Vaccination topics
Updated 27 January 2025
1. Diseases targeted by core-vaccination in the UK
The British Small Animals Veterinary Association (BSAVA) recommends that, in the UK, core vaccines for dogs include:
- Canine distemper virus (CDV)
- Canine adenovirus/infectious canine hepatitis (CAV)
- Canine parvovirus (CPV)
- Leptospirosis
The current BSAVA position statement on companion animal vaccination is available on the BSAVA website.
1.1 Canine Distemper Virus (CDV)
Canine distemper is a highly contagious and particularly severe viral infection of dogs and other carnivores including foxes and ferrets. The fox acts as a potential wildlife reservoir of infection. The disease has been recognised for at least two centuries whilst the first modified live canine distemper vaccines were not developed until the 1940s. All the current UK products are live attenuated vaccines. A recognised problem with the development of the first vaccines was post-vaccinal encephalitis causing neurological symptoms. These adverse reactions were seen especially from vaccine strains propagated in canine cell cultures (Carmichael, 1997) and were resolved by manufacturing the vaccines using alternative strains on less reactive cell lines. This form of adverse reaction is extremely rare with modern CDV vaccines (Appel, 1999). Despite being safe for use in dogs, some vaccine strains can remain virulent when used to immunise other species and care must be exercised when using live attenuated vaccines in ferrets, for example. As a modern disease, canine distemper usually occurs in younger dogs and although older animals can be affected, the chronic and classic forms (e.g. hardpad) of the disease is extremely rare.
The virus is unstable in the environment (compare with canine parvovirus (CPV) see 2 below) and thus the main route of infection is via secretions from infected animals. Some dogs may continue to shed the virus for several months if they survive initial infection and this has assisted the spread of the disease in local canine populations. In addition, assuming the epidemiology is similar in the feral fox, wherever the two species come into contact there is a risk of disease transfer. Historically, routine vaccination has successfully controlled the disease in most areas of the UK. Cases of confirmed canine distemper have recently been reported in the UK in unvaccinated puppies and the risk of re-emergence of canine distemper in the absence of vaccination remains (Adamantos and Warman, 2014; Davies 2015).
Clinical signs of infection with virulent canine distemper virus
In general, three distinct clinical syndromes are recognised following canine distemper infection:
-
Mild infection: Affected dogs show only mild clinical signs such as a temperature rise (pyrexia), listlessness and reluctance to eat for a period of time. Such generalised symptoms often go unremarked by the owner.
-
Generalised Infection: This is the severe form of the disease and clinical signs are usually characteristic of the disease. Affected dogs may develop a discharge from the nose and eyes, become depressed and pyrexic. Some diarrhoea and sickness occur commonly. Characteristic chronic (later) signs include the development of thickened, highly keratinised pads on the feet (hence the term “hardpad” used to describe the disease) and in puppies defective enamel development (distemper teeth) on their permanent dentition is seen.
-
Nervous disease: Dogs may develop encephalomyelitis (inflammation of brain and spinal cord) with variable presenting signs that tend to be progressive. Paralysis, seizures, ataxia and muscle spasms may be observed. A reported complication in chronic infections is “old dog encephalitis” in which a dog develops characteristic neurological signs months or years after apparently recovering from infection.
UK authorised vaccines for canine distemper
Vaccines containing live attenuated CDV in combination with other vaccine antigens to protect against a range of other diseases are available. After primary vaccination in puppies between the ages of 6 and 12 weeks, recommended re-vaccination schedules for dogs range from 1-3 years according to the brand of vaccine used. With some UK vaccines, if used as recommended, there is no requirement to re-vaccinate dogs at twelve months of age. The current WSAVA recommendation for puppies is for initial core vaccination at 6-8 weeks of age, then every 2-4 weeks until 16 weeks of age or older and revaccination (booster) at either 6 months or 1 year of age, then not more often than every 3 years (Day et al., 2016).
For animals already infected with the virus, there are no specific medicinal products authorised for therapeutic treatment. Only supportive treatment can be given to dogs showing signs of disease. Administration of a vaccine once infection has occurred will not influence the development of the disease.
1.2 Canine Parvovirus (CPV)
In 1978 a new infectious disease appeared in dogs (canine parvovirus (CPV-2)) and developed into an epizootic affecting the entire canine population. Infection occurred in all ages of dogs and manifested itself in acute forms of infection that are now well recognised. As a new infectious disease in dogs, CPV demonstrated an epidemiology typical of a disease outbreak in a population of highly susceptible animals (i.e. without any previous immunity to the disease). The emergence of parvovirus in dogs was reviewed in 2010 which confirms the origin of CPV-2 remains unproven despite various theories having been put forward to explain the emergence of the virus (Hoelzer & Parrish, 2010). In the intervening years, CPV-2 has undergone further evolution into a number of recognised sub-types or strains. CPV-2a appeared in the late 1970s and CPV-2b in the mid-1980s in the UK. A new variant CPV-2c appeared more recently in 2000 and is thought to be the predominant virus variant in Italy and Germany and present at high rates in Spain and France but to date CPV-2c has not been identified in the UK (Decaro et al., 2011). CPV is endemic in the UK with recorded outbreaks of CPV-2, 2a and 2b (although the original type CVP-2 has now disappeared from the field having been replaced by the other variants). Despite the increasing number of sub-types there is a high degree of cross-protection between virus types and there is an emerging consensus amongst many scientists that current vaccines are protective against all of the currently known circulating strains of CPV (Truyen, 2006; Spibey et al., 2008; Siedek et al., 2011; Decaro and Buonavoglia, 2012; Wilson et al., 2013). The CPV vaccines which are authorised in the UK are derived from either the original CPV-2 virus or CPV-2b isolates. However, a number of manufacturers of these vaccines have supported additional claims regarding protection of their vaccine strains against CPV-2c.
CPV is transmitted by direct contact with infected dogs or indirectly through faecal contamination. The virus is a very stable virus and can survive in the environment for many months. Therefore transmission does not necessarily require close contact between dogs and may be spread by transfer on clothing or other objects.
Clinical signs of infection with virulent canine parvovirus
There are two predominant clinical syndromes associated with CPV infection:
-
Enteric form: Enteritis (inflammation of the small intestine). All ages of dog can be affected but it is most common in younger animals, especially pups. Mortality and morbidity depend on many factors, but death can be very rapid in very susceptible individuals. Clinical signs include vomiting, diarrhoea and marked depression.
-
Cardiac form: Myocarditis (inflammation of the heart muscle). This form of infection tends to occur in very young dogs when the heart muscle (myocardium) is still developing as the virus multiplies in rapidly dividing cells and the dam has no immunity to CPV infection. Clinical signs will vary depending on the severity of infection but range from sudden death to typical signs associated with heart failure.
UK authorised vaccines for canine parvovirus
There are a number of authorised vaccines in the UK to protect against infection and these are listed in the attached table. All CPV vaccines currently authorised on the UK market are live attenuated vaccines and are frequently included with other antigens, in multivalent vaccines, to provide protection against a range of canine diseases. However, vaccines containing just CPV are also available. Details of the vaccination schedules recommended for puppies and older dogs are provided in the Table in Annex I. After primary vaccination in puppies between the ages of 6 and 12 weeks, re-vaccination schedules for dogs range from 1-3 years according to the brand of vaccine used. With some CPV vaccines authorised in the UK there is no requirement to re-vaccinate dogs at twelve months of age. The current WSAVA recommendation for puppies is for initial core vaccination at 6-8 weeks of age, then every 2-4 weeks until 16 weeks of age or older and revaccination (booster) at either 6 months or 1 year of age, then not more often than every 3 years (Day et al., 2016).
In addition to a wide choice of CPV vaccines there is also a specific product that has been authorised for the treatment of an active infection. Virbagen Omega is a novel product that has been authorised throughout Europe to reduce mortality and clinical signs of parvovirus (enteric form) in dogs and can be used in dogs as young as one month of age. Omega interferon is of feline origin and the exact mechanism of action is unknown but is thought to enhance non-specific defences of the body against infection.
1.3 Canine Adenovirus (CAV)
Two closely related canine adenoviruses are associated with disease in dogs:
-
CAV-1 is infectious canine hepatitis (ICH) or “Rubarth’s disease” (named after the researcher who identified the infection in 1947). The cause is canine adenovirus 1 (CAV-1). The virus may also be associated with other forms of the disease including those affecting the respiratory system, kidney, the neonatal foetus and the eye (Appel, 1999).
-
CAV-2 is associated with respiratory disease and, in unvaccinated dogs, is one of the potentially infectious viral agents that is involved in the disease complex called ‘Kennel Cough’.
The first modified live vaccines were derived from CAV-1 isolates in the 1950s but there were a number of unacceptable side effects associated with CAV-1 vaccines including ‘blue eye’ and kidney pathology. In the 1970s CAV-1 vaccines were replaced by CAV-2 based versions which not only provided protection against infection with CAV-2 but from CAV-1 infection, with the added benefit of a much-reduced risk of adverse reactions, including ‘blue eye’.
Urine, faeces or saliva from infected dogs are the main sources of infection of ICH (CAV-1) and dogs who appear to have recovered from the disease may continue to shed virus in their urine for at least six months. CAV-2 is transmitted between dogs via the respiratory route. CAV-1 related disease has become uncommon in regions where routine vaccination is used effectively.
Clinical signs of infection with virulent canine adenovirus
-
CAV-1 infection in unvaccinated dogs may lead to a severe form of acute hepatitis. Less acute forms of the disease are difficult to diagnose, and dogs may present with only a mild pyrexia (increased body temperature) and inappetence. The abdomen is often painful due to the large, inflamed liver. In severe cases, dogs are depressed, may vomit and die suddenly. If the disease progresses to a chronic form the animal is likely to become jaundiced due to extensive liver damage. The characteristic sign of ‘blue eye’ in an infected animal is caused by immune complexes that form in the cornea of the eye. It is usually a transient sign.
-
CAV-2 virus is one of several infectious agents that may be involved in the disease complex commonly known as ‘Kennel Cough’. Infection is associated with laryngotracheitis producing the typical hacking cough. Symptoms are highly variable depending upon the variety of infectious organisms that become involved, with severe infections often leading to secondary bacterial infection of the lungs and trachea producing the pneumonia typical of the Kennel Cough syndrome. CAV-2 is considered to be one of the primary infectious agents that may trigger the onset of a Kennel Cough outbreak.
UK authorised products for canine adenovirus
There are a number of authorised vaccines in the UK to protect against infection and these are listed in the attached table. All CAV vaccines utilise live attenuated virus and are frequently formulated with other virus antigens, for ease of administration, to provide protection against a range of canine diseases. Details of the vaccination schedules recommended for puppies and older dogs are provided in the Table in Annex I. After primary vaccination in puppies between the ages of 6 and 12 weeks, revaccination schedules for dogs range from 1-3 years according to the brand of vaccine used. With some UK vaccines there is no requirement to re-vaccinate dogs at twelve months. The current WSAVA recommendation for puppies is for initial core vaccination at 6-8 weeks of age, then every 2-4 weeks until 16 weeks of age or older and revaccination (booster) at either 6 months or 1 year of age, then not more often than every 3 years (Day et al., 2016).
There are no specific products authorised for the treatment of affected animals and administration of a vaccine once infection has occurred will not influence development of the disease. Only supportive treatment can be given to dogs showing signs of disease.
1.4 Canine Leptospira
Leptospirosis is a serious bacterial infection of world-wide significance. The classification of this particular bacterium has undergone many alterations as there are a large number of antigenic types (called serovars), with some related serovars belonging to different bacterial species (Maele et al., 2008). The two most common leptospires that infect dogs in the UK are L. canicola and L. icterohaemorrhagia and all vaccines in the UK incorporate these serogroups. However, in other countries different serovars have been identified as being the important infections of dogs. These include L. grippotyphosa, L. bratislava and L. pomona, and some vaccines have been tailored to include these serovars. The epidemiology of these leptospires in the UK is largely unknown. However, there have been reports that cases of leptospirosis have been caused by the serovar Bratislava in the south-west (Bovens, 2014; Wilson et al., 2015). The authors report that the majority of the affected dogs had been vaccinated with a bivalent vaccine and were up to date with vaccination when they developed the disease, some of the dogs were unvaccinated and none had received a tetravalent vaccine (Wilson et al., 2015). Leptospire organisms are known to affect several species of mammal including dogs, cattle, pigs, rats, foxes and man being relatively well known.
Leptospires are excreted in the urine of infected animals, symptomless carriers and maintenance hosts for months or throughout life. Rodents, in particular, are recognised carriers of some leptospiral serovars and, therefore, the organism is considered ubiquitous throughout the UK. Leptospires can survive well for many months in water and are considered to be common in waterways and ditches.
Infection can cause serious disease in many species and is a potentially life-threatening disease in humans. Weil’s disease is a severe form of human leptospirosis leading to jaundice following liver damage and kidney failure. Leptospirosis is a notifiable disease. During 2013, 50 confirmed cases of leptospirosis were reported in humans the UK. Twenty-three cases in England and Wales were acquired indigenously, and 24 were acquired through travel (with the largest number of cases returning from Southeast Asia (n=16) including Borneo, Cambodia, Indonesia and Thailand). Four of the indigenous infections were likely to have been acquired through occupational activities (including a fish farmer, a livestock farmer, rubbish recycling site worker and a pet food factory worker). A further 17 cases were likely to have been acquired through recreational or non-occupational exposures to rodent-infected or contaminated environments. There was no risk factor information available for the remaining three cases. Two of the indigenous cases were fatal. Further information may be accessed in the annual UK Zoonoses Reports.
Cases of canine leptospirosis in the previous 12 months were reported in 14.61% of UK veterinary practices surveyed in a recent study (Ball et al., 2014) (13 practices out of 89 which returned questionnaires). The authors note that all but one of the cases occurred in non-vaccinated dogs, highlighting the importance for dogs in the UK to maintain a current vaccination. Over 60% (8/13) of the cases resulted in fatality.
The WSAVA vaccination guidelines suggest that vaccination against leptospirosis should be restricted to geographical areas where a significant risk of exposure has been established or for dogs whose lifestyle places them at risk. The British Small Animal Veterinary Association (BSAVA)recommends that, in the UK, core vaccines for dogs include leptospirosis.
Clinical signs of leptospirosis
The clinical signs of infection with leptospira serovars in dogs are highly variable ranging from mild sub-clinical (hidden) infections to severe peracute (fatal) infections. The clinical signs of acute and subacute infections have been reviewed extensively by van de Maele et al., 2008. Typical signs of infection include vomiting, lethargy, raised temperatures, abdominal pain and diarrhoea. Infection of the liver and the resulting chronic damage may lead to neurological signs and haemorrhaging into mucosa. Of particular concern is the chronic pathological damage to both liver and kidney caused by sub-acute and sub-clinical infection. This leads to both kidney and liver malfunction or failure in middle or old age.
UK authorised vaccines for canine leptospires
Leptospires differ from many of the other disease agents commonly found in canine vaccines because they are bacteria and not viruses. All UK authorised vaccines for use in dogs are products containing killed (inactivated) cultures or purified antigens of some or all of Leptospira interrogans serogroup Canicola, Leptospira interrogans serogroup Icterohaemorrhagiae, L. interrogans serogroup Australis serovar Bratislava and L. kirschneri serogroup Grippotyphosa serovar Dadas and are indicated to reduce infection, clinical signs and death. Inactivated vaccines in particular may contain various adjuvants to enhance the immune response. Leptospirosis vaccines are available for use either as stand-alone vaccinations or in combination with other multivalent vaccines.
As is characteristic for inactivated bacterial vaccines, the primary vaccine course for leptospiral vaccines requires two doses of vaccine administered 2-4 weeks apart to provide protective levels of immunity. In addition, annual re-vaccination is required to maintain protective levels of immunity. Laboratory challenge studies, used to support applications for a UK marketing authorisation, have demonstrated the degree of effectiveness of 12 monthly re-vaccination intervals. Published studies, using authorised products, add further support to the acceptance of a duration of immunity (DOI) of at least 12-14 months for several of the available canine leptospiral vaccines in the UK (Klaasen et al., 2003; Minke et al., 2009).
The WSAVA Guidelines recommend a primary vaccination schedule in puppies of an initial dose at 8 weeks of age or older followed by a second dose 2-4 weeks later and a primary vaccination schedule in adults of two doses given 2-4 weeks apart. An annual revaccination schedule is recommended.
Unlike the viral vaccines, the circulating antibodies in the blood are relatively short-lived following vaccination and, therefore, serological tests do not correlate well with protection and cannot be used reliably to indicate immunity.
2. Non-core UK authorised vaccines
The British Small Animals Veterinary Association (BSAVA) recommends that, in the UK, non-core vaccines for dogs include:
- Bordetella bronchiseptica +/- canine parainfluenza virus (CPi, “Kennel Cough” vaccine). Vaccination should be considered for dogs before kennelling or other situations in which they mix with other dogs (e.g. dog shows, training classes)
- Rabies - legal requirement for dogs travelling abroad / returning to the UK under the Pet Travel Scheme
- Canine Herpes Virus for breeding bitches
- Leishmaniasis before travelling to endemic areas
- Borrelia burgodorferi (Lyme disease) for dogs at high risk of exposure
The current BSAVA position statement on companion animal vaccination is available on the BSAVA website.
2.1 Canine parainfluenza virus (CPi)
This virus is another of the infectious agents considered to be part of the group of organisms associated with the Kennel Cough syndrome (see also Bordetella bronchiseptica below). Modified live virus (MLV) vaccines exist in the UK for the control of infection with CPi and they are available in combination with other canine vaccine antigens to enhance protective immunity against respiratory disease or as part of a multivalent vaccines to protect against a number of core and non-core diseases. Vaccination reduces the severity of the clinical symptoms associated with respiratory infection. CPi vaccine is also capable of reducing the amount of viral shedding during infection and, therefore, reduce the capacity for infection spread. The immunity produced is relatively short-lived, compared to many of the other common canine vaccine components and annual or possibly more frequent vaccination is usually recommended.
2.2 Bordetella bronchiseptica
This is a bacterial, rather than viral, canine respiratory disease. However, unlike the inactivated bacterial leptospiral vaccines this is a live attenuated bacterial vaccine formulated to protect against associated respiratory infection. B. bronchiseptica infection is also one of the infectious agents in tracheobronchitis syndrome or Kennel Cough commonly seen in dogs. These vaccines are designed to be administered intra-nasally to stimulate a rapid, protective mucosal immunity against respiratory disease to prevent infection at the primary site of bacterial invasion. However, the duration of immunity for live attenuated mucosal vaccines is relatively short lived, with only up to one year’s protection being provided following intra-nasal administration. Kennel Cough is a very common, multifactorial, contagious respiratory disease which results in a characteristic hacking cough and the potential for more serious pneumonic damage to the lungs. Generally, the cough can persist for weeks and can be life-threatening in puppies or animals with other medical complications. The agents responsible for Kennel Cough are spread through coughing or aerosol infection and are transmitted through close contact wherever dogs come together. Vaccination reduces the severity of infection and the shedding of infectious particles but does not necessarily prevent infection.
3. Other UK authorised vaccines
3.1 Canine herpes
A purified sub-unit vaccine, containing glycoproteins of canine herpes virus adjuvanted in mineral oil, is available for the active immunisation of bitches to prevent mortality, clinical signs and lesions in puppies resulting from canine herpes virus infection. This is a specialised vaccine designed principally for use in breeding bitches. Puppies acquire the virus in the first few days following birth and infection results in serious illness and, in many cases, early neonatal death. The virus is one of the infectious agents believed to contribute to the condition of young puppies commonly called fading puppy syndrome. The primary course of vaccination consists of two doses of vaccine, the first administered during the bitch’s season (or 7–10 days after the presumed date of mating) and the second injection is given within two weeks of the anticipated whelping date. The intent of vaccination is to generate antibodies in the early lactation of the bitch (colostrum) to provide the puppies with a maternally derived immunity to infection. Re-vaccination during subsequent pregnancies with the complete primary vaccination schedule is recommended. Canine herpes vaccines are not listed in the WSAVA Guidelines and are commonly used by dog breeders in bitches where the herpes virus is suspected of being the cause of early neonatal death. Herpes virus is transmitted between dogs chiefly in saliva and infection in the adult dog is associated with very mild or no clinical signs.
3.2 Canine coronavirus (canine enteric coronavirus) (CECOV)
Authorised multivalent vaccines are available in the UK incorporating inactivated antigens intended to generate immunity to the enteric form of canine coronavirus infection. CECoV, as a primary agent, is associated with symptoms of mild enteritis, although more virulent strains have been identified. The virus can exacerbate infection associated with other enteric pathogens, especially canine parvovirus in young dogs. Vaccination reduces infection with wild-type virus, of which there are two recognised genotypes (CECoV-I and II, Decaro et al., 2009). Canine coronavirus vaccines are not recommended by the WSAVA Guidelines. A random survey of dogs presenting at veterinary practices in the UK revealed a prevalence of CECoV of nearly 3% (Stavisky et al., 2010).
3.3 Rabies virus
Inactivated rabies vaccines are used in dogs primarily to meet the requirements of The Pet Travel Scheme (PETS). Rabies is a notifiable disease and a serious zoonosis (risk to human health). The UK is free of disease as a result of an historical eradication strategy and stringent import controls on animals entering the UK. PETS is an official system that permits pet dogs, cats, and ferrets from certain countries to enter the UK without quarantine as long as they meet the requirements of the PETS. It allows UK dog owners to travel to specified countries with their dogs and return with them to the UK. Under the scheme requirements, animals must be micro-chipped and vaccinated against rabies virus. Primary vaccination is usually a single dose providing a duration of immunity of up to 2-3 years. As a point of information, to permit effective epidemiological surveillance, rabies vaccines authorised for use in wildlife, are prohibited for use in the UK, despite being authorised by the EU.
Rabies vaccines are considered a core vaccine by the WSAVA guidelines where vaccination is required by statute or in areas where the disease is endemic. However, in the UK they are only required for dogs travelling outside of the country and, therefore, rabies vaccination is not considered a core vaccine for the UK.
4. Epidemiological comments
4.1 Related to the use of core and non-core canine vaccines and the control of the associated diseases
There is limited information available on the incidence and prevalence of canine diseases driving the use of core and non-core vaccines of companion animals. However, the likelihood of wildlife reservoirs of infection, international travel, anecdotal evidence and veterinary knowledge strongly suggest that cases of these diseases do still occur, and they cannot be expected to be eliminated despite many years of vaccination. For example, the presence of Infectious Canine Hepatitis (CAV-1 infection) in red foxes in England was described by Thompson et al. (2010) and the wild animal reservoir can be expected to at least maintain the prevalence of CDV, CPV and leptospirosis. For the non-core diseases, several publications have shown a continuing high incidence of Kennel Cough particularly amongst dogs kept in or exposed to a kennel environment (Chalker et al., 2003; Erles et al., 2003; Erles et al., 2004).
An ongoing pharmaceutical industry funded project, CICADA (Computer-based investigation of Companion Animal Disease Awareness), aims to collate information submitted by veterinary practices on numbers of both confirmed and unconfirmed (suspected) reports of major infectious disease. During the nine months leading up to February 2014 veterinary practices participating in this survey reported 974 cases of Kennel Cough, 79 cases of canine parvovirus, 27 cases of leptospirosis, 2 cases of infectious canine hepatitis and 1 case of canine distemper. However, caution is needed in interpreting these data or using it to make decisions regarding vaccination due to the limitations in the way the data is collected and collated. For example, the veterinary practices which are participating may not be representative of all practices or regions in the UK as it is a voluntary reporting system. However, from these data it can be concluded that these diseases do still occur and still pose a potential threat to inadequately vaccinated dogs.
A Veterinary Products Committee (VPC) working group recommended in 2002 that manufacturers and other organisations should be encouraged to obtain data on disease incidence and duration of immunity in the field. A number of initiatives should eventually improve the information available to veterinary surgeons and the pet owning public. An ongoing collaborative project between veterinary schools, veterinary general practices and other partners in the UK involves the development of standard nomenclature and data coding in electronic patient records. This will enable the linking together of data from many different veterinary practices throughout the UK for the purposes of disease surveillance in companion animals. A second ongoing project, SAVSNET (Small Animal Veterinary Surveillance Network), aims to collate laboratory results and to survey data from veterinary practices throughout the UK using the concept of ‘one practice’ management software. These projects should enable researchers to estimate disease prevalence in veterinary practice using client populations of companion animals.
However, it is important to be aware that this will only represent the population of dogs visiting veterinary practices, which in itself is a biased population. This population sample has a much higher probability of having been vaccinated regularly and, therefore, may not truly represent the general dog population which would include those dogs which have never visited a veterinary practice and have never been vaccinated. An under-representation of those dogs not visiting a veterinary surgeon may introduce bias into the results of the study, possibly making the prevalence of these diseases appear less frequent.
Epidemiological field studies to determine duration of immunity (DOI) can be carried out prospectively or retrospectively, each method having advantages and disadvantages. Prospective studies would need to recruit a large number of dogs and continue following each of these dogs for several years. Therefore, such studies are likely to be very expensive and there is a high potential of ‘loss to follow-up’ (e.g. owners moving house, dropping out of the study). Retrospective studies, where dogs could be recruited into the study based on the number of years since their last vaccination, are less resource intensive but rely more heavily on the use of questionnaires and accurate records. In both study types it would be important to look at other factors which are likely to confound DOI results. One significant factor, for example, would be the incidence (high or low) of a field challenge with an infectious organism. If a dog has been vaccinated and is then exposed to field challenge this is likely to boost the level of immunity and result in a perceived longer DOI. Therefore, in both study types, it would be necessary to obtain information from the dog owners about the lifestyle of their dogs in order to try to evaluate the effect of confounding factors. Epidemiological studies therefore can be of use in risk assessment in clinical circumstances but there are significant challenges to extrapolating these findings to the regulatory procedures to determine the DOI of specific vaccines.
There are also significant differences between individual animal vaccination programmes and population immunity. Both influence the risk of exposure to infection of the individual and thus the likelihood of disease. In its simplest form a population of vaccinated animals restricts the spread of an infectious disease and reduces the risk of exposure to infection. This benefits both vaccinated and unvaccinated animals. A vaccinated animal in a susceptible population may, however, be immune but may find its immunity overwhelmed by a challenge from a significant outbreak of the disease.
Immunity therefore does not necessarily signify a freedom from disease. With some vaccines the ambition is simply to reduce the severity of the disease (e.g. Kennel Cough vaccine) rather than to protect fully the animal from the symptoms of the disease. In some cases the ambition is to reduce the dissemination of the disease-causing organism.
5. Overview of requirements for regulation
All canine vaccines on the UK market must meet the standards set by The Veterinary Medicines Regulations 2013. This provides assurance that vaccine products have been manufactured to an acceptable quality, can be used safely both from the animal and owner perspective and achieve the level of protection claimed by the manufacturer. Legislation sets stringent technical standards for the manufacture and quality control of all veterinary vaccines.
6. Additional guidance
The Pharmacopoeias lay down minimum standards for specific veterinary vaccines and provide guidance on production, quality control tests and studies to demonstrate safety and efficacy.
Each and every authorised vaccine in the UK must be supported by its own specific data package submitted by the manufacturer. Each vaccine strain of a particular manufacturer is considered to have unique biological properties and, therefore, must have its own corresponding quality, safety and efficacy package to support the authorisation. The WSAVA guidelines appear to assume common biological properties for certain groups of canine vaccines and could only be used to support data packages if applicants can demonstrate relevance to their particular vaccine product. An assumption that all vaccines are the same would ignore basic principles of immunology and vaccinology.
The rigorous controls applied to the manufacture of veterinary vaccine ensure that any potential risks to the animal, their owners and the environment are minimised. Veterinary vaccines are biological products that pose inherent risks owing to the source of starting material used for the manufacture of the vaccine. To illustrate this risk, vaccines are manufactured from seed materials in most cases originally derived from diseased animals. The seed materials are grown in cultures using a variety of biological materials, often sourced from animal tissues and, therefore, the risks of a vaccine containing an extraneous agent (unwelcome contaminant) are high. To prevent this, the manufacture of products according to the principles of Good Manufacturing Practice (GMP), the application of quality control tests on every batch and pre-authorisation tests on seed materials are the principal controls to ensure the quality of authorised veterinary vaccines and the exclusion of extraneous agents.
The risks for killed/inactivated products are much reduced compared to the modified live virus (MLV) vaccines. Principally this is because, during the manufacture of killed vaccines, the process of inactivation (a process using a chemical added to destroy the microorganism without affecting its ability to stimulate the immune system) should also destroy the majority of potential contaminants. Published reports of contaminated vaccines (Roth, 1999) causing disease in animals indicate that risks can be minimised but not eliminated. Continual improvement in the manufacture and controls applied to veterinary vaccines is the aim of both the industry and regulators.
Laboratory and field safety studies must be conducted for all vaccines before they are marketed. Laboratory studies are designed to demonstrate the safety of the vaccine in the youngest puppy intended for vaccination or any special categories of dog that are to be included in the authorisation, such as pregnant bitches. Such tests use a single dose, an overdose and repeated doses to cover all potential safety concerns when administered by the veterinary surgeon in the field. The studies are designed to evaluate the local and systemic reactions following administration of the vaccine to the most sensitive groups. Any observed reactions are described on the publicly available Summary of Product Characteristics (SPC) available on our Product Information Database.
The most common are local injection swellings which resolve in a few days. More severe reactions are possible but extremely rare. Nevertheless, all potential adverse reactions of this type are taken into account when deciding whether to authorise the vaccine.
For MLV vaccines there are additional studies to investigate the possibility of the vaccine organism reverting to virulence (and producing a clinical infection) and any potential to disseminate within the animal or, more seriously, to spread into the environment and thus prove a risk to other animals and humans. Once completed satisfactorily the laboratory studies are followed by field studies to confirm the safety profile established during the laboratory studies and in addition, using a range of breeds and much larger numbers of animals. Specific safety studies may be required under the guidance within the European Pharmacopoeia. One example would be the requirement to demonstrate any immunosuppressive property (depression of the natural immune system) of a particular vaccine strain. This is a natural property of some pathogenic strains of viruses. There are specific European Pharmacopeial monographs for CDV, CAV, CPV, CPi and canine leptospirosis that establish minimum standards for quality, safety, potency and immunogenicity.
Laboratory and field efficacy studies are also performed to demonstrate the onset and duration of immunity of the vaccine. To establish the onset of immunity, puppies at the minimum age for vaccination receive a primary vaccination course and the time interval to develop a protective immune response is established by either viral challenge or serology. This period is usually one to two weeks but can occur more rapidly for mucosal (e.g. nasal) vaccines such as those authorised for preventing Bordetella infections (Kennel Cough). Maternal antibodies found in young puppies may interfere with the immune response to a vaccine and manufacturers must demonstrate the ability of their vaccine strains to overcome the neutralising effects of these antibodies. As a result of these studies the effects of maternally derived antibodies (MDA) on the recommended vaccination schedule will be evaluated to determine the age by which the vaccine can be expected to be effective in pups. Vaccination schedules are designed to minimise the risk of puppies acquiring a clinical infection, taking account of MDA and the risk of acquiring the disease from the environment. Primary vaccination schedules in puppies are usually completed between 10-12 weeks of age, although this may be delayed where MDA levels are high. The WSAVA Guidelines offer general advice on this, and recommendations made there can be applied, taking account of local epidemiological information, the vaccine used and veterinary advice.
The duration of immunity established through research is a minimum period of duration and the actual duration of immunity may be much longer. However to establish the maximum duration of immunity that might be applied across the wide range of husbandry systems would require animals to be isolated for very long periods of time and this raises considerable concerns about animal welfare, veterinary ethics and cost. Any scientific research should only be conducted where the benefit to the wider population of animals can be justified. Therefore, extending any laboratory studies to derive DOI beyond 3 years poses significant questions as to the value of such studies and the benefit they would offer to the wider population of pet animals.
7. Public information and transparency
Since October 2005 regulatory authorities have been required to prepare and publish Public Assessment Reports (UKPARs) which provide information on the manufacture of the vaccine and the scientific studies that were assessed to support the safety and effectiveness of the product.
For UK authorised vaccines public assessment reports (UKPARs) are available for all canine vaccines authorised after October 2005 on the VMD’s Product Information Database.
8. Comparison with WSAVA guidelines
The Summary of Product Characteristics (SPC), a publicly available document produced during the authorisation of a veterinary vaccine, provides guidance on the authorised use of the product and includes information which may help the veterinary surgeon when prescribing the product.
SPCs for UK authorised products are available on the VMD’s Product Information Database.
The WSAVA vaccination guidelines were developed by a panel of veterinary experts and are based on experience, expert opinions and scientific data that may or may not have been published. They are general guidelines intended for world-wide interest and are not written with reference to data packages for specific vaccines.
These guidelines provide useful guidance to clinicians but should be read in conjunction with the specific product SPC. The WSAVA guidelines are, therefore, supplementary information to UK authorised vaccine SPCs and for the most part are complementary.
The work that supports the recommendations in the WSAVA guidelines has been reviewed by and updated in the recent version in the Journal of Small Animal Practice 2024; guidelines for the vaccination of dogs and cats – compiled by the Vaccination Guidelines Group (VGG) of the World Small Animal Veterinary Association.
A critical issue is the duration of immunity of core MLV vaccines. The recommendations for use of the MLV components of most UK authorised products are in general accord with the re-vaccination schedules recommended in the WSAVA guidelines. However, there are instances where a conflict might be perceived to exist in terms of vaccination schedule recommendations.
For example:
-
For primary vaccination of puppies the WSAVA recommend up to three doses of vaccine. The majority of UK vaccines recommend two vaccinations for the puppy.
-
The WSAVA guidelines recommend a booster at either 6 months or 1 year of age after completing the primary vaccination for all core-components, defined as; canine distemper, parvovirus and adenovirus, followed by a booster vaccination frequency of not more than every three years. For UK authorised vaccines this is only included on some SPCs where the data submitted indicated that a first annual booster is necessary; for other products, the interval recommended between booster vaccinations following the primary course of vaccination is 3 years. However, whatever the SPC states, a veterinary surgeon should take account of the recommendations and warnings on the SPC alongside any specific risk factors for the individual animal when devising the optimum vaccination schedule for the young adult. This may follow either the recommendation for a first full yearly booster, as may be specified in the SPC of some products and WSAVA guidelines, or this may prove to be unnecessary.
-
The WSAVA guidelines state that, because of the presence of maternally derived antibodies, the immune response to vaccination before 12 weeks of age is likely to be weak and suggest that, where animals are vaccinated so that the primary course of vaccination is finished by 10 weeks of age, caution is required in terms of the exposure of these animals to other animals. There are a number of UK authorised products with SPCs which specify a primary course of vaccination that ends at a minimum age of 10 weeks. These claims will have been supported by data demonstrating the ability of the vaccine to overcome the inhibitory effects of maternally derived antibodies. However, should a veterinary surgeon choose to observe the advice in the WSAVA guidance, the SPC does not preclude the administration of the vaccine to puppies older than the minimum age stated on the SPC. There are appropriate warnings concerning the need to take into account the effect of the presence of maternally derived antibodies (MDA), especially if the levels are expected to be high. For some products there are recommendations to delay starting the vaccination course if high levels of MDA are expected. All of these statements will be supported by suitable data.
-
The WSAVA guidelines recommend that the primary vaccination schedule consists of three vaccinations finishing at 14-16 weeks of age. SPCs for vaccines in the UK usually recommend two vaccinations. Data will have been presented to the VMD to demonstrate the effectiveness of two vaccinations in both sero-negative puppies and those with levels of maternally derived antibodies that reflect the levels expected in the field. Veterinary surgeons can, however, make their own risk assessment and decide to administer a third vaccination where they consider this necessary.
-
The WSAVA guidelines do not recommend the use of canine coronavirus (CCV) vaccines as the prevalence of clinical cases of confirmed CCV disease does not justify vaccination. For authorised canine vaccines containing either CCV or a strain of feline corona virus (FCV) the benefit/risk assessment was considered favourable during assessment of the product.
-
The WSAVA guidelines recommend annual re-vaccination intervals for canine leptospira vaccines. The UK authorised products for leptospira recommend annual revaccination and this reduced frequency of vaccination is justified by the data supporting the vaccine authorisation.
In the UK, most veterinary surgeons will use vaccination schedules based on the authorised SPCs. However, some account of the WSAVA guidelines may be taken.
Earlier WSAVA guidelines included claims for durations of immunity of nine years or longer for CPV-2, CAV-2 and CDV MLV vaccines. Much of the work to support these extended claims, beyond the 3 years established by most manufacturers, has been reviewed (Schultz, 2006; 2010). He has reported studies assessing the minimum duration of vaccinal immunity in more than 1,000 dogs vaccinated with products from all the major US veterinary biological companies, using either serology or challenge in selected groups. For CDV, CPV and CAV, antibodies are suggested to be well correlated with protection, with the mere presence of antibodies in actively immune dogs demonstrating protective immunity.
These studies were conducted in dogs kept in their natural, as well as virus-free, environments. The longest period of time that antibodies have been shown to persist in natural environments is 14 years for CDV, 14 years to CAV-1 and 10 years for CPV-2. In environments free from virus, protection from challenge has been demonstrated 9 years post-vaccination with CDV and CPV-2 with antibodies persisting for this period at levels not significantly below titres following primary vaccination. The minimum established DOI for CAV-2 vaccines appears to be 9 years based on serology and 7 years with challenge. For CDV there appears to be a suggested strain variation with Rockborn or Snyder Hill strains having extended DOIs compared to vaccines produced using Onderstepoort strains.
Confounding these observations and claims is the lack of detail reported in the primary scientific literature for these studies and as a result a thorough scientific analysis of the data is not possible without the provision of the raw data. For example, it is not possible to ascertain the number or age of the puppies at the time of vaccination, their immunological status or the vaccination protocol and products administered. The serological methods are not described, nor are the clinical signs or the detailed observations following challenge. Whilst the evidence as reported is persuasive, much of the data would not meet the usual standards of scientific scrutiny reserved for peer reviewed primary literature.
It is, however, recognised that there is an increasing body of scientific literature and opinion that suggests the DOIs of the core vaccines (as defined by the WSAVA guidelines) for dogs may be considerably longer than the authorised claims for existing vaccines on the EU market. Nevertheless regulatory and scientific requirements restrict extrapolation of generic claims, like the data discussed, to specific products. Despite this conflict, veterinary surgeons in the UK may take account of recommendations in the WSAVA guidelines and scientific journals when devising optimum vaccination schedules for their clients’ pets, after making an individual benefit:risk assessment of the impact. However a shift to a revised vaccination regime may potentially prove ineffective in maintaining the control of any of the diseases mentioned.
9. Safety and failures
9.1 VMD’s Pharmacovigilance system
Veterinary medicines legislation is designed to ensure that decisions on whether a product is safe and effective for use in either food producing, or companion animals are based on robust science. Changes in veterinary medicines legislation have placed greater emphasis on pharmacovigilance which has been defined by the World Health Organisation as the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any medicine-related problem.
Since the relationship between an adverse effect and the use of an individual veterinary medicine may be largely circumstantial, the basis of veterinary pharmacovigilance is the identification of reporting trends. One of the aims is the detection of new safety signals in relation to the use of a veterinary medicine. A signal consists of information about a possible new causal association, or a new aspect of a known association between an adverse event and a veterinary medicine. The regular review and analysis of adverse events in a pre-defined time period for one specific product in a particular species might lead to the identification of potential signals when, for example:
- an increase in the number of adverse events in a short period is observed
- an increase in the frequency of a particular clinical sign is recorded, compared with the expected frequency for that sign
- a clinical sign not previously associated with the product is reported
Pharmacovigilance databases are designed for surveillance not for detailed epidemiological investigation. Acute reactions which develop over a short period are more likely to be detected than chronic problems taking months or even years to become apparent. In the latter case there could be a number of confounding factors making it difficult to conclusively establish a causal relationship between the veterinary medicine and an adverse effect. Specific epidemiological studies may be necessary to answer certain questions.
For a veterinary medicine to be considered safe, its expected benefits should be markedly greater than any associated risks of harmful reactions. All veterinary medicines can cause reactions; however, it is important to note that most animals treated with veterinary medicines suffer no observable serious side effects. Idiosyncratic drug reactions occur with veterinary medicines as they do with human medicines. Such reactions cannot be explained by the known mechanisms of action of the agent involved and develop unpredictably in susceptible animals only. Such reactions are generally thought to account for up to 10% of all adverse reactions in human medicine. They are difficult to study because of their sporadic nature and the lack of valid models. While the mechanism of most idiosyncratic drug reactions is thought to be immune mediated toxicity, they are not confined to immunological products.
The fact that both immediate and longer-term adverse events may occur make the benefits of vaccination for a healthy animal more difficult to assess especially as the prevalence of a disease against which a vaccine will protect may not be measurable with any degree of certainty. In such cases a decision regarding vaccination of a healthy dog is largely a matter of judgement on the part of the owner following advice from their veterinary surgeon. It is acknowledged that more information is needed on the prevalence of canine diseases in the UK to enable veterinary surgeons and their clients to make more meaningful benefit/risk assessments on whether to vaccinate an individual animal.
Data on adverse reactions reported to the VMD involving different types of veterinary medicines are published in the Pharmacovigilance Annual Review on gov.uk and occasional articles and letters on specific topics of interest or concern.
Over 187 million doses of the vaccines currently available on the UK market have been sold for use in dogs between 2002 and October 2022, and during this period, there were 30,097 incidents of adverse events reported that were assessed to be either probably or possibly related to the use of a vaccine. It is acknowledged that any pharmacovigilance system is primarily reactive, and under-reporting is an inevitable feature. However, under-reporting will apply equally to all products and, given that changes in the incidence rates of adverse events are the useful indicators of issues which need to be investigated, it is the clinical detail in the reports and the trends and patterns of adverse events that are far more important tools in the science of pharmacovigilance. Therefore under-reporting is not a significant issue. Nevertheless, the VMD devotes considerable resource each year to encourage reporting of adverse events.
Further information on the VMD’s Pharmacovigilance system can be found on Pharmacovigilance of Veterinary Medicinal Products in Great Britain.
9.2 Analysis of suspected adverse event reports
Between 2002 and October 2022, over 187 million doses of dog vaccines currently authorised have been sold in the UK and 41,644 incidents of adverse event have been reported following the use of authorised dog vaccines. This figure includes reports with insufficient information to be classified as either probably or possibly related to product use, but association with product use cannot be discounted. This represents a rate of 22.2 incidents per 100,000 doses (0.022%).
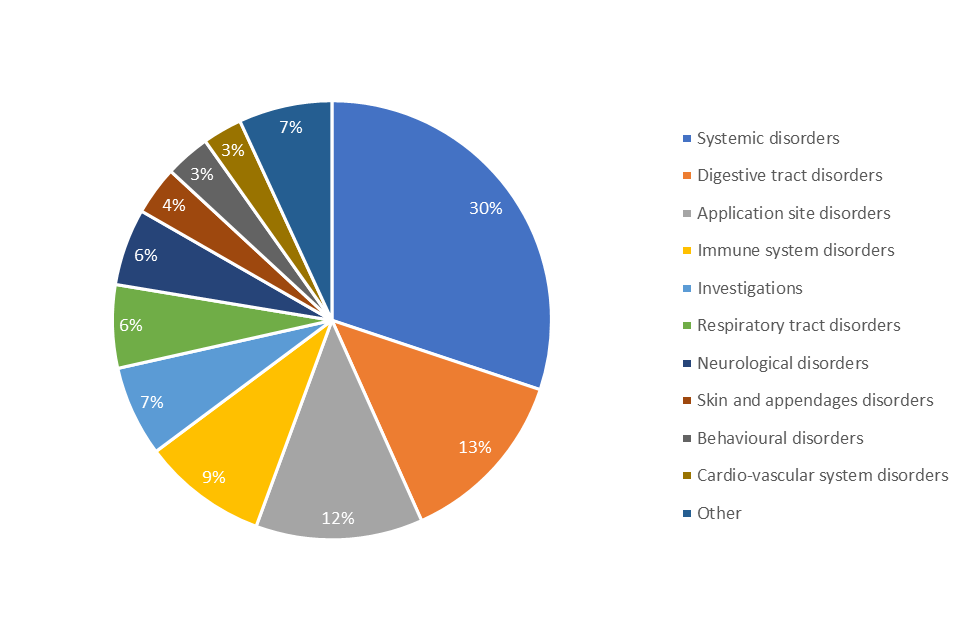
Table 1 details the 20 most commonly occurring clinical signs in reports for currently authorised dog vaccines between 2002 and October 2022. The terminology used in the table reflects the system of recording adverse events. The Table only includes information from the 30,097 cases where there was assessed to be a probable or possible association with the product, and it should be noted that each report may be associated with more than one animal and more than one disorder may be attributed to each animal.
Figure 1 shows the distribution of the types of symptoms reported in relation to the use of vaccines in dogs. Systemic disorders represent the most frequently reported clinical signs.
There are a number of vaccines authorised in the UK containing either two (L2) or four (L4) strains of Leptospira. Based on the most recent periodic safety update report data received for each product until July 2022, the incidence of adverse animal reactions for all L2 vaccine products combined is 0.017%; for L4 vaccine products this figure is 0.055%. In other words, the VMD has received fewer than 2 adverse reactions for L2, and fewer than 6 for L4, for every 10,000 doses sold. This includes every suspected adverse event reported, even cases that were considered unclassifiable or were later found to be unrelated to the vaccine. The overall incidence of suspected adverse reactions for both L2 and L4 vaccine products is therefore considered to be rare. The veterinary surgeons and the client should discuss and agree a vaccination programme for an individual animal. This should be based on the local epidemiological situation and risk of leptospirosis, balanced with the potential risks as outlined in the SPC.
The purpose of pharmacovigilance is to protect animal health and to ensure the balance of benefits and risk remains favourable. Adverse reactions to canine vaccines are rare with less than 23 reports per 100,000 doses. Whilst acknowledging a level of under reporting that is unquantifiable, the benefits of vaccination are considered significantly greater than the risks of infection by the ever present canine infectious diseases in the UK.
Table 1. Adverse events recorded between 2002 and October 2022 for UK authorised dog vaccines. The Table includes the 20 most frequently reported clinical signs (symptoms).
| Clinical sign | Disorder type | Incidence Rate per 100,000 doses sold |
|---|---|---|
| Lethargy | Systemic | 4.6 |
| Emesis | Digestive tract | 3.4 |
| Allergic oedema | Immune system | 2.4 |
| Hyperthermia | Systemic | 2.2 |
| Anorexia | Systemic | 2.1 |
| Injection site oedema | Application site | 1.9 |
| Diarrhoea | Digestive tract | 1.6 |
| Malaise | Systemic | 1.3 |
| Anaphylaxis | Immune system | 1.2 |
| Injection site pain | Application site | 1.2 |
| Injection site infection | Application site | 1.2 |
| Pale mucous membrane | Systemic | 1.2 |
| Injection site mass NOS* | Application site | 0.9 |
| Cough | Respiratory tract | 0.8 |
| Ataxia | Neurological | 0.8 |
| Lack of efficacy | Systemic | 0.8 |
| Muscle tremor | Neurological | 0.8 |
| Pruritus | Skin | 0.8 |
| Vocalisation | Behavioural | 0.7 |
| Tachypnoea | Respiratory tract | 0.7 |
*NOS – Not otherwise specified, for example, not fully described
Figure 1. Breakdown of SAEs by disorder type reported after vaccination of dogs
9.3 Veterinary Products Committee (VPC)
Working Group on Feline and Canine vaccination 2002
The Working Group was set up by the Veterinary Products Committee (VPC) in response to concerns in both the public domain and scientific community about possible health risks related to the routine vaccination of dogs and cats. The working group concluded that vaccination plays a very valuable role on the prevention and control of the major infectious diseases in cats and dogs. Although adverse reactions to vaccination occasionally occur, including lack of efficacy, the Working Group Veterinary Products Committee working group report on feline and canine vaccination concluded that the overall risk/benefit analysis strongly supports their continued use.
The Working Group also observed that there was evidence to support a longer duration of immunity following vaccination than the one year which, at that time, was typically recommended on the product literature.
10. Practice Overview of Canine Health (POOCH) 2004
In 2006, the Animal Health Trust published the results of an epidemiological investigation into whether there was a temporal association between canine vaccination and ill-health. Random selections of dogs were evaluated from a number of veterinary practices in order to evaluate whether there was an increase for ill-health in dogs receiving a recent vaccination. Nearly 4,000 questionnaires were received from 28 veterinary practices. Within three months of vaccination, there was no significant association with ill-health. The study did not attempt to address the health status of vaccinated versus non-vaccinated dogs due to the difficulties in identifying a sufficient number of unvaccinated animals registered with veterinary practices Practice Overview of Canine Health (POOCH) / Veterinary Industry News / VetClick
11. Immunology and duration of immunity
11.1 Modified Live Virus (MLV)
A vaccine is generally defined as a formulation of live attenuated organisms, killed organisms or other antigens designed to elicit an immune response in an animal to protect against infection, clinical signs or mortality from infectious diseases. With the emerging development of live recombinant vaccines the distinction between conventional MLV and killed vaccines is less obvious, with the recombinant vaccines behaving in many respects like MLV vaccines but with few disadvantages. There are currently no live recombinant vaccines authorised for dogs on the UK market.
Conventional MLV vaccines have a number of advantages over killed vaccines and have been mostly produced for viral diseases owing to the relatively ease at producing stable attenuated products. The only live attenuated bacterial vaccines marketed in the UK are for Kennel Cough caused by Bordetella bronchiseptica. MLV vaccines, in general, produce a more rapid onset and longer duration of immunity compared to killed products probably because the immune response to MLV vaccines more closely mirrors the response to infection with disease and, therefore, a strong active immunity is produced in the vaccinated animal. Killed vaccines may incorporate an adjuvant to enhance the immune response and usually require a two-dose primary vaccination course to achieve the required level of protection.
The main risk for MLV vaccines is their potential to revert to virulence and cause disease in vaccinated animals. Such vaccines would pose a greater potential risk to pregnant or immunosuppressed animals. MLV vaccines also tend to be less stable if stored incorrectly and pose a greater risk of being contaminated with other undesirable microorganisms.
11.2 Notes on the immune response to vaccines
The immune system can be broadly divided into the innate and adaptive immune systems. Interaction between the innate immune system, which responds quickly and non-specifically to a pathogen and the adaptive immune response, which acts in an antigen-specific manner, is essential for the induction of an effective immune response to pathogens (Palm and Medzhitov, 2009).
Most vaccines do not prevent infection but reduce the severity of the illness associated with disease. A live vaccine contains a virus that has been modified to lose its disease-causing ability (attenuated). Killed vaccines are attenuated through a process that results in their death.
In general, the live viruses in MLV vaccines undergo limited cycles of development within the animal. They provide rapid protection and can overcome circulating maternal antibodies more effectively than inactivated vaccines because they are structurally very similar, or in many cases identical, to the disease-causing pathogen and undergo a similar development within cells. In addition, they stimulate the correct type of immune response, providing protection for long periods. The main disadvantages of MLV vaccines include the possibility of reverting to a disease-causing form and the possibility of producing disease in immunocompromised patients. Killed vaccines have the obvious advantages of being safer in immunocompromised patients and not reverting to virulence. However, they are generally not processed by the immune system in the same way as the live organism and, therefore, do not stimulate strong immune responses. As they don’t stimulate the complete immune response, they require the addition of adjuvants (non-specific immunostimulants) and require frequent re-vaccination to maintain immunity.
11.3 Immunological memory
Immunological memory is the ability of the adaptive arm of the immune system to recognise and respond more rapidly to an antigen that it has encountered previously (Zinkernagel et al, 2006). The protective immunity provided by vaccines is dependent on the magnitude and nature of this response. The response can be best measured by controlled challenge studies, testing the immunity of a vaccinated animal by inoculating live pathogenic organisms. However, the ultimate determination of a vaccine’s merits comes from controlled tests conducted under field conditions. Antibodies have a short half-life and continual replenishment is required to maintain stable long-term protective immunity characterised by high levels of neutralizing antibodies (Wrammert and Ahmed, 2008). Despite the importance of long-lived antibody responses, relatively little is known about how the response is maintained. The mechanisms by which long-lived immunity is maintained are currently active fields of research.
11.4 Serology as an alternative to vaccination
Serology is the qualitative or quantitative diagnosis of antibodies to a particular disease organism or antigen in serum. Serological tests are used to diagnose infection or determine the immunological response to a vaccine. Serological surveys may be used in epidemiological investigations to determine the prevalence of a particular disease in the field.
Serological tests can be used to measure the antibody-mediated immune response to many disease organisms or vaccines and are becoming increasingly used to determine whether a vaccinated animal is protected to reduce the frequency of re-vaccination as a result of perceived associated risks. Interpreting the serological titre to determine protection to a particular disease is dependent on the ability to correlate the results derived from a particular test with protection. For CDV, CPV and CAV there appear to be a relatively strong correlation between neutralising antibody titre and protection (VPC Working Group, 2002). Yet perversely, animals with high levels of circulating antibody may still be susceptible to infection, whilst some animals may be protected even in the absence of antibodies.
Serology is used extensively in human medicines to assess DOI of some vaccines where protective levels of antibody are known, as it is not possible for ethical reasons to conduct challenge studies. Thus standardised and validated in vitro serological tests that correlate with protection are available to determine the immunological status of a patient. In the veterinary field DOI is usually established by challenge; the “gold standard” for demonstrating protection from infectious disease. Unfortunately, there are no international standards or prescribed serological tests for most of the canine diseases. Tests vary considerably between laboratories and are difficult to standardise. Diagnostic laboratories must validate their own in-house tests and establish thresholds for re-vaccination based on their expertise and scientific opinion. In the absence of clearly defined protective titres, interpretation of serological tests must be treated with care and advice sought on equivocal results. Whilst the WSAVA guidelines suggest any measurable titre in an actively immune vaccinated animal is correlated with protection, the evidence to support such conclusions appears weak.
Serological testing as an alternative to vaccination has been reviewed by several notable experts in the field of veterinary diagnostics (Burr, 2006; Tizzard and Yawei, 1998; Twark and Todds, 2001; Ottiger et al., 2006; Dodds et al., 1999). In vitro tests, such as virus neutralisation tests (VNT) and haemagglutination inhibition tests (HIT), are preferred techniques as they measure functional aspects related to virus infection. Enzyme-linked immunoabsorbent assays (ELISAs) have a number of advantages over VNT and HIT but do not necessarily measure titres of neutralising antibodies. Thus, the basis of a test can be an important consideration before interpreting the results.
The conclusion has to be that serology provides useful additional information on the immune status of an animal but should not be treated in isolation to other determinants for deciding upon re-vaccination. The fact that a particular titre may be well correlated with protection does not ensure the animal is protected. The application of statistical correlations to the individual will, by the very nature of statistics, expose some to the risks of disease. The risks of over-vaccination are arguably relatively small compared to the risks of exposing susceptible animals to life-threatening infections.
11.5 Vaccine-induced auto-immune disease
Immune mediated diseases and the association with vaccine reactions have been reviewed by Day (1999, 2006), Pedersen (1999) and the VPC Working Group (2002). Vaccine reactions are generally classified into one of four recognised categories: Type I-IV. The immunological theories behind such reactions would suggest that such adverse events should only occur following an immunological reaction to a previously exposed vaccine antigen, adjuvant, excipient or other production remnants such as bovine serum. However, immune mediated reactions can also follow the administration of a primary dose of vaccine and the exact mechanisms for such a reaction are unknown.
Type I hypersensitivity reactions
These involve an immune mediated reaction that releases potent inflammatory mediators and other chemicals that trigger an anaphylactic reaction in the affected animal. The reactions are usually acute, with the clinical signs appearing within minutes or hours of vaccination. Typical signs reported are facial oedema, shock, lethargy, respiratory distress and diarrhoea. Severe anaphylactic reactions may result in death. Urticaria (hives), facial oedema and anaphylactic shock are specific clinical manifestations of Type I hypersensitivities.
Type II hypersensitivity reactions
These involve the binding of the animal’s own antibodies to cells or a cell matrix. The formation of auto-immune antibodies is thought to involve a number of complex immunological mechanisms. Secondary immune mediated haemolytic anaemia (IMHA) has been associated with vaccination but may also occur following infection, neoplasia or administration of medications. In this condition, auto-antibodies are produced against the animal’s own red blood cells but the immunological mechanism by which vaccines may produce such a response is not yet established. Confirmation of IMHA is dependent on the demonstration of autoantibodies and, therefore, not all reports of such adverse events can be recorded accurately if confirmatory diagnostic tests have not been performed. For the period 2005-2010, 57 suspect adverse reaction reports of immune mediated reactions were submitted to the VMD. Subsequently only 25 of these reports were attributed to immune mediated reactions. Other clinical manifestations of Type II disease include immune mediated thrombocytopenia (IMTP) (autoantibodies to blood platelets) myasthenia gravis (autoantibodies to muscle nerve receptors) and pemphigus (foliaceous & vulgaris) (auto-antibodies to epidermal proteins).
Type III hypersensitivity reactions
These result from the formation of circulating complexes of antigen and antibody that deposit in certain organs or tissues in the body leading to inflammatory reactions and destruction of cells and associated matrix. The deposition of immune complexes usually results in inflammation of the blood vessels. The reaction is dependent on the continued presence of both antibody and antigen with the latter being derived from infection, vaccination, medication or exposure of ‘self-antigens’ through disease. Infections will inevitably result in the formation of immune complexes as the body aims to rid itself of a foreign invader. Some examples of well-known immune complexes provide clinical signs such as:
-
‘Blue Eye’ a well-documented manifestation of a Type III reaction following infection with CAV-1 or administration of some of the early CAV-1 vaccines. Replacement of CAV-1 by CAV-2 vaccines have minimised the risk of such adverse events with just twelve reports of ‘blue eye’ reported to the VMD since 2005.
-
Reactions involving type III immune mediated mechanisms have also been demonstrated following rabies virus vaccination.
-
Systemic lupus erythematosus (SLE), a disease characterised by the development of antinuclear antibodies (ANAs).
-
Drug-induced arthritis has been reported as a Type III reaction, but the evidence of similar vaccine associated immune-mediated syndrome is sparse. The VMD has received two reports of pemphigus associated with vaccination during the last five years.
-
Type IV hypersensitivity reactions or “delayed-type hypersensitivity” are cell, rather than antibody, mediated. These diseases are usually relatively slow to develop and are dependent on the cell-mediated arm of the immune system.
12. British Small Animal Veterinary Association (BSAVA) Position
The current BSAVA position statement on companion animal vaccination is available on the BSAVA website.
The BSAVA endorsed the Veterinary Products Committee (VPC) report (2002) and the Committee for Veterinary Medicinal Products (CVMP) statement (2003) on canine and feline vaccination and advised members that they should consider the recommendations made in these reports when discussing with owners the relative risks and benefits of vaccination policy.
The BSAVA is a member organisation of the WSAVA and supports the vast majority of WSAVA Guidelines for the Vaccination of Dogs and Cats.
13. Animal Boarding Establishments vaccination requirements
The VMD does not regulate the vaccination requirements laid down by animal boarding establishments. Local Authorities issue licences to proprietors of boarding kennels and catteries under the provisions of The Animal Welfare (Licensing of Activities Involving Animals) (England) Regulations 2018 in England.
The 2018 Regulations do not specify specific vaccination requirements for animals to be admitted to boarding establishments. The vaccination related conditions are introduced under Schedule 2 9. (4) where it is stated that “All reasonable precautions must be taken to prevent and control the spread among the animals and people of infectious diseases, pathogens and parasites…”.
In July 2018 Defra published comprehensive guidance to accompany the 2018 regulations, to which local authorities must have regard, so that a consistent approach was maintained in the issuing of licences and the enforcement of the legislation by local authorities.
The guidance, which was drafted by put together by various organisations including representatives from local authorities, veterinary associations and the pet industry, provides comprehensive advice to local authorities and licencees on what needs to be done to meet the minimum welfare standards set out in the 2018 Regulations. The aim of the licence conditions is to ensure high standards of animal health and welfare.
The guidance notes for conditions for providing boarding in kennels for dogs relating to The Animal Welfare (Licensing of Activities Involving Animals) (England) Regulations 2018. This guidance is aimed at local authority inspectors in England. Recommendations include:
- An up-to-date veterinary vaccination record must be seen to ensure that dogs have current vaccinations against canine parvovirus, canine distemper, canine adenovirus/infectious canine hepatitis, leptospirosis and other relevant diseases. Vaccination against diseases such as kennel cough (Bordetella bronchiseptica/Canine parainfluenza virus) may be required by the establishment.
- Certification from a veterinarian of a recent protective titre test may be accepted instead of a booster vaccination as required by the establishment. The certificate must state that it is valid for the current period. It is the decision of the kennel proprietor whether to accept such a certificate.
- Primary vaccination courses must be completed at least 2 weeks before boarding.
- Vaccines used must be licenced for use in the UK. Homoeopathic vaccination is not acceptable.
14. Promotion of vaccines
The VMD does not facilitate or promote any pharmaceutical company’s campaign on national vaccination of pets. The VMD regulates the authorisation and supply of veterinary medicinal products and there are very specific controls on certain legal categories of products. All vaccines for dogs are prescription only medicines with a legal category POM-V. A veterinary medicinal product that has been classified as a POM-V may only be supplied once it has been prescribed by a veterinary surgeon following a clinical assessment of an animal or group of animals under the veterinary surgeon’s care. Further information on legal categories of veterinary medicines can be found in the Veterinary Medicines Guidance Notes.
These Guidance Notes also include restrictions on advertising POM-V medicines:
-
Advertisements for POM-V products may only feature in publications aimed at veterinary surgeons, pharmacists, veterinary nurses and professional keepers of animals. It is not acceptable to promote specific POM-V products directly to members of the public.
-
Advertising information aimed at the general public may not include the brand name of a POM-V product in relation to treatment, but it may name active substances and contain a small strapline at the top or bottom of the article stating ‘this information was provided by [company] makers of [product].
-
The displaying of a poster for a specific POM-V product in a public place, such as, a veterinary surgeon’s waiting room or a shop wall, would be considered as advertising material aimed at the general public and is, therefore, illegal. However, information/educational material that does not contravene the points raised above would be acceptable.
Vaccination reminder cards produced by the manufacturer of a specific product(s) are permitted in certain circumstances. It is acceptable to send a complimentary vaccination reminder card to a client which features a company ‘strapline’ , for example, ‘Brought to you by [company name], makers of [product brand name]’. Any document that features information about a single product is deemed to be advertising. The VMD is not aware of any promotion of the national campaign that has promoted the vaccination of pet animals in an inappropriate way.
