The UK Rare Diseases Framework
Published 9 January 2021
Ministerial foreword
The UK Rare Diseases Framework aims to ensure that the lives of people living with rare diseases continue to improve. We will work across the 4 nations of the United Kingdom to ensure that rare disease patients receive the best possible care, building on the commitments in the UK Strategy for Rare Diseases and major advances in the diagnosis and treatment of rare diseases. This framework will develop positive change in how we diagnose, treat and care for patients with a rare disease.
Although rare diseases may be individually rare, they are collectively common, with 1 in 17 people being affected by a rare disease at some point in their lives. In developing this framework, we have put the needs of patients at the forefront. Through the National Conversation on Rare Diseases which informed the framework, the rare diseases community has helped us to identify 4 key priority areas to bring about real change for patients.
We will build upon recent advances in diagnostic technologies, most notably in genomics and data analysis, to help patients receive a final diagnosis faster and reduce the ‘diagnostic odyssey’ faced by so many. We will increase awareness of rare diseases amongst healthcare professionals while ensuring that those involved in patient care are provided with the appropriate education and support will also be critical in improving the quality of care received. As many rare diseases often cut across multiple clinical specialities and care providers, we will also work to remove unnecessary barriers to improve the coordination of care throughout the patient journey and utilise new technology and digital tools, and continue to build on the UK’s world-leading research and life sciences to improve access to innovative treatments and specialist care.
We will ensure the patient voice remains central to the implementation of the framework, building on the close collaboration we already see across the national and international rare disease community. This will include working to understand how the experience of patients and their families throughout the coronavirus (COVID-19) pandemic can shape framework implementation. Importantly, we will work collaboratively to ensure that the needs of rare disease patients are appropriately reflected across wider government policy, including mental health and social care.
We will sustain, improve and foster opportunities for international collaboration; continuing to offer an attractive environment to global researchers, clinicians and pharmaceutical companies, for the benefit of patients. We will support industry and researchers to better understand rare diseases, develop new affordable treatments, and improve the pathway to reaching patients on the frontlines of clinical care.
To facilitate effective implementation of these priorities across all 4 UK nations, each nation will develop and publish a plan detailing the steps they will take to meet the framework aims in a way best suited for their population and health system. The plans will be developed in continued collaboration with the rare disease community as well as across nations, government and organisations.
This framework sets out a community-led vision to build upon existing UK strengths and improve areas of weakness to deliver better health outcomes and improve the lives of those living with rare diseases.
Lord Bethell of Romford
Parliamentary Under Secretary of State for Innovation
Department of Health and Social Care
Robin Swann
Minister for Health
Northern Ireland Executive
Vaughan Gething
Minister for Health and Social Services
Welsh Government
Mairi Gougeon
Minister for Public Health, Sport and Wellbeing
Scottish Government
Introduction
A rare disease is defined as a condition which affects less than 1 in 2,000 people. It is currently estimated that there are over 7,000 rare diseases, with new conditions continually being identified as research advances. While 80% of rare diseases have an identified genetic origin, they can also be caused by disordered immunity, infections, allergies, deterioration of body tissues and organs or disruption to development while in the womb.
Although rare diseases are individually rare they are collectively common, with 1 in 17 people being affected by a rare disease at some point in their lifetime. In the UK this amounts to over 3.5 million people. It is therefore important that the NHS and other services provide this large and diverse patient population with the best possible care.
Rare diseases can be both life-limiting and life-threatening, and disproportionately affect children. 75% of rare diseases affect children and more than 30% of children with a rare disease die before their fifth birthday. Rare disease patients and their families can face a lifetime of complex care and living with a rare disease can also have a huge impact on someone’s education, financial stability, mobility and mental health. It is vitally important that the voice of rare disease patients is included when developing wider policy.
In 2013, the UK government and the 3 devolved administrations published the first UK Strategy for Rare Diseases (the strategy). The strategy represented a step-change in the way we think about rare diseases and respond to the challenges faced every day by rare disease patients and their families. The strategy was widely welcomed by the rare disease community and was a major collaborative milestone, with all 4 nations working together towards common goals to improve the lives of rare disease patients and those who care for them.
Since publication of the strategy, there has been much progress to reflect on with new challenges and opportunities emerging. The coronavirus (COVID-19) pandemic in particular has brought many of the challenges faced by the rare disease community into sharp relief. It is vital that the government continues to support those living with and caring for those with rare diseases, many of whom are especially vulnerable to coronavirus (COVID-19) through the current and any future pandemics. There will be opportunities to learn from the coronavirus (COVID-19) pandemic and the experiences of rare diseases patients and to reflect on this learning in shaping the broad commitments of this new framework and wider national responses to infectious disease outbreaks.
There have been a number of important achievements from across the UK since the strategy was published, leading positive change for the lives of many rare disease patients. Some examples of this brilliant work are outlined in the case studies below.
One of the most significant developments has been the revolution brought about by advances in genomics. Through the delivery of the ground-breaking 100,000 Genomes Project, the UK has been at the forefront of harnessing the potential of cutting-edge genomic science for rare disease patients, particularly when it comes to diagnosis. The 100,000 Genomes Project has helped 1 in 4 patients with an undiagnosed rare diseases receive a diagnosis for the first time.
Pioneering work in genomics is already being integrated into frontline healthcare with the announcement of the NHS Genomic Medicine Service (GMS) in partnership with Genomics England, the Precision Medicine Scotland Innovation Centre and NHS Scotland Laboratory Services Genetics Consortium in Scotland, Genomics Partnership Wales and the development of genomic medicine services in Northern Ireland supported by the GenOCEANIC (Genomics Open Core Engine for Accelerating Northern Ireland Care) IT project.
On 26 September 2020, the government published Genome UK: the future of healthcare, a landmark publication setting out how the genomics community will come together to harness the latest advances in genetic and genomic science, research, and technology for the benefit of patients. This includes the aim to analyse 5 million genomes, including sequencing of 1 million whole genomes from the NHS and UK Biobank, making the NHS the first national healthcare system in the world to offer whole genome sequencing as part of routine care, in particular for adults and children with rare diseases and cancer. The NHS GMS in England will be supported by the National Genomic Informatics Service (NGIS) and a network of 7 NHS Genomic Laboratory Hubs (GLHs) to deliver an integrated system for genomic testing, as well as a National Genomic Test Directory of genomic tests for specified cancers and rare diseases that will be updated annually.
Already NHS England and NHS Improvement is successfully using whole exome sequencing tests to rapidly diagnose rare diseases in critically ill babies and children, a test which has been shown to double the chance of diagnosis. Under the new Wales Infants and childreN’s Genome Service (WINGS), NHS Wales became the first country in the UK to introduce rapid whole genome sequencing to rapidly diagnose rare diseases in critically ill babies and children. The roll-out of whole genome sequencing under the GMS in England will help to diagnose patients with rare diseases more quickly and end the wait for many patients and their families. Where possible, patients will also be given the opportunity to take part in research via the National Genome Research Library which will further develop our understanding of underlying causes of rare diseases and lead to advances in care and treatments.
The UK has also made important strides in the treatments made available for rare disease patients. The Early Access to Medicines Scheme (EAMS) was launched in 2014 to give people across the UK early access to new medicines that do not yet have a marketing authorisation, when there is a clear unmet clinical need. Since its launch, rare diseases patients living with Duchenne muscular dystrophy and haemophilia have benefited from the scheme with earlier access to life-changing treatments. In Scotland, access to new medicines for rare, very rare and end of life conditions has significantly increased through a new ultra-orphan medicines pathway introduced in October 2018, ensuring those with the rarest diseases will get faster access to new treatment where appropriate. The Welsh Government’s £80 million New Treatment Fund, introduced in 2017, has significantly speeded up access to innovative new medicines in Wales, with rare diseases treated by medicines in the Fund including cystic fibrosis, Fabry disease, Gaucher disease and Batten disease. NHS England have also worked with the National Institute for Health and Care Excellence (NICE) to implement 2 managed access agreements (MAA) for patients with spinal muscular atrophy and Batten disease.
At the heart of these developments has been the empowerment of the patient voice. With the establishment of the UK Rare Disease Policy Board and Forum and groups such as the 100,000 Genomes Project Patient Participant Panel, the Rare Diseases Advisory Group, government has been able to work hand in hand with the rare diseases community to deliver policies centred around the patient experience and need. Ongoing collaboration between patients and policy makers will be vital moving forward in order to build on these successes.
UK case studies
Wales
Engagement with the rare disease community: Genomics Cafes across Wales
The first Genomics Cafes were held in June 2019. Organised by Wales Gene Park on behalf of Genomics Partnership Wales, Genomics Cafes are free events for people affected by a rare or genetic condition and held in various locations across Wales. Genomics Cafes are a relaxed and informal opportunity for individuals to meet others, find out more about new advances in genomic medicine in Wales and advise Genomics Partnership Wales how they can be better supported. Genomics Cafes are a networking opportunity, and guest speakers are also present to highlight new initiatives and give attendees the chance to shape activities in genomics. Since the COVID-19 restrictions were introduced in March 2020, these cafes have moved to an online platform with the first virtual cafe taking place in May and monthly thereafter. Attendance has been increasing with very positive feedback from attendees on the content and format of the events.
Co-production: Patient and Public Sounding Board initiative
Genomics Partnership Wales (GPW) and its key partners including NHS Wales, is committed to working in an open and transparent manner with patients and the public with personal or family experience of rare disease. A Patient and Public Sounding Board was established in 2019 and membership includes individuals from across Wales with varied experience of genetic conditions including rare disease. The aim is to use their collective experiences to shape and add value to the work of the Genomics Partnership and future precision medicine services in Wales. Experts from within GPW provide information to frame discussions on different aspects of genomics such as research consent and service developments. This ensures that we remain focused on the needs of our patients and the Welsh population in delivering the aspirations of our Genomics Strategy.
Northern Ireland
The power of Whole Genome Sequencing – a case study from Northern Ireland
Following involvement in the 100,000 Genomes Project, many families have received results from Whole Genome Sequencing that has put an end to their “Diagnostic Odyssey”. This is illustrated by the story of a family from NI, whose names have been changed to John and Kate. They are in their early 30s, healthy, with no family history of note. In Kate’s first pregnancy antenatal scans showed a severe abnormality of the foetus’s brain development and unfortunately Beth died at 9 weeks of age. Genetic and metabolic tests came back normal, and a diagnosis was not possible.
In their second pregnancy, antenatal scans showed almost identical findings to the first; baby Ella was born and died after just 6 weeks. This recurrence meant that an autosomal recessive condition was likely and that any future babies would have a 25% risk of being affected. Despite multiple additional gene tests, as well as consulting with UK and international experts, a diagnosis could not be reached. Without a diagnosis, neither a prenatal diagnostic test, nor preimplantation genetic diagnosis (PGD), could be offered. Kate and John did not feel they would be able to undertake another pregnancy, and this had an enormous psychological effect on them.
Through 100,000 Genomes Project 2 changes in a gene called FLVCR2 in Ella’s DNA were identified. This was subsequently confirmed in Beth’s DNA, and Kate and John were both gene carriers. FLVCR2 causes an extremely rare condition called Fowler syndrome. Establishing the diagnosis made a massive difference to Kate and John, both psychologically and practically. They feel that Beth and Ella did not die for “no reason”; their brief lives had meaning. They have been referred for PGD, which will hopefully allow them to have a healthy unaffected baby in the future.
Scotland
A Scottish family share their experience of the benefits of a targeted analysis of a clinical exome test funded by the Scottish Government Bridge project
We found out from a scan that our daughter was to be born with clubfeet. She has needed multiple casts and operations to bring her feet into the position for walking and running. When she was 2, we asked for further tests as we suspected her feet were a symptom of something more. Skeletal scans showed that bone growth in her arms, hips, thighs, knees and feet was abnormal, but could not pinpoint the underlying cause.
The team at Aberdeen Sick Children’s hospital were brilliant and our surgeon referred us to clinical geneticist Dr Zosia Miedzybrodzka for blood tests. These showed that all 3 of us have a faulty SLC26A gene. This can cause a spectrum of physical problems, including failure to grow and joint pain. Through genetic counselling, we learned about our daughter’s future, the possibility of our son being a carrier and the implications for his future, as well as the options for any future pregnancies.
Most importantly, identification of the faulty gene gives us the opportunity to investigate already available, or emerging, possible treatments. A researcher in Italy has shown that mice without SLC26A grow better on an over-the-counter supplement called NAC. It’s cheap, readily available and is licensed to treat another childhood onset condition, so a trial of treatment has the potential to reverse the damage to our daughter’s bones.
Now, because of the Scottish Genomic programme and in particular getting the right genetic test, we have the opportunity to enhance our daughter’s growth, and maybe reduce the development of painful joints. We are so grateful for this opportunity to share our experience so that others may also benefit.
England
Improving data management for congenital anomalies and rare diseases: NCARDRS
The National Congenital Anomaly and Rare Disease Registration Service (NCARDRS), led by Public Health England, was formed in 2015 and records people with congenital anomalies and rare disease across the whole of England. Data is now collected from 244 healthcare providers across England on over 1400 rare diseases and congenital anomalies. This data is gathered from a range of sources to ensure high ascertainment and population coverage. Automated data feeds have been established from national routine data sources such as Hospital Episode Statistics (HES) and Office of National Statistics (ONS), and robust and reliable data feeds were developed from all the Regional Cytogenetics Laboratories in England. NCARDRS is well-aligned with the Genomic Medicine Service and existing data feeds are mapped against the NHS Genetic Test Directory.
NCARDRS have worked in partnership with clinical and patient groups to establish and validate new data sources and support efforts to improve patient outcomes. One example of these partnerships is the Registration of Complex Rare Diseases - Exemplars in Rheumatology (RECORDER) project. RECORDER is a collaboration between NCARDRS and the Division of Epidemiology and Public Health at the University of Nottingham which aims to identify and register people with rare autoimmune diseases. This improves surveillance and our understanding of these conditions and is vital to support high-quality clinical care.
NCARDRS continue to meet with patient groups to discuss collaborations to expand the number of rare disease collected and/or share data with existing registers. In addition, an interim email-based system was launched to support patient self-reporting and patient groups have piloted the system on a larger scale, which include processes for confirmation of diagnoses by treating clinicians. This system will inform the development of a web-based self-reporting system.
UK Rare Diseases Framework
While the last 7 years has seen significant progress, we recognise there is still work to be done. Many rare disease patients still face a long diagnostic odyssey and there are areas from the previous strategy where progress can be built upon. As individual rare diseases affect small numbers of people who are geographically dispersed, for this population more than any other it is vitally important we work together across the UK and internationally to deliver our aim of improving the lives of those living with rare diseases.
To demonstrate our ongoing commitment to the rare diseases community, and to build on the achievements of the previous strategy, we, the governments of all 4 UK nations have worked together with the rare disease community to design a new UK Rare Diseases Framework. This framework identifies the key priorities for rare diseases going forward and creates a vision for the future which is shared by all 4 UK nations to address health inequalities, improve the quality and availability of care, and improve the lives of people living with rare diseases. The framework will be followed by nation-specific action plans which will provide more detail about the steps each nation will take to achieve the high-level aims set out here.
Our approach
To identify the priorities for the next 5 years, we undertook a programme of engagement to understand the main challenges for those living and working with rare diseases across the UK and how these could be addressed. In October 2019 government launched the National Conversation on Rare Diseases Survey to seek the views of patients, their families, clinicians, researchers and rare diseases patient organisations. The survey ran for 6 weeks and received a remarkable 6,293 responses from the community. The results of the survey can be found at Annex A. Following the survey, an Editorial Board of policy officials, representatives from clinical practice and patient organisations was formed to formally identify and refine the priorities and underpinning themes for the new framework. These ideas were further tested through stakeholder engagement with patient organisations, clinicians, researchers and industry representatives and were put to the UK Rare Disease Policy Board and Rare Diseases Advisory Group for discussion.
4 key priorities have been identified for the next 5 years. Work in these areas will be supported by 5 further underpinning themes which will be vital for delivering results. The UK Rare Diseases Framework is being developed in 2 key phases.
Phase 1
Phase 1 is this document – the UK Rare Diseases Framework. This framework sets out our 4 priorities and 5 underpinning themes for improving the lives of those living with rare diseases across the UK. It sets out a high-level vision for each of these priority areas, shared by all UK nations, providing a strategic direction for the UK’s work on rare diseases across the next 5 years, at which point it will be reviewed.
Phase 2
In Phase 2, each nation will develop an action plan, highlighting steps they will take to meet the aims of the framework in accordance with their own arrangements. Health is a devolved matter and therefore each individual nation has the flexibility to deliver the aims of the framework in the way which is most effective for their population. These action plans will be developed in close collaboration with the rare diseases community through additional engagement and will be reviewed regularly (every 1 to 2 years). Importantly, we will work to reduce health inequalities, including taking steps to meet the needs of people with disabilities where these are different from the needs of other people. In the spirit of continued UK collaboration and to ensure best practice across the UK, each nation will follow a set of core principles when delivering action plans and implementing the framework.
Priorities
4 key priorities have been identified for the next 5 years, which have been highlighted as major challenges by the rare disease community. Progress in these areas will be vital in order to meet our commitment to improve the lives of those living with rare diseases. Here we set out the importance of addressing each of these areas and the high-level outcomes that we will achieve.
Priority 1: helping patients get a final diagnosis faster
For people living with a rare disease, getting the right diagnosis is key to appropriate management of their condition. It can enable greater treatment choice and reproductive decision making and can link individuals to vital information and support through patient organisations.
However, getting the right diagnosis has been consistently highlighted as one of the most significant challenges faced by both the genetic and non-genetic rare disease community. Currently, it can take years to receive a final diagnosis, and some people living with a rare disease may never receive one at all. This ‘diagnostic odyssey’ often involves multiple referrals, inconclusive tests, and sometimes incorrect diagnoses before a final diagnosis is obtained. Without the right diagnosis, it is difficult for patients to receive the best treatment to manage the symptoms of their condition and may miss out on treatments that target their underlying disease.
Screening programmes identify healthy people in the population who are asymptomatic but have an increased risk of developing a disease or condition. The aim is to detect and provide further tests or treatment at an earlier stage with the objective of improving outcomes. The UK National Screening Committee (UK NSC) advises Ministers and the NHS in all 4 countries on potential screening programmes, appraised against its internationally-recognised criteria to consider viability, effectiveness and acceptability. The UK NSC will only recommend a screening programme where this is shown to do more good than harm.
Newborn Blood Spot screening is also offered to all babies at 5 days old to test for 9 serious but rare conditions, including congenital hypothyroidism, sickle cell disease, cystic fibrosis and inherited metabolic diseases. While most babies will have a normal result, a small number of babies will screen positive and will be referred for further tests or treatment as required. The UK NSC run an annual open call for new screening proposals, which can include proposals for additions to the Newborn Blood Spot screening. The UK NSC recently reported that there is clear potential for genomics in the testing for many of the conditions currently included in the blood spot test. Together with our world-leading genomic infrastructure embedded in the NHS, this makes the UK uniquely placed to conduct a high quality, large-scale research programme to determine whether and how sequencing should be implemented for screening in newborns.
Advances in genomics and diagnostic services have allowed us to make significant strides towards shortening the diagnostic odyssey for many rare disease patients. In the future, we expect that new, validated genomics approaches and diagnostic tools will contribute to further improvements in diagnosis and screening, including improved recognition of which patients should undergo advanced genetic testing. This will be further strengthened by the aim set out in Genome UK to make it easier to return findings from research to better inform clinical practice for rare disease patients. It is also critical to support patients with non-genetic rare diseases, and the healthcare professionals treating them, to reach a diagnosis as soon as possible. We must ensure diagnosis rates continue to improve, including utilising advanced diagnostic technologies and tools where possible.
Our vision is for rare disease patients across the UK to get a final diagnosis faster and for research into previously unrecognised conditions to identify new rare diseases and provide new diagnoses.
Priority 2: increasing awareness of rare diseases among healthcare professionals
Awareness of rare diseases among healthcare professionals is also raised by the rare disease community as a key challenge. At the start of a patient’s journey, the first point of call is often their GP. When faced with a patient with unusual or unexplained symptoms, many GPs will not routinely have the knowledge or experience to correctly identify that they are suffering with a rare condition. Similarly, paramedics or A and E staff are not likely to be familiar with correct emergency procedures when a rare disease patient needs emergency hospital care. With over 7,000 rare diseases, no healthcare professional can receive training on every rare disease. It is therefore important that they are aware of rare diseases more widely, alert to considering them, and are provided with the education and resources that can help them recognise rare diseases in patients and be aware of potential specialist treatment needs.
With our ambition to create the most advanced genomic healthcare system in the world, healthcare professionals need to be offered opportunities to continue to improve their understanding of genomics, particularly for its application in diagnosing rare diseases. Additional education and training, specific and relevant to their role, will allow clinicians to effectively recommend appropriate tests and be skilled in the interpretation of results to inform patient care. It is particularly crucial for healthcare professionals to be conscious of rare diseases and supported with the right training and materials to know when to consider whether a patient has a non-genetic rare condition, which often rely on a clinical diagnosis.
Our vision is for healthcare professionals to have an increased awareness of rare diseases and use of genomic testing and digital tools to support quicker diagnosis and better patient care.
Priority 3: better coordination of care
As many rare diseases are chronic and affect multiple body systems, those living with rare disease, whether diagnosed or undiagnosed, face multiple hospital appointments and complex condition management. The management of their condition may require the expertise of multiple different specialists, who could be spread across different hospitals, and individuals may also have regular interaction with other services such as GPs and social care. Parents of children with rare conditions often face a significant care burden, needing time off work to look after their children and take them to appointments, and there can be challenges in ensuring continuity of care when transitioning between paediatric and adult services. Therefore, coordination of care is essential to ensure care is effectively managed, the burden on patients and their carers is minimised, and healthcare professionals are working together to provide the best possible joined up and high-quality care.
There are many potential benefits of using advances in technology and new digital tools to support better care coordination, allowing patients to access services remotely and enabling specialists from across the system to easily share information and discuss tailored care plans. The use of virtual multidisciplinary team meetings, telemedicine, video appointments and alert cards are starting to be implemented across some rare disease services and are all great examples of using technology to support better care coordination. The coronavirus (COVID-19) pandemic has necessitated the use of more virtual appointments, and there is great potential to build on this infrastructure going forward, while considering the implications of digital inequalities.
Our vision is for rare disease patients to experience better coordination of care throughout the patient journey.
Priority 4: improving access to specialist care, treatments and drugs
Due to the nature of rare diseases, providing access to safe, high-quality specialist care and treatments can present challenges including some patients having to travel significant distances to access specialist centres. Patients need to have access to expertise in the treatment and care of their rare disease where available and there are opportunities to develop innovative models of care across the healthcare system so that patients have their care delivered as locally as possible.
Very few rare diseases have established treatments, but where they do exist, they can be life-changing, significantly improving prognosis and/or quality of life. The development of new treatments by pharmaceutical companies is complex, from understanding the basic science of a disease in small populations to the use of novel clinical trial methodologies, which can be reflected in high drug prices. Assessment of, and access to, rare disease medicines for small patient populations can provide challenges to health technology assessment bodies (such as NICE, Scottish Medicines Consortium and the All Wales Medicines Strategy Group) due to limited and uncertain data and challenges for the NHS in finding the balance between the need for treatment for all patients against fixed resources.
The government recognises the global nature of research and innovation and aims to sustain, improve and foster opportunities for international collaboration. It is essential that the UK can offer an environment that will attract substantial investment in high value life science products of the future, and that will attract discovery scientists from global pharmaceutical companies to the UK. This aligns with ambitions to attract and retain global investment, science, research and innovation talent to the UK that are set out in the UK Life Sciences Industrial Strategy published in 2017 and reiterated in its 2020 update.
Ensuring continued development and improved access to specialist expertise, treatments and drugs will require innovation and we must harness opportunities to fully realise our global potential, and to signal our commitment to innovation and collaboration. The UK Research and Development roadmap sets out an ambition for the NHS to take a greater role in seeding and adopting evidence based innovative new treatments, including the work of the EAMS, the Accelerated Access Collaborative (AAC) and the expansion of the Innovative Medicines Fund, which will be subject to a public engagement exercise in the first quarter of 2021. In addition, NICE is reviewing the methods and processes it uses in the development of its technology appraisal and highly specialised technologies recommendations. NICE is being ambitious in the scope and breadth of this review and will continue to welcome contributions from all stakeholders throughout.
The UK is a world leader in science, with a world-class research infrastructure, and an increasing number of innovative drugs and treatments are being developed for rare diseases. It is vital that we continue to collaborate internationally, support researchers and industry to better understand rare diseases, and to develop new affordable treatments, establish their clinical and cost effectiveness, and improve the pathway for rare diseases treatments reaching patients on the frontlines of clinical care.
Our vision is for rare disease patients to have improved access to specialist care, treatments and drugs.
Learning from coronavirus (COVID-19)
Coronavirus (COVID-19) has brought unprecedented challenges to the healthcare system and the rare disease community, which was hit badly due to the impact of the pandemic on diagnostics, treatments, research and displacements within the patient pathway. Many rare disease patients were identified, or were able to self-register for support, through the government shielding programme and it will be important to work with the rare disease community to understand their experiences with coronavirus (COVID-19) and how this can shape the commitments developed under each of the priorities in this framework. There are many links between the issues raised by the rare disease community during the national conversation survey, the priorities in this framework, and the challenges faced during coronavirus (COVID-19). A personal diagnosis and awareness among health care professionals, for example, became important for receiving relevant shielding advice and access to coordinated specialist care became virtual.
Many of the measures brought in due to coronavirus (COVID-19), such as the increased use of technology and virtual appointments, will be beneficial for the rare diseases community in the long-term but we must also learn where we can do better. There will be opportunities to learn from coronavirus (COVID-19) and ensure that the experiences of the rare disease community feed into the implementation of the priorities in this new framework and wider national responses to infectious disease outbreaks.
Underpinning themes
In order to achieve the above outcomes, we recognise that there will need to be a range of supporting activities across the health and social care system. To support these key enablers, 5 underpinning themes have been identified in which work will continue to be progressed to support the priorities of the framework and improve the lives of those living with rare diseases.
Patient voice
Patients, their families and carers, and the organisations that represent them live with the realities of a rare disease every day. They have a great amount of knowledge and lived experiences to share which can be hugely valuable to policy makers and service providers when designing services for rare disease patients. We will continue to put the patient voice at the heart of its decision making and collaborate closely with patients and patient organisations. Therefore, any commitments will be developed in consultation with patient representatives, giving particular consideration to ensuring representation from those whose voices can often go unheard, including patients from black, Asian and minority ethnic (BAME) or disadvantaged backgrounds.
National and international collaboration
Due to the small numbers of patients with individual rare diseases, both national and international collaboration is absolutely essential to support research and patient care, particularly for ultra-rare diseases with only a few patients in the UK. It is only through working together as all 4 nations of the UK, with our European neighbours and international partners that we will be able to undertake robust research and develop the best care for rare disease patients. We are committed to continuing collaboration with the rare diseases community across the world including patients, doctors and industry to share knowledge and ideas to improve outcomes.
Pioneering research
The UK is a global leader in scientific research and innovation, attracting international industry investment with world class life sciences and research infrastructure. Scientific advancements have underpinned many of the recent breakthroughs for rare disease patients and harnessing the potential of cutting-edge science to better understand the underpinning disease mechanisms of rare conditions and enable development of new treatments will be vital to build on this progress. This year the UK government published the UK Research and Development Roadmap outlining plans to revitalise UK research and development and have set out an ambition to increase public funding for research and development, including for rare diseases, to £22 billion per year by 2024/25 and to reach 2.4% of GDP spent on research and development by 2027.
Through the UK’s thriving life sciences sector, research councils, research charities, the National Institute for Health Research in England, the Chief Scientist Office in Scotland, Health and Care Research Wales, and the Public Health Agency’s Health and Social Care Research and Development division in Northern Ireland, we will continue to support and invest in innovative research for rare diseases and ensure that the outcomes are translated into frontline clinical care.
Digital, data and technology
Similar to advances in science, new technologies also have the ability to revolutionise care for patients across the NHS with particular benefit for rare disease patients. For example, telemedicine and video conferencing are already being trialled in parts of the NHS and have the potential to significantly reduce the burden on rare disease patients and their families travelling to different appointments across the country. Digital and online resources, examples of which are already provided by some patient organisations, could also prove invaluable to healthcare professionals as a resource for understanding how best to care for a rare disease patient. When working towards the aims of the framework, we will utilise the benefits technology can bring to rare disease patients and consider how digital tools could be appropriately used to improve efficiency and patient experience and support research.
Effective data interoperability and the ability to easily share and access patient data and registries will also be important for supporting multidisciplinary teams discussing patient care and researchers developing new treatments. Robust rare disease registries, such as NCARDRS in England, the Congenital Anomaly Register and Information Service (CARIS) in Wales and Congenital Anomalies and the Rare Diseases Registration and Information Service for Scotland (CARDRISS) in Scotland are also crucial in supporting researchers, clinicians, patients and service commissioners and can also be key to identify non-genetic rare diseases, which do not benefit from screening programmes or genomic testing.
Wider policy alignment
Improving the lives of those living with rare diseases goes beyond healthcare. Due to the nature of their condition, many rare disease patients require housing adjustments, social care, financial aid, mental health support and special educational needs support. Wider policy development in these areas must reflect the needs of those with rare conditions. Caring for rare conditions also requires specially trained staff, including nurses, care-workers and consultants. With the increasing use of genomics in healthcare, the need for staff trained in genetics and genomics is particularly great. Therefore, workforce and long-term succession planning must also consider the needs of rare disease patients in both health and social care.
As well as addressing the priorities in the UK Rare Diseases Framework, we will work to ensure that the needs of rare disease patients are recognised in wider policy development, whether that be mental health, social care, specialist educational support, or long-term workforce planning. Given the importance of genomics in improving diagnosis for some rare diseases, and to support new research unto potential treatments, the framework will align closely with Genome UK, the government’s strategy for genomic healthcare, as well as other relevant strategies and policies. Policy documents on wider work which will have a positive impact on the support available to rare disease patients, including work to tackle health inequalities, will be signalled to in each nation’s action plan.
Principles of the UK Rare Diseases Framework
All 4 UK nations have signed up to the UK Rare Diseases Framework and have therefore agreed to collaborate to achieve the outcomes set out above. However, as health is a devolved matter, each nation will deliver these aims in a way which is most effective for their respective populations. Therefore, each nation will set out an action plan detailing the steps they will take to meet the aims of the framework within their own arrangements.
In order to ensure cross-border collaboration and maximise the benefits of the framework for the rare diseases community, each nation will follow the below core principles when delivering action plans and implementing the framework. Each nation will:
Deliver the aims of the UK Rare Diseases Framework under each of the priorities and underpinning themes:
Priorities:
- Ensuring patients get the right diagnosis faster
- Increasing awareness of rare diseases among healthcare professionals
- Better coordination of care
- Improving access to specialist care, treatments and drugs
Underpinning themes:
- patient voice
- national and international collaboration
- pioneering research
- digital, data and technology
- wider policy alignment
Consider where action plans can contain specific and measurable commitments under each focus area and regularly review commitments (every 1 to 2 years).
Develop policy commitments with expertise, in close collaboration with patients and others living and working with rare diseases.
Ensure any impacts on health inequalities are considered when developing action plans.
Ensure that the experiences of rare disease patients during the coronavirus (COVID-19) pandemic are reflected in the development of action plans and implementation of framework priorities and themes.
Ensure that the voice of the rare diseases community is recognised across the system and that work as part of the UK Rare Diseases Framework is aligned with other relevant policy development, such as mental health and social care.
Work collaboratively across nations to share knowledge and best practice.
Review progress made towards the aims of the framework every 5 years and update priorities when necessary.
Next steps
Following the publication of the UK Rare Diseases Framework, all 4 nations will develop action plans which will set out how the priorities identified in the framework will be addressed, taking into account the underpinning themes. These action plans will be developed according to the principles of the UK Rare Diseases Framework and we will work closely with the rare diseases community to ensure the commitments developed are actionable and measurable. Where possible, each nation will aim to publish the action plans in 2021.
Annex A: results of the national conversation on rare diseases survey
In July 2019, a national conversation on rare diseases was announced to capture views from the rare diseases community to form the basis of the new rare diseases framework. To initiate the conversation, a national survey was developed to identify the major challenges faced by those living and working with rare diseases. Findings were discussed with the UK Rare Diseases Policy Board and UK Rare Diseases Framework Editorial Board, as well as with NHS England’s Rare Diseases Advisory Group (RDAG).
Engagement on the survey findings, framework priorities and underpinning themes was also undertaken with patients via a UK-wide patient roundtable Chaired by the Deputy Chief Medical Officer for England and through separate engagement with clinicians, industry, regulatory and research bodies. This engagement reinforced that the priorities identified by the survey were reflective of the lived experience, with wide-ranging support for the priorities and themes included in the framework.
National conversation survey
Survey design
The Department of Health and Social Care (DHSC) developed a national survey with the aim of identifying the major challenges faced by people living and working with rare disease. DHSC worked closely with rare disease patient organisation stakeholders, arms-length bodies including NHS England and policy colleagues across government to develop surveys targeting 5 rare disease stakeholder groups:
- People living with a rare disease
- Family members and carers of people living with a rare disease
- Rare disease patient organisations
- Healthcare professionals working with rare disease, including clinical academics and researchers
- Life sciences industry professionals working in drug and therapy development for rare disease
Surveys were designed to take between 5 to 10 minutes to complete and asked respondents some demographic questions including the name and nature of the disease they lived or worked with. Respondents were asked to select their top 3 challenges in order of importance from a list provided. Comparable lists were provided for patient and healthcare professional groups. Healthcare professional respondents were also able to answer the survey from a research perspective if they were involved in rare disease research. Researcher and industry professional respondents were presented with challenges specific to those fields. All respondents were also able to provide free-text responses to highlight any challenges they felt were missing from the list and what they felt could be done to address the challenges they had selected. Lists of challenges and free text questions for each respondent group are shown in Annex B.
Survey questions underwent cognitive testing with respondent groups ahead of the survey launch. The survey was disseminated via extensive engagement with stakeholder groups, who shared the survey with their networks. The survey was open for 6 weeks, closing at the end of November 2019, and was available in the Welsh language.
Approach to analysis
As respondents could select up to 3 challenges, these were ‘weighted’ for analysis, so that challenges selected as the most important received 3 points, as the second most important received 2 points, and the third most important received 1 point. This allowed for identification of the challenges that were most frequently ranked in the top 3. It should also be noted that the issue of coordination of care was separated into 3 separate challenges in the challenge list; coordination of care between different hospital specialities, between primary and secondary care and between health and social care services. For the purpose of analysis, the coordination of care challenges were grouped into one challenge. Analysis was undertaken for the UK as a whole.
Survey responses
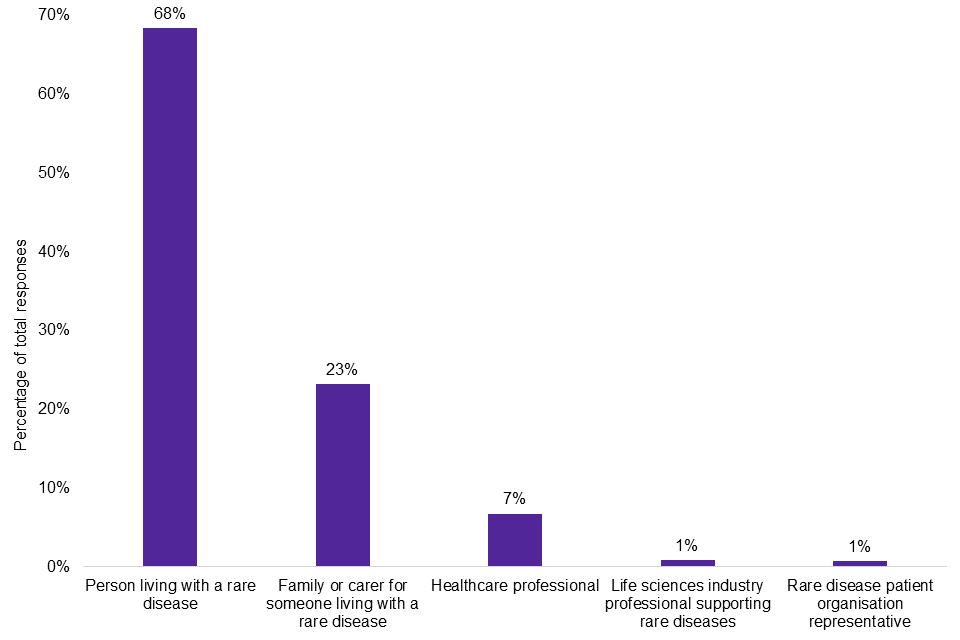
There were 6,293 responses to the survey. As shown in Graph 1, the majority of responses came from individuals living with a rare disease (68%), while their family members and carers were the second highest group (23%).
Graph 1: survey responses by group
Survey analysis
Patient groups and healthcare professionals – top challenges
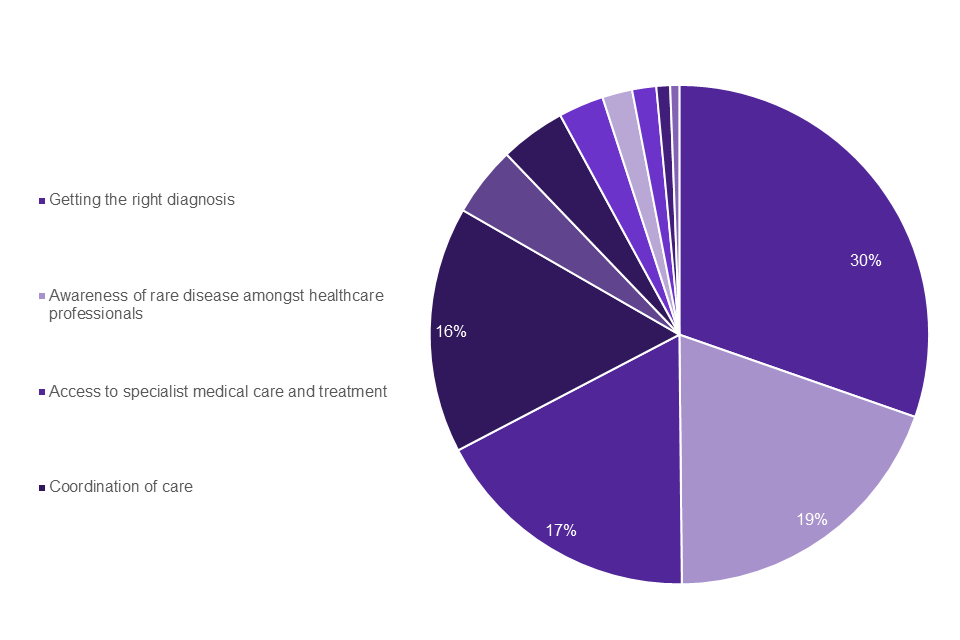
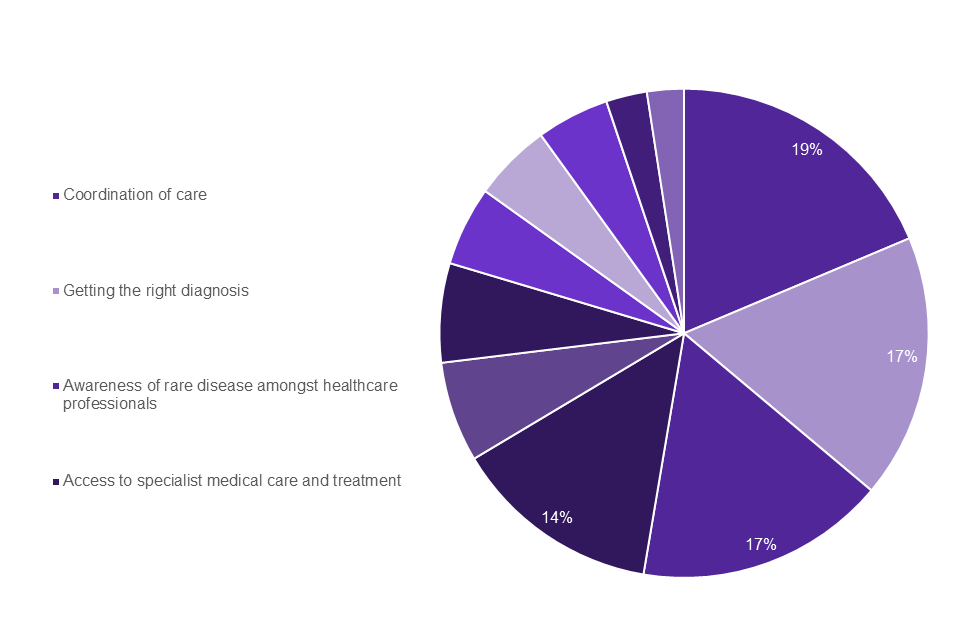
The top 4 weighted challenges identified were the same across the patient groups and healthcare professionals. As shown in Graph 2 the top 4 challenges that appeared most frequently in the top 3 selected by individuals living with a rare disease were: getting the right diagnosis (30%); Awareness amongst healthcare professionals (19%); access to specialist medical care and treatment (17%); coordination of care (16%). The top 4 challenges for family members and carers (Graph 3) were the same: coordination of care (19%); getting the right diagnosis (17%); awareness amongst healthcare professionals (17%); access to specialist medical care and treatment (14%).
Graph 2: people living with a rare disease top challenges
Graph 3: family members and carers top challenges
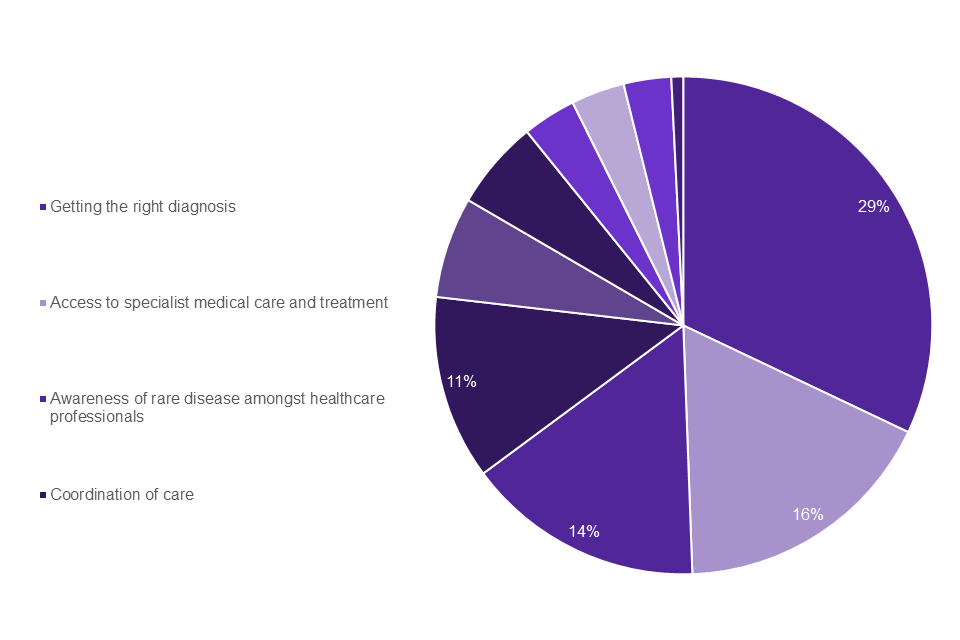
Graph 4 shows that the top 4 challenges identified by individuals living with a rare disease as well as family members and carers were also reflected by patient organisations: getting the right diagnosis (29%); access to specialist medical care and treatment (16%); awareness amongst healthcare professionals (14%); coordination of care (11%).
Graph 4: rare disease patient organisations top challenges
For healthcare professionals, the same top 4 challenges were identified (Graph 5): coordination of Care (18%); diagnosis (18%); awareness amongst healthcare professionals (14%); access to specialist medical care and treatment (11%). Table 1 shows a full breakdown of weighted challenges for all 3 patient groups and healthcare professionals.
Graph 5: healthcare professionals top challenges
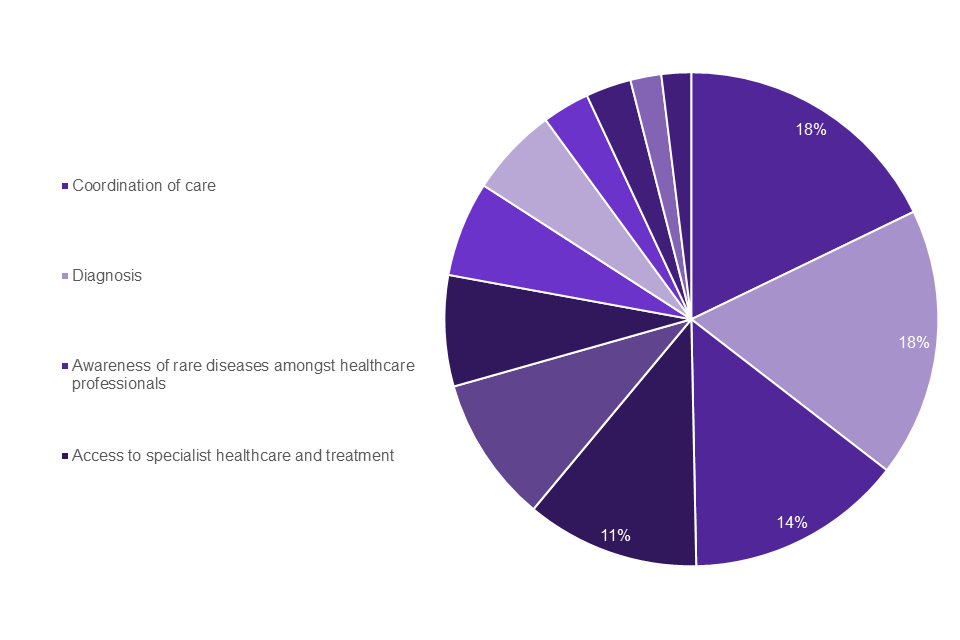
Table 1: percentage of challenges appearing in top 3 selection for the 3 patient groups and healthcare professionals
| Challenge | Patient group: individuals | Patient group: family and carers | Patient group: patient Organisations | Healthcare professionals |
|---|---|---|---|---|
| Getting the right diagnosis | 30% | 17% | 29% | 18% |
| Awareness amongst healthcare professionals | 19% | 17% | 14% | 14% |
| Access to specialist medical care and treatment | 17% | 14% | 16% | 11% |
| Coordination of Care | 16% | 19% | 11% | 18% |
| between GP and hospital care | 9% | 7% | 5% | 8% |
| between different hospital specialists | 6% | 7% | 4% | 7% |
| between health services and social care services | 1% | 4% | 2% | 3% |
| Access to drugs | 5% | 5% | 9% | 7% |
| Availability of information about rare diseases | 4% | 7% | 3% | 3% |
| Availability and provision of wider support for rare disease e.g. social care provision, special education support | 4% | 12% | 8% | 10% |
| Ability to participate in research opportunities, including clinical studies and trials | 2% | 2% | 3% | 3% |
| Opportunities to access medical opinions from international experts | 2% | 3% | 1% | n/a |
| Transition from child to adult services | 1% | 5% | 6% | 6% |
| Training on how to diagnose and treat patients with rare diseases | n/a | n/a | n/a | 6% |
| Opportunities to collaborate with specialists from across the UK | n/a | n/a | n/a | 2% |
| Opportunities to collaborate with international experts | n/a | n/a | n/a | 2% |
Patient groups and healthcare professionals – free text
The majority of responses to the free text question, asking respondents whether there were any challenges missing from the list, stated challenges that had already been listed. However, these responses largely served to reinforce the top challenges selected, with many highlighting awareness amongst healthcare professionals and access to drugs. Notably, a small but significant number of responses to this question from individuals living with a rare disease highlighted mental health support as a missing challenge. This was reflected in the responses from family members and carers, patient organisations and healthcare professionals, which also highlighted mental health support as a missing challenge.
Of those who answered the free text question asking respondents what they felt could be done to address the challenges they had identified in the list provided, improved training and awareness amongst healthcare professionals was consistently highlighted across the 3 patient groups and healthcare professionals.
Quotes from patient groups and healthcare professionals
“To obtain my diagnosis after 3 decades I’ve had to self-advocate and self-diagnose using online research. I’m a medical doctor… No one joins the dots in multi-system involvement even if provided.”
“Living with extremely painful life-altering rare conditions regularly leads to depression, but not enough mental health provision is available apart from pills! Anti-depressants do not normally work well for patients whose depression is caused by extreme chronic pain and disability and so more counselling should be available. I was referred to a ‘general’ neurologist and, after 2 years of being given a different diagnosis every time I saw him, elected to see a different neurologist privately before finally getting an accurate diagnosis and treatment plan.”
“More recently qualified medical professionals seem to have a much better attitude, more empathetic, more willing to listen to the patient and less likely to prejudge. Also, far more knowledgeable in rare conditions and, when not familiar, take the time to read up about them. I believe this is because medical training has improved and so in time I hope the issues regarding attitude and awareness will improve.”
Caveats and considerations
Of the respondents who identified as living with a rare disease, the majority were white females over the age of 45. While this is not representative of the rare disease community as a whole, this is typical of the demographic groups most likely to respond to online surveys. Family members and carers responding to the survey were caring for an equal split of female (49%) and male (50%) patients, the majority (58%) of whom were under the age of 15 years. In addition, responses were received from 48 rare disease patient organisations, who represent the wider rare disease community, including organisations representing primarily BAME individuals living with a rare disease.
It should also be noted that additional analysis was undertaken to ensure that the more ‘common’ rare diseases, which formed a significant proportion of responses from individuals living with a rare disease, did not skew the data for this group. It was found that the top challenges identified by individuals living with a rare disease remained the same regardless of whether respondents were living with a ‘common’ or ‘rare’ rare disease.
Clinical academics and life sciences industry professionals – top challenges
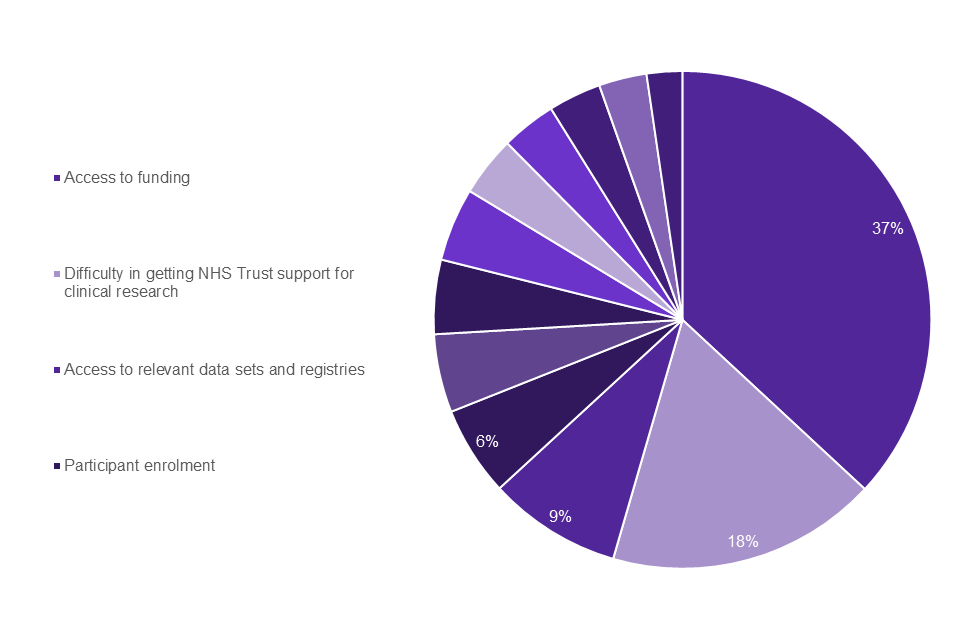
64% of healthcare professionals responding to the survey also stated that they were involved in rare disease research as clinical academics. The top 4 challenges identified by clinical academics, shown in Graph 6, were: access to funding (37%); difficulty in getting NHS trust support for clinical research (18%); access to relevant data sets and registries (9%); participant enrolment in research (6%).
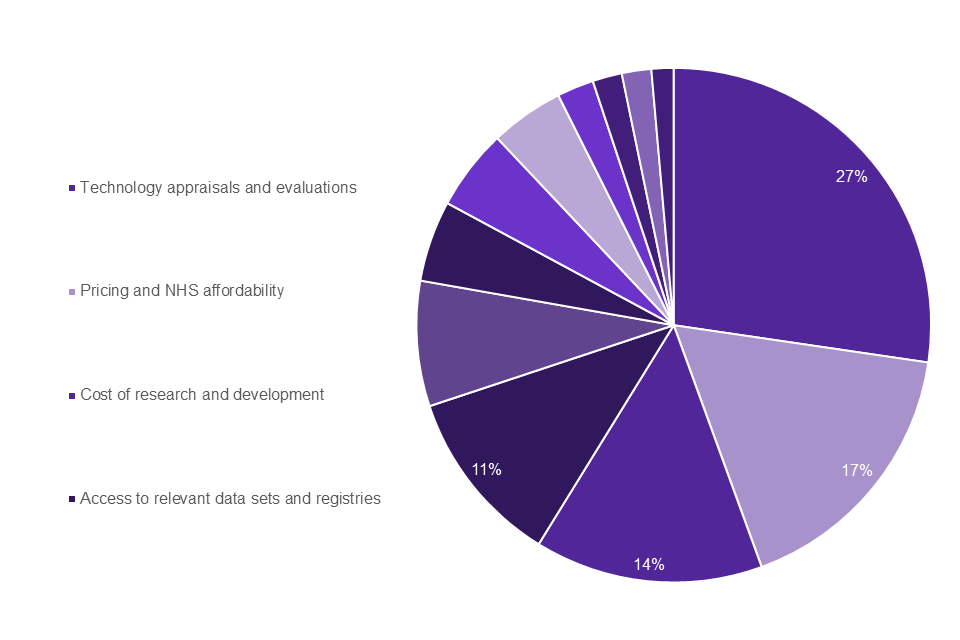
As shown in Graph 7, the top 4 challenges identified by life sciences industry professionals working to develop rare disease treatment and therapies were: technology appraisals and evaluations (27%); pricing and NHS affordability (17%); cost of research and development (14%); access to relevant data sets and registries (11%).
Graph 6: clinical academic top challenges
Graph 7: life sciences professionals top challenges
Clinical academics and life sciences industry professionals – free text
Of the clinical academics who responded to the free text questions, over one third identified having the necessary time and resources to be able to participate in research. This was reflected by similar numbers of responses to the question asking what could be done to address the challenges identified, highlighting that more dedicated research time and administrative support was needed. There were too few responses to the free text questions from life sciences industry professionals to be able to identify any significant themes.
Quotes from clinical academics and industry professionals
“Patients and families with rare diseases should be given the same care and access to therapies as everyone else. This means allowing innovative and sometimes expensive therapies to be made available once deemed safe and effective by the competent Regulatory Authorities.”
“Deliver an approach which encourages innovation and early access, but which maintains the need for an evidence base of benefit derived through real world evidence. Stimulate incremental disease improvements through access granted and allow for a more pragmatic approach to HTA in these disease areas where patient cohorts are often tiny and distinct.”
Annex B: national conversation on rare disease survey challenge lists and free text questions
The sections below list the questions asked in the survey followed by the response options.
Section 1: challenges list for patient group (individuals living with a rare disease, family members and carers, patient organisations).
Question: What do you think are the greatest challenges faced by people living with rare diseases?
- getting the right diagnosis
- awareness of rare disease amongst healthcare professionals
- access to specialist medical care and treatment
- coordination of care between GP and hospital care
- coordination of care between different hospital specialists
- coordination of care between health services and social care services
- access to drugs
- availability of information about rare diseases
- availability and provision of wider support for rare disease e.g. social care provision, special education support
- ability to participate in research opportunities, including clinical studies and trials
- opportunities to access medical opinions from international experts
- transition from child to adult services
Section 2: challenges list for healthcare professionals
Question: As a healthcare professional, what do you think are the greatest challenges you face when caring for patients with a rare disease?
- diagnosis
- access drugs
- access to specialist healthcare and treatment
- coordination of care across primary and secondary care
- coordination of care across secondary care specialties
- coordination of care between health services and social care services
- transition from child to adult services
- training on how to diagnose and treat patients with rare diseases
- awareness of rare diseases amongst healthcare professionals
- availability of relevant patient information
- availability and provision of wider support for rare disease e.g. social care provision, mental health support services, special educational support
- patient access to research opportunities, including clinical studies and trials
- opportunities to collaborate with international experts
- opportunities to collaborate with specialists from across the UK
Section 3: challenges list for clinical academics
Question: What are the greatest challenges you have faced in undertaking rare disease research?
- access to funding
- industry and/or charity engagement
- not working in a research active NHS Trust
- difficulty in getting NHS Trust support for clinical research
- participant enrolment
- patient awareness, engagement and involvement in research
- health care professional awareness and uptake of research evidence
- health care professional promotion of research opportunities to patients
- access to relevant data sets and registries
- opportunities for international collaboration
- opportunities for UK collaboration
- advances in technology
Section 4: challenges list for life sciences industry professionals
Question: What do you think are the greatest challenges faced in developing treatments or interventions for patients with rare diseases?
- cost of research and development
- EMA authorisation process
- technology appraisals and evaluations
- pricing and NHS affordability
- approval for companion diagnostics
- identifying potential clinical trial participant
- recruiting clinical trial participants
- evidence base requirements
- health care professional promotion of research opportunities to patients
- capacity of research sites
- patient and public engagement
- opportunities for international collaboration
- opportunities for UK collaboration
- advances in technology
- access to relevant data sets and registries
Free-text questions
Please give any challenges we missed from the list that you would have included in your top 3 challenges.
What do you think could be done to address the challenges you have selected?
