement), habituation and socialisation of animals.
iv. Describe techniques/procedures including, for example, injection, sampling and dosing techniques (routes/volumes/frequency), dietary modification, gavage, tissue biopsy, behavioural tests, use of metabolic cages.
v. Describe how to perform minor techniques and relate appropriate sample volumes and sampling frequencies for the relevant species.
vi. Describe the need for rigour and consistency in conducting scientific procedures and the correct recording and handling of samples.
vii. Describe appropriate methods for the assessment of the welfare of animals with respect to the severity of procedures and know what appropriate action to take.
viii. Recognize that refinement is an on-going process and know where to find relevant, up-to-date, information.
ix. Describe the biological consequences of transport, acclimatization, husbandry conditions and experimental procedures on the species concerned and describe how these can be minimised.
8. Minimally invasive procedures without anaesthesia – species specific (skills) (part of Module PILA (skills))
This module delivers practical elements of training relevant to Module covering minimally invasive procedures without anaesthesia – species specific (theory). Practical training for minor procedures can be taught through a number of methods using different tools which are available and designed for the purpose (this is likely to include synthetic animal models and the use of cadavers). The module should be designed in such a way that it will enable the trainee to attain a level of proficiency such that, when commencing work under supervision, s/he should cause no pain, suffering, distress or lasting harm to the animal.
Learning Outcomes
Trainees should be able to:
i. Select and explain the best methods for common procedures (such as blood sampling and administration of substances) including route/volume/ frequency as appropriate.
ii. Demonstrate that s/he can handle and restrain the animal in the best position for the technique.
iii. Perform minor techniques under supervision, in a manner that does not inflict unnecessary pain, suffering, distress or lasting harm.
9. Ethics, animal welfare and the 3Rs (level 2) (Module E2)
This module provides guidance and information to enable individuals designing procedures and projects to look, in detail, at different aspects of ethics and the 3Rs and to apply the principles learned to the ethical and welfare issues raised by the use of animals in scientific procedures within their own programme of work.
The purpose of this module is to address the fact that those designing procedures should command a deeper and broader understanding of the general issues. Thus, the main difference between level 1 and level 2 Modules on “Ethics, animal welfare that the 3Rs” is not necessarily the topics to be covered (which have not been repeated here) but rather that some of them are addressed in more detail and with a greater expectation for the Learning Outcome itself. For example at level 1 there are elements the trainee should know and be able to describe, which at level 2 the trainee should have a more detailed understanding and be able to discuss. This module also prepares individuals so that they are able to keep themselves informed in order to continuously apply the 3Rs to their work as new methods and approaches evolve.
Learning Outcomes
Trainees should be able to:
i. Understand that there is a broad range of ethical, welfare and scientific perspectives on the use of animals in scientific procedures, and that thinking on all of these matters evolves over time and is influenced by culture and context.
ii. Understand that this means there is need for on-going critical evaluation of the justification for using animals and of implementation of the 3Rs at all stages of the life of a project.
iii. Recognise that there are ethical limits to what it is considered permissible to do under ASPA.
iv. Explain that ASPA requires that the justification for programmes of work is assessed by weighing potential adverse effects on the animals against the likely benefits; that harms to animals must be minimised, and benefits maximised.
v. Understand and provide the information necessary to enable a robust harm/benefit assessment to be performed; and explain why they personally consider that the potential benefits outweigh the likely adverse effects.
vi. Understand the need to communicate appropriate information to a wider public audience, and be able to prepare an appropriate non-technical project summary to facilitate this.
vii. Describe the importance of disseminating information that will promote understanding of ethical issues, good animal welfare, good science and application of the 3Rs.
10. Design of procedures and projects (level 1) (part of Module PPL)
This module is a pre-requisite for people who will be designing projects but it is also beneficial for scientists who have some involvement in designing the procedures that they carry out. The module comprises information about experimental design concepts, possible causes and elimination of bias, statistical analysis and information about where expertise can be found to assist with procedure, design, planning and the interpretation of results.
Learning Outcomes
Trainees should be able to:
i. Describe the concepts of fidelity and discrimination.
ii. Explain the concept of variability, its causes and methods of reducing it (uses and limitations of isogenic strains, outbred stocks, genetically modified strains, sourcing, stress and the value of habituation, clinical or sub-clinical infections, and basic biology).
iii. Describe possible causes of bias and ways of alleviating it (e.g. formal randomisation, blind trials and possible actions when randomisation and blinding are not possible).
iv. Identify the experimental unit and recognise issues of non-independence (pseudo-replication).
v. Describe the variables affecting significance, including the meaning of statistical power and “p-values”.
vi. Identify formal ways of determining sample size (power analysis or the resource equation method).
vii. List the different types of formal experimental designs (e.g. completely randomised, randomised block, repeated measures [within subject], Latin square and factorial experimental designs).
viii. Explain how to access expert help in the design of an experiment and the interpretation of experimental results.
11. Design of procedures and projects (level 2) (part of Module PPL)
This module provides a relevant level of understanding of the legal and regulatory framework within which projects are constructed and managed, and of their legal responsibilities.
The trainee must be able to identify, understand and respond appropriately to the ethical and welfare issues raised by the use of animals in scientific procedures generally, and specifically within their own programme of work.
The trainee should be able to develop, direct and control a programme of work in order to achieve its stated objectives, while ensuring compliance with the terms and conditions of any regulation governing the project. This includes implementation of the 3R’s throughout the programme of work.
Learning Outcomes
Trainees should be able to:
Legal issues
i. Describe in detail the main components of ASPA regulating the scientific use of animals; in particular, explain the legal responsibilities of those designing procedures and projects and those of other persons with statutory responsibilities under ASPA (e.g. the person responsible for compliance, veterinarian, animal care staff, training officers).
ii. List the key purposes of other legislation and associated guidelines that impact on the welfare and use of animals. This includes legislation/guidelines relating to: veterinary care, animal health, animal welfare, genetic modification of animals, animal transport, quarantine, Health & Safety, wildlife and conservation.
Good scientific practice
iii. Describe the principles of a good scientific strategy that are necessary to achieve robust results, including the need for definition of clear and unambiguous hypotheses, good experimental design, experimental measures and analysis of results. Provide examples of the consequences of failing to implement sound scientific strategy.
iv. Demonstrate an understanding of the need to take expert advice and use appropriate statistical methods, recognise causes of biological variability, and ensure consistency between experiments.
v. Discuss the importance of being able to justify on both scientific and ethical grounds, the decision to use living animals, including the choice of models, their origins, estimated numbers and life stages. Describe the scientific, ethical and welfare factors influencing the choice of an appropriate animal or non-animal model.
vi. Describe situations when pilot experiments may be necessary.
vii. Explain the need to be up to date with developments in laboratory animal science and technology so as to ensure good science and animal welfare.
viii. Explain the importance of rigorous scientific technique and the requirements of assured quality standards such as GLP.
ix. Explain the importance of dissemination of the study results irrespective of the outcome and describe the key issues to be reported when using live animals in research e.g. ARRIVE guidelines.
Implementing the 3Rs
x. Demonstrate a comprehensive understanding of the principles of replacement, reduction and refinement, and of how these ensure good science and good animal welfare.
xi. Explain the importance of literature and internet searches, discussion with colleagues and with relevant professional bodies in identifying opportunities for applying each ‘R’.
xii. Describe relevant sources of information relating to ethics, animal welfare and the implementation of the 3Rs.
xiii. Explain how to use different search tools and methods of search.
xiv. Describe examples of alternative methods and research strategies that replace, avoid or complement the use of animals in different types of research programme.
xv. Identify, assess and minimise all of the welfare costs to animals throughout the animals’ lifetime (including adverse effects relating to sourcing, transport, housing, husbandry, handling, procedures and humane killing); Explain and give examples of welfare assessment protocols.
xvi. Define and apply appropriate humane end-points; establish suitable criteria to identify when the humane endpoint has been reached.
xvii. Describe possible conflicts between Refinement and Reduction (e.g.in the case of re-use) and the factors that need to be considered to resolve this conflict.
xviii. Define the requirements for, and controls on, re-homing of animals; identify any relevant re-homing guidelines.
Responsibilities
xix. Explain the need to be aware of local arrangements relating to project licence management, e.g. procedures for ordering animals, accommodation standards, disposal of animals, safe working practices and security, and the actions to take in the event of unexpected problems arising with any of these.
20. Anaesthesia for minor procedures (Module PILB)
This module provides guidance and information to individuals who, during their work with animals, will need to apply sedation or short-term anaesthesia for a brief period and mild pain level procedure.
The objectives of this module are:
-
to introduce the course candidates to the administration of anaesthetics to laboratory animals;
-
to discuss anaesthesia under the following broad headings (pre anaesthetic considerations; effects of anaesthetic agents; anaesthetic administration; regional/local/ general anaesthesia; anaesthetic emergencies; recovery from anaesthesia);
-
to provide information on the effects of drugs used during anaesthesia;
-
to consider the potential adverse effects of anaesthesia and on recovery;
-
to discuss anaesthetic emergencies and their treatment and
-
to identify when anaesthesia may compromise science.
The Learning Outcomes aim to give the minimum knowledge necessary for the appropriate and safe application of such a sedation or brief anaesthesia, with simple induction, basic maintenance for the purpose of performing minor procedures such as illustrated defined below:
-
Simple induction process (e.g. chamber induction or simple IP administration, no requirement for endotracheal intubation) and
-
Basic “hands on” and “observational” monitoring of anaesthetic depth; maintenance is anticipated to be uncomplicated at a stable anaesthetic depth and maintenance rate.
-
Brief duration (up to about 15 minutes in a rodent species – maintenance of anaesthesia for imaging - if the anaesthesia is expected to last longer than this, the trainee would require further modules)
-
Use for minor procedures only - non-invasive / superficial procedures only (skin level, no access to body cavities unless terminal anaesthesia is used), superficial venous access and taking a blood sample, identification using SC microchip or, tail tipping (limited biopsy of tip of tail), anaesthesia for restraint.
-
No pain or short / minor pain level,
-
No high-risk or sensitive animal.
Learning Outcomes
Trainees should be able to:
i. Define sedation, local and general anaesthesia.
ii. Identify the three components of the triad of anaesthesia and understand that different anaesthetic agents produce these to different degrees.
iii. Define balanced anaesthesia and indicate that this is best achieved by using drugs in combinations to achieve all components of the anaesthetic triad to an acceptable degree.
iv. Relate why and when sedation or anaesthesia might be used for restraint.
v. List the factors to be considered in pre-anaesthetic evaluation of animals - how to perform a basic health check, consider physiological or pathological status of the model they are working with and how these may influence the choice of anaesthetic agent.
vi. Discuss the relative merits / drawbacks and principles of selection of different agents and their application, including calculation of doses, in relevant species, including injectable and volatile agents (or dissolved agents in the case of aquatic species), including local anaesthesia regimes.
vii. Indicate the importance of minimising stress prior to anaesthesia in reducing the likelihood of complications due to anaesthesia.
viii. Recognise when premedication is beneficial to incorporate into an anaesthetic regime.
ix. Describe and demonstrate the correct set-up, operation and maintenance of anaesthetic equipment appropriate to the species concerned.
x. Evaluate and appreciate the different levels and planes of anaesthesia (voluntary excitement, involuntary excitement, surgical anaesthesia (light, medium & deep), excessively deep).
xi. List the factors indicating that an animal is suitably anaesthetized (stable and of appropriate depth) to enable procedures to be undertaken and what actions should be taken if an adverse event occurs. This will include basic “hands on” and “observational” anaesthetic monitoring techniques, including assessment of reflexes appropriate for species.
xii. Describe methods of optimising post anaesthetic recovery (e.g. heat blankets, analgesia, reversal agents, access to food and water, environmental conditions) to ensure a smooth and rapid recovery from anaesthesia.
xiii. Demonstrate an understanding of safe / good working practices with regard to use, storage and disposal of anaesthetic and analgesic agents.
21. Advanced anaesthesia for surgical or prolonged procedures (part of Module PILC)
This module is linked, but not exclusively, to the “surgery” module. “Surgical procedures” include all procedures not defined as “Minor procedures” in the Preamble to the anaesthesia for minor procedures module . Prolonged is defined as any duration greater than 15 minutes, which may require additional or continuous dosing (including anaesthesia for imaging).
This module also discusses the alleviation of pain during painful procedures such as surgery, through the use of anaesthetic and analgesic drugs. Anaesthesia is also used for producing muscle relaxation, suppressing reflexes, and producing loss of consciousness for purposes other than prevention of pain perception. For example, anaesthesia is required for MRI, CT scans and other minimally invasive imaging modalities.
Because of the wide variability of laboratory animal species and strains, as well as anaesthetic agents, an appropriate anaesthetic regimen should be developed in consultation with a veterinarian.
If not used for restraint alone, the need to use an anaesthetic to perform a procedure implies that the procedure would be painful for an awake animal. In addition there may be some residual pain after the animal recovers from the anaesthetic and analgesics should be used. Some drugs described here appear in both the anaesthesia and surgery modules.
Learning Outcomes
Trainees should be able to:
i. Relate why and when anaesthesia might be used, including additional factors relevant for long term anaesthesia.
ii. Relate the need for and list the factors to be considered in pre-anaesthetic evaluation of animals, including acclimatisation.
iii. Discuss the use of pre-anaesthetic agents and analgesics as part of a balanced anaesthetic regime.
iv. Indicate that a range of drugs are commonly used for premedication and the induction and maintenance of anaesthesia in relevant laboratory species, and identify where to get advice on the different drug available and their use.
v. Describe how an animal’s concurrent pathology may require specific anaesthetic regimen, monitoring or nursing care.
vi. Indicate types of agents used for the induction and maintenance of general anaesthesia, their advantages and disadvantages and when each might be used.
vii. Describe how anaesthetic agents interact to produce the three components of the anaesthetic triad to different degrees, and how balanced anaesthesia might be best achieved by using combinations.
viii. Demonstrate a sufficient understanding of anaesthetic agents having a low analgesic effect, potentially requesting the use of an additional analgesia.
ix. List the factors to be considered when monitoring anaesthesia both for anaesthetic depth and physiological stability. Indicate how to determine that an animal is sufficiently deeply anaesthetised to enable painful procedures to be undertaken, and what action should be taken if an adverse event occurs.
x. List methods which can used to assist monitoring of anaesthesia (e.g. ECG, BP, Urine output, Oxygen saturation, CO2) and how these can be monitored.
xi. Monitor anaesthetic depth and the animals’ vital signs, using both clinical signs, and electronic apparatus if appropriate.
xii. Describe and demonstrate the correct set-up, operation and maintenance of anaesthetic and monitoring equipment appropriate to the species concerned.
xiii. Demonstrate competence in maintaining and interpreting records of pre- and post-anaesthetic induction and whilst an animal is anaesthetised, as well as in managing the animal care adequately.
xiv. Indicate the problems that may occur during anaesthesia, and understand how to avoid these, or manage them if they occur.
xv. Demonstrate an understanding of mechanical ventilation.
xvi. Describe methods to optimise post anaesthetic recovery to ensure a smooth and rapid recovery from anaesthesia, as in Basic Module but with additional methods required, including analgesia and fluid replacement, for animals having undergone lengthy anaesthesia of surgical procedure.
xvii. Consider the consequences of anaesthesia and the surgical procedures on recovery.
xviii. Appreciate how the choice of anaesthetic agent will determine the rate of recovery and describe how duration and quality of anaesthesia governs the rate of recovery.
xix. Describe the problems that can arise (in the post-operative period), and indicate how to avoid these, or manage them if they occur.
xx. Discuss how to integrate a program of pain management into an overall scheme of perioperative care.
xxi. Indicate some of the problems associated with pain recognition and pain management in animals.
xxii. Demonstrate a sufficiently detailed understanding of analgesics to be able to administer safely, including routes of administration and potential adverse effects.
xxiii. Demonstrate an understanding of safe / good working practices with regard to use, storage and disposal of anaesthetic and analgesic agents.
22. Principles of surgery (part of Module PILC)
This module covers principles of pre-operative animal assessment and care, preparations for surgery including equipment preparation and aseptic technique and the principles of successful surgery.
The module provides information about possible complications, post-operative care and monitoring along with details of the healing process.
It also covers more practical elements for example the demonstration of commonly used instruments and provide an opportunity for trainees to practice some of the practical aspects of surgical technique, such as methods of suturing, using appropriate non-animal models.
Learning Outcomes
Trainees should be able to:
i. Explain the relevance and need for pre-operative assessment and, where appropriate, conditioning.
ii. Identify sources of reference for good surgical practice.
iii. Describe the process of tissue healing and relate to this to the importance of asepsis and hygienic practices, wound creation, the principles of tissue handling and selection of a suitable surgical approach.
iv. Discuss possible causes of delayed or impaired wound healing or other post- surgical complications and describe ways in which these can be avoided or, if they occur, treated.
v. Describe in general terms how personnel, animals, instruments and equipment should be prepared for aseptic surgery.
vi. List the principles of successful surgery (e.g. Halstead’s principles) and indicate how to achieve these.
vii. Describe the characteristics of different, commonly-used instruments, suture materials and needles.
viii. Relate the importance of good technique in accessing surgical sites, handling tissues and repairing incisions.
ix. Indicate the characteristics of different suture patterns and their applicability to different situations.
x. Demonstrate how to place a suture correctly.
xi. Describe common post-surgical complications and their causes.
xii. Relate the principles of post-surgical care and monitoring.
xiii. Describe the planning of surgical procedures and discuss the competencies required of all personnel involved.
xiv. Demonstrate competence in surgical techniques, including ablations and incisions and their closure by methods appropriate to the tissue concerned.
xv. Describe particular aspects of care appropriate for animals before, during and after surgical or any other potentially painful intervention.
23. Advanced animal husbandry, care and enrichment practices (Module NACWO)
This module delivers a more in-depth knowledge of animal care practices aimed at those taking the responsibility as the named person responsible for the welfare and care of the animals in an establishment.
Learning Outcomes
Trainees should be able to:
Demonstrate a thorough understanding of how animal welfare is maintained in the animal unit
i. Describe how environmental conditions may need to be varied according to the species, age, and life stage or specific care conditions (e.g. peri- operative care, immune-deficient animals, genetically altered strains).
ii. Discuss the possible effects of an uncontrolled environment on animal welfare and experimental results.
iii. Discuss how environmental enrichment is achieved.
iv. Explain how the 3Rs contribute to the continuous improvement of welfare, husbandry and enrichment practices.
Know suitable environmental conditions for laboratory animals and how they are monitored
v. Describe suitable environmental conditions and enrichment for the relevant animal species and how these conditions are monitored.
vi. Be able to use environmental measure equipment, read charts, graphs or tables generated by environmental monitoring equipment and evaluate potential problems.
Explain how the organisation of the animal facility maintains an appropriate health status for the animals and the scientific procedures
vii. Describe suitable routines and housing conditions or laboratory animals housed for different scientific purposes.
viii. Explain how routines and housing conditions may change given specified conditions.
ix. Evaluate the use of barriers in controlling the animals’ health status.
Identify potential disease risks in the animal facility
x. Describe a health-screening programme suitable for the animals in their care.
xi. Discuss potential sources of disease in the animal facility.
xii. Recognise examples of laboratory animal parasites.
xiii. Describe the life cycle of some common laboratory animal disease organisms.
Evaluate methods for minimising the risks from potential disease organisms
xiv. Explain methods for minimising the risk from disease organisms.
xv. Apply suitable disease control methods under specified conditions.
Devise appropriate breeding programmes for laboratory animals given specified condition
xvi. Summarise the basic breeding data of common laboratory animals.
xvii. Describe in detail suitable breeding programmes for named species under specified conditions.
xviii. Select suitable future breeding stock.
Evaluate methods for determining oestrus, checking mating has taken place and confirming pregnancy in a range of laboratory species
xix. List methods for determining oestrus, mating and confirming pregnancy in laboratory animals and evaluate their effectiveness.
Analyse breeding performance
xx. Analyse breeding cards/data to describe the breeding performance of a breeding group.
xxi. Describe any identified problems and suggest appropriate remedial actions.
Explain the use and problems associated with genetically altered animals [where appropriate to the species concerned]
xxii. Explain how genetically altered animals are used for research purposes.
xxiii. Describe the potential problems associated with the use of genetically altered animals.
xxiv. Describe methods for producing genetically altered animals.
Know procedures for the safe and legal transportation of animals
xxv. Identify the key pieces of legislation controlling the transportation of animals.
xxvi. Describe the procedures, equipment, legislative responsibilities and responsible persons in transport of animals.
xxvii. Explain how health status & animal welfare standards are maintained throughout the transport.
Accurately apply the legislation that governs the use of research animals
xxviii. Summarise the key aspects of the legislation protecting laboratory animals.
xxix. Discuss how the legislation controls the use of animals for scientific purposes.
24. Named Veterinary Surgeon (Module NVS)
This module provides basic guidance and information for the veterinarian at the entry Named Veterinary Surgeons (NVS) level. As applies to all veterinarians, NVS’ are expected to develop and enhance their skills through continuing professional development. Other training opportunities could be developed as needed for veterinarians to complete their expertise as NVS, depending on the programme of the establishment (e.g. involvement in training/supervision/assessment; media communication on responsible use of animals in science; species-specific husbandry and veterinary care).
This module focuses on the principles of veterinary management of animal health and welfare for animals maintained, bred and/or used for scientific purposes ensuring that the NVS understands the role of the vet in the research environment according to professional obligations.
There may be elements of training that can be exempted on the basis of a gap-analysis of the individual’s previous educational background and experience.
The objectives of this module are to:
-
cover the basic principles of (rather than species-specific) components of a programme of veterinary care specifically in relation to the care and use of animals for research, which are:
-
Movement of animals and its implications
-
Animal care, health and management
-
Assessment of well-being
-
Recognition and alleviation of pain, suffering and distress
-
Relevance of the choice of animal models
-
Design of procedures and projects
-
Implementation of the 3Rs
-
Use of medicines
-
Surgical and non-surgical interventions
-
Anaesthesia and analgesia
-
Euthanasia
-
Occupational health and safety (zoonosis, allergies, etc.)
-
consider the importance of routine veterinary visits and factors enabling the determination of an adequate frequency for the visits;
-
discuss the balance between animal treatment and the need to ensure valid scientific results;
-
appreciate how to identify ethical issues associated with biomedical research;
-
consider the role of the vet in advising on choice of animal model and model refinement;
-
discuss the role of the vet in advising on the implementation of humane end- points;
-
discuss the principles of management of veterinary communications and decisions;
-
review the opportunities to gather further veterinary information in Laboratory animal medicine and science.
Learning Outcomes
The trainees should be able to:
Legislation
i. Summarise the statutory duties and professional requirements of the NVS.
ii. Compare the roles, responsibilities and interactions of those working within an establishment and explain the legal composition and the role of Animal Welfare and Ethical Review Body.
iii. Explain the role of the veterinarian in directing prescription, order, storage and dispensing and disposal of medicines for animals maintained at authorised establishments and used in procedures.
iv. Describe the role of the NVS in the import and export, and transport of laboratory animals.
v. Outline legislative controls on the creation and use of Genetically Altered Animals.
Ethics, Animal Welfare and the 3Rs
vi. Define the 3Rs principles and provide examples of application of each to a breeding/supplier/user establishment; in particular, discuss the alleviation of pain and potentially lasting harm.
vii. Justify the importance of good animal health and welfare (with regards to the scientific outcomes and societal or moral reason) and recognise the relationship between health and welfare and scientific validity.
viii. Identify sources of information relating to ethics, animal welfare and veterinary information enabling the implementation of the 3Rs.
ix. Explain the need for a culture of care and the individual’s role in contributing to this.
x. Explain how the NVS can contribute to the dissemination of information that will promote understanding of ethical issues, good animal welfare, good science and application of the 3Rs.
xi. Identify the criteria used in making a harm-benefit analysis and be able to apply them.
xii. Identify the role of the NVS in advising on choice of animal model and model refinement.
Animal Care, Health and Management
xiii. Relate the purposes of a routine animal house visit and how to deal with issues arising.
xiv. Outline the preparation required for routine visits.
xv. Formulate the information to be included in health records and reports to the animal care staff and others.
xvi. Summarise basic principles of disease surveillance, prevention and management in laboratory animals and the principles of health monitoring schemes, including information on relevant microorganisms infecting laboratory animals such as their classification, the potential impact on research and animal health, their zoonotic potential, their prevention, diagnosis, treatment and eradication, as well as the clinical appearance, aetiology and pathology of common laboratory animal diseases.
xvii. Outline the requirements for health screening,
xviii. Outline appropriate management and control strategies for biosecurity and disease outbreak in laboratory animals.
xix. Describe an overview of the principles of laboratory animal husbandry, outlining the main principles of cage/enclosure design and construction and the advantages and disadvantages of different types of caging system.
xx. Explain the principles relating to the choice of appropriate environmental conditions and types of environmental enrichment used for laboratory animals.
xxi. Describe the different methods by which the relevant animals are allowed to be killed, the influence different methods can have on scientific outcomes and on how to select the most appropriate method.
xxii. Outline the principles of hygiene/disinfection/sterilisation that apply to the laboratory animal facility including the parameters influencing water quality, how to check for water quality and how to interpret results.
xxiii. Demonstrate an awareness of the main hazards that may be encountered in a laboratory animal facility and the role of the NVS in minimizing the risks.
xxiv. Describe key biological characteristics and features of relevant species and recognize factors that may impact their care or use as laboratory animal.
xxv. Discuss the creation and use of genetically altered animals in research including common types of GA animals and uses in research and different ways to create and evaluate GA animals, as well as how these are designated according to international guidelines for nomenclature.
Anaesthesia, analgesia, surgery
xxvi. NVS.26. Demonstrate adequate knowledge of the management of anaesthesia, analgesia and surgery in the context of animals used for scientific purposes.
xxvii. Relate the factors influencing choice of anaesthetic protocols in different situations.
xxviii. Describe the specific issues arising from experimental surgery and identify the role of the NVS in relation to experimental surgery.
The principles of veterinary communications
xxix. Define strategies for effective communication and explain how these promote animal welfare and good science.
xxx. Review the opportunities to gather further veterinary information in laboratory animal medicine and science.
50. Introduction to the local environment (establishment) for persons taking specific roles under ASPA (Module Local)
This Module provides the necessary understanding of the local structure, key roles and their related tasks as well as appreciation as to how these contribute to the welfare of animals, good science, implementation of the 3Rs, and the establishment of the culture of care.
Learning Outcomes
Trainees should be able to:
i. Discuss how the scope and the spirit of UK legislation and guidelines pertain to the care and use of animals for scientific purposes in your establishment.
ii. Describe the local organogram and your role within it.
iii. Distinguish the roles, responsibilities and interactions of those working under ASPA within the establishment.
iv. Relate the tasks of the Animal Welfare Ethical Review Body and describe your role in contributing to these tasks.
v. Analyse ways in which your role can contribute towards the promotion and implementation and dissemination of the 3Rs at your establishment.
vi. Discuss the importance of proactive approach to, and mechanisms of communication, as a tool to promote the 3Rs and the culture of care.
-
These are the GB Regulator and NI Regulator for ASPA ↩
