Veterinary Pharmacovigilance in the UK Annual Review 2019 – a summary of veterinary adverse events
Published 21 December 2020
Pet owners, farmers, animal carers, vets and anyone else interested in animal welfare should read this review. It gives information about the reports of side effects we received during the year. These reports were usually about animals, but sometimes people had a side effect after giving a medicine to an animal, or after accidentally touching or taking an animal medicine.
The annex to this review lists the changes made to medicine information leaflets during the year.
1. Highlights
During 2019, we improved 55 of the medicine information leaflets included with animal medicines. The changes made were based on adverse event reports received. They ensured that the benefits of using these medicines continued to outweigh any risks. They also made users better informed about the medicines they are using.
The VMD received 7,118 adverse event reports during 2019, a 0.6% decrease from the previous year. Compare this number with the millions of doses of different types of veterinary medicines used during that year.
The VMD would like to thank owners and veterinary professionals for continuing to report adverse events as without these reports, this valuable work would not be possible.
Detailed information about the reports received can be accessed through VMD’s adverse events dashboard.
2. Introduction
Veterinary pharmacovigilance is the monitoring of all adverse event (AE) reports, both adverse reactions and lack of expected efficacy (LEE), for emerging patterns of undesirable effects, following the use of veterinary medicines.
Without the information submitted by reporters, we would not be able to continually make users better informed about the medicines they are using.
Within the UK, vets, animal owners and other people who work with animals, administer many millions of doses of different types of veterinary medicine to animals every year. In a small number of cases, an AE occurs. This may occur during, or sometime after, the use of a medicine.
The reports described in this review are those received after everyday use of veterinary products in the United Kingdom, by animal owners, vet practices and farm workers; this review does not include reports associated with scientific studies.
During 2019, VMD received and assessed 7,118 adverse event reports. This was a slight decrease on the previous year, compared to a 6.5% increase from 2017 to 2018.
Acting on the adverse event information received; the product information leaflet enclosed with medicines was improved for 55 products.
Most of the reports received described events that occurred in animals during or after the use of authorised veterinary or human medicines. Many reports involved the use of multiple products, some of which may not have been authorised medicines.
Some reports described reactions experienced by humans exposed to products used to treat animals. Others involved the detection of the residues of veterinary medicines in a food product intended for human consumption, usually milk, before it entered the food chain.
This Summary provides an overview of the adverse events received in 2019, and a list of the product information leaflet changes (See Annex).
3. Number and types of reports received.
3.1 The overall number of adverse event reports received in 2019 was similar to 2018.
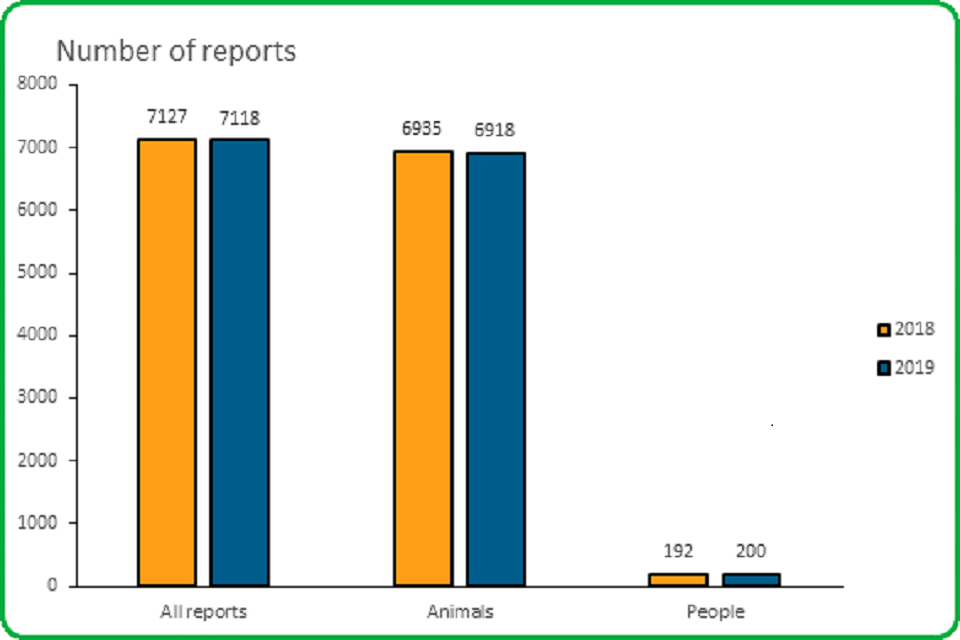
The overall number of adverse event reports received in 2019 decreased slightly compared to 2018
- The total number of reports received in 2019 decreased from 7,127 in 2018 to 7,118; a decrease of 9 (0.1%)
- The number of animal reports in 2019 decreased from 6,935 in 2018 to 6,918; a decrease of 17 (0.2%)
- The number of reports involving people increased from 192 in 2019 to 200; an increase of 4.2%
3.2 The size of the change in animal report numbers varied from species to species.
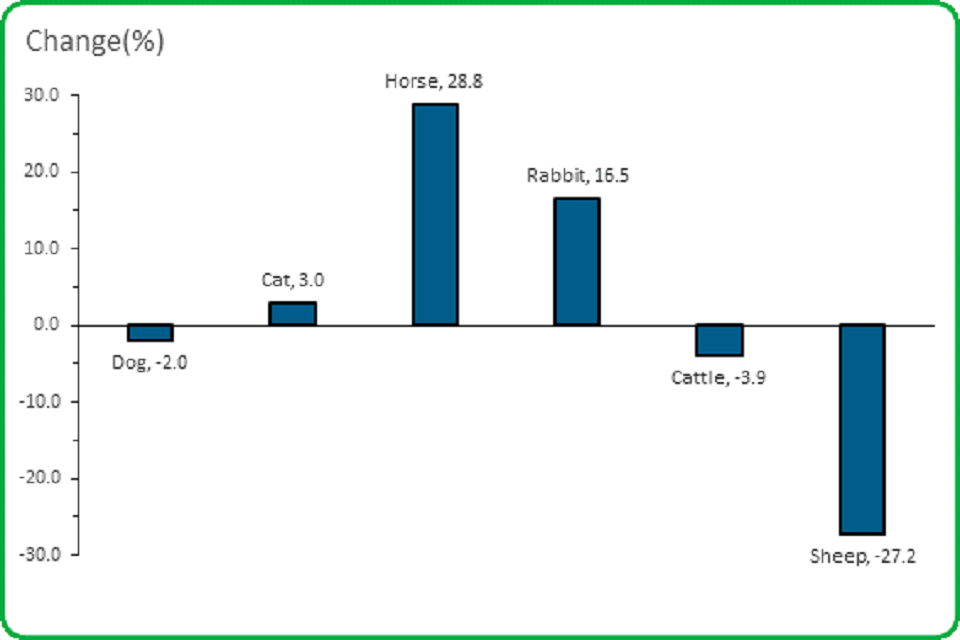
A large increase in the number of all reports involving horses was offset by a large decrease in those involving sheep
- The number of reports involving dogs in 2019 decreased by 2.0%
- The number of reports involving cats in 2019 increased by 3.0%
- The number of reports involving horses increased by 28.8%
- The number of reports involving rabbits increased by 16.5%
- The number of reports involving cattle decreased by 3.9%
- The number of reports involving sheep decreased by 27.2%
3.3 The overall number of lack of expected efficacy (LEE, medicine not working) reports was almost the same, but for the species most often reported they increased to varying degrees, except for sheep.
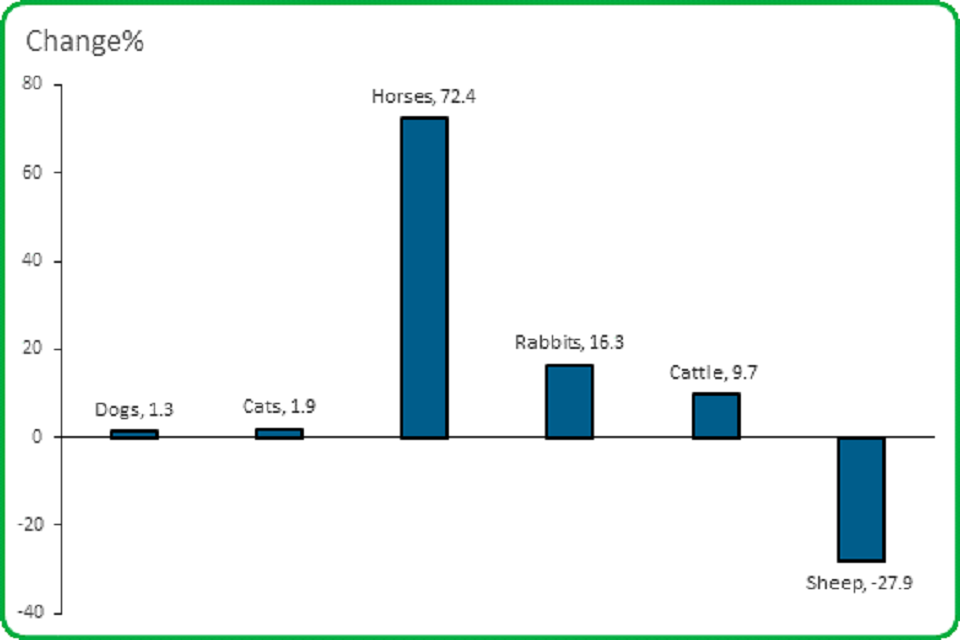
The number of lack of expected efficacy (LEE) reports increased for all species, except sheep
- There was a 1.3% increase in LEE reports involving dogs compared to 2018.
- There was an 1.9% increase in LEE reports for cats
- There was a 72.4% increase in LEE reports for horses
- There was a 16.3% increase in LEE reports for rabbits
- There was a 9.7% increase in LEE reports for cattle
- There was a 27.9% decrease in LEE reports for sheep
In 2019, the number of LEEs reported in rabbits increased again, as they had done in the previous year, but the increase was not as marked. Almost all reports in these years were associated with vaccines for myxomatosis and/or rabbit haemorrhagic disease (RHD).
For horses, there were a similar number of LEE reports involving the use of euthanasia products compared to 2018. However, there was also a group of reports involving Equine flu vaccines in the first quarter of the year following an equine flu outbreak that occurred early in the year.
Many of the cases were linked, having originated from horses in a small number of yards. In most of these cases, there was insufficient vaccination history provided to determine the role of the vaccine involved.
3.4 The number of safety (adverse reaction) reports increased in three of the six most often reported species.
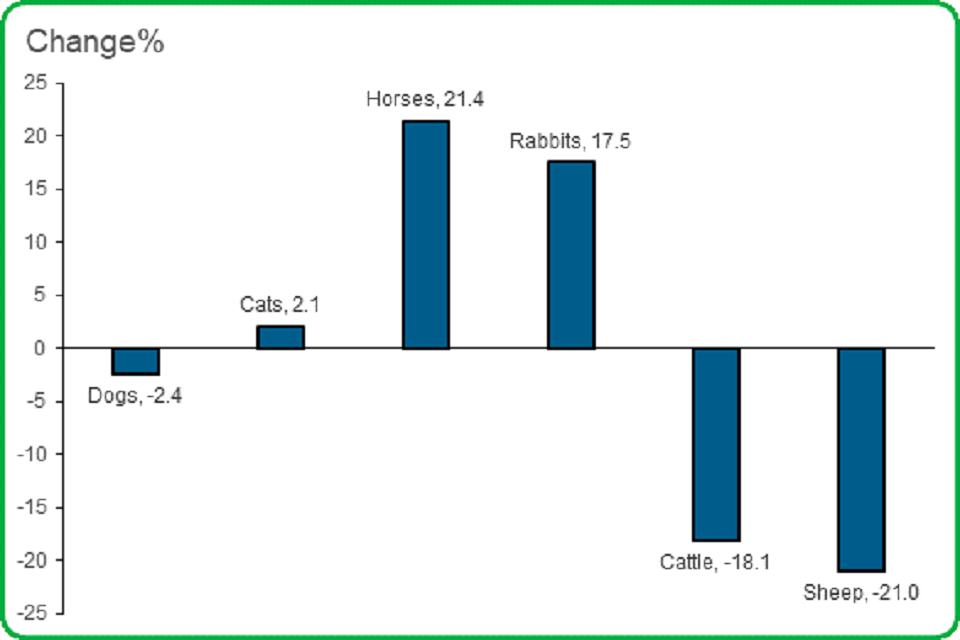
The number of safety reports for both cats and dogs were almost the same as in 2018. There were large increases for Horses and Rabbits, and large decreases for Cattle and Sheep.
- The number of safety reports received involving dogs decreased by 2.4%
- Safety reports for cats increased by 2.1%
- Safety reports for horses increased by 21.4%
- Safety reports for rabbits increased by 17.5%
- The number of cattle safety reports decreased by 18.1%
- The number of sheep safety reports decreased by 21.0%
Over 50% of the products reported in horse safety cases were vaccines and most of these were flu vaccines, following on from the equine flu outbreak early in the year.
Reports involving the use of products with neurological indications were also quite common (almost 20% of products), with treatments for equine Cushing’s disease, sedatives and painkillers reported most.
69% of products reported in rabbit safety reports were vaccines against myxomatosis and rabbit haemorrhagic disease. Other types of product were, in decreasing order of prevalence, injected general anaesthetics, pain killers (predominantly buprenorphine), and sedatives (mostly medetomidine).
42% of products reported in cat safety reports were inactivated viral vaccines. All classes of anti-parasiticides were represented in safety reports, with endectocides mentioned most often followed closely by ectoparasiticides, with anthelmintics and anti-protozoal products reported least often.
Products associated with thyroid therapy were also reported, with anti-thyroid preparations reports being 7-fold higher than systemic cortico-steroid preparations.
Vaccines to control infections by severe, life-threatening diseases present in the UK dog population, recommended by the British Small Animals Veterinary Association (BSAVA), were most often reported in this species.
Products for treating or preventing infestation by internal or external parasites were reported. External parasiticides were reported most often, with newer products that act systemically being reported more often than older ones that are applied to the dog’s skin. This may reflect a change in product usage rather than a change in risk.
4. Products reported and animals affected.
4.1 Most products involved in animal adverse event reports were authorised veterinary medicines.
This is to be expected as non-authorised products should only be used where a suitable authorised product is not available and VMD is only responsible for monitoring adverse events involving authorised veterinary medicines.
Vets are required to use veterinary medicines authorised in the UK to treat animals, unless there is no suitable medicine available. Using their clinical judgement, they can decide to use a:
- medicine authorised in the UK for use in people
- veterinary medicine not authorised in the UK
- specially prepared (extemporaneous) medicine
In 2019 96.2% of the products mentioned in animal adverse event reports were UK authorised veterinary medicines.
Of the remaining 3.8% of products:
- 1.3% were UK medicines authorised for use in people
- 0.4% were extemporaneous medicines
- 0.2% were imported medicines
- 0.3% were veterinary medicines that are exempt from marketing authorisation requirements
- the remaining products were either unidentified products or those without medicinal value, such as microchips or sutures, household pesticide sprays or disinfectants.
4.2 Immunological products were the veterinary medicines most often associated with animal adverse event reports.
- 45.2% of medicines were for aiding the immune system
- 17.6% were for the control of parasites
- 9.1% were for the nervous system, mostly anaesthetics
- 8.1% were for controlling hormone balance
- 6.2% were for treatment of bone and joint problems
- 4.5% were for treating digestive and metabolic problems
Other products included:
- 2.6% treatments for skin problems
- 2.4% treatments to prevent or treat infection, other than vaccines
- 1.4% treatments for heart or circulation problems
- 1.0% for eye or ear problems
- 0.8% for bladder problems
- 1.2% for cancer treatments, fluids, vitamins and treatments for breathing problems
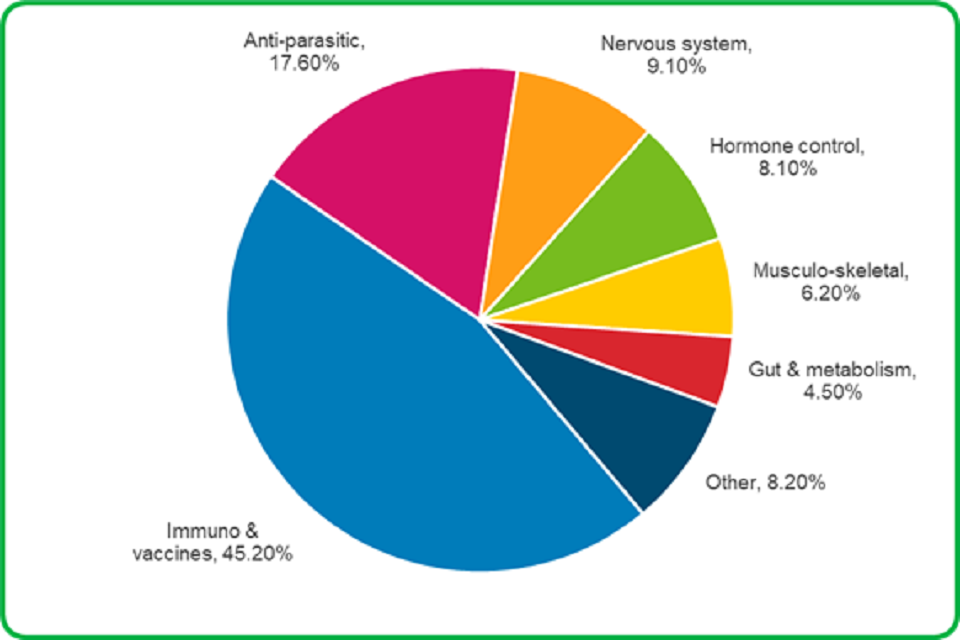
Immunological products were the veterinary medicines most often associated with animal adverse event reports.
4.3 The number of reports of lack of expected efficacy (LEE) was much smaller than the number of reports of adverse reaction for all major species, except for cattle and sheep.
| Animal | Number of Adverse Reaction reports (species %) | Number of LEE reports (species %) | ||
|---|---|---|---|---|
| Dogs | 3,578 (92.0%) | 312 (8.0%) | ||
| Cats | 1,572 (96.7%) | 53 (3.3%) | ||
| Horses | 272 (84.5%) | 50 (15.5%) | ||
| Rabbits | 275 (82.8%) | 57 (17.2%) | ||
| Cattle | 127 (36.0%) | 226 (64.0%) | ||
| Sheep | 83 (33.5%) | 165 (66.5%) |
Explore the data using our interactive data dashboard.
Interactive Data Dashboard
5. Important messages
5.1 For anyone administering veterinary medicines
- Obtain veterinary medicines from a reputable source. Look for the VMD Accredited Internet Retailer Scheme logo if you are buying medicines online.
- Follow the instructions for use provided in the package leaflet, including any advice to avoid harm coming to you whilst administering the medicine, or in the time after administration.
- Report a problem with an authorised veterinary medicine to the Marketing Authorisation Holder or to us. We cannot take regulatory action in relation to a medicine without sufficient evidence of a problem. Social media does not provide the evidence we need.
- Report a problem with a medicine especially prepared for your animal (an extemporaneous product) to the pharmacist/vet/manufacturer of that medicine or to us.
- Use appropriate safety equipment when administering medicines that may be harmful to your own health.
- Use appropriate personal protective safety equipment or animal restraints when administering medicines to animals that may harm you or cause you to injure yourself with a needle if they move unexpectedly.
- Seek immediate medical attention if you accidentally inject yourself with an oil-based vaccine. Also report the incident to us, with as much information as possible.
- Be aware of the hazards posed by some surgery-only medicines, for example, inhaled anaesthetics are a risk to unborn children.
- If you are planning to euthanase a horse, follow the product leaflet guidance and have a secondary plan available, in case the original method does not achieve the required outcome. Wear head protection.
- Reduce the chance of accidental exposure by keeping animal medicines out-of-sight and reach of children (and animals) and separate from personal medicines.
- Clean and dry any dosing equipment thoroughly between uses, particularly if used with different medicines.
- Dispose of empty medicine containers promptly, in accordance with labelling instructions. Oral horse medicine syringes are attractive to dogs.
- If you suspect your animal has been poisoned by a veterinary medicine, seek advice from your vet or the Veterinary Poisons Information Service, then report the adverse event to us. If you have been affected, seek medical advice first, and then report to us.
5.2 For people who work or play with treated animals
- Never allow animals recently treated with topical medicines, such as spot-ons, collars, to share a bed with people.
- Always ensure that spot-on anti-parasitic products are completely dry before allowing anyone, including other pets, to kiss, cuddle or groom the treated animal.
- Do not allow your dog to run free in areas inhabited by farm animals or horses. These animals can excrete medicine residues that could be harmful to your dog, if ingested.
- Do not allow animals recently treated with topical medicines to enter streams, rivers or other water courses, as these medicines can be fatal to water life.
6. Annex
6.1 Product information changes relating to pharmacovigilance
The following tables list the changes made to product literature resulting from the assessment of Adverse Event report information. Without the information submitted by reporters, we would not be able to continually make users better informed about the medicines they are using, and reduce some of the risks associated with the use of those medicines.
We thank all reporters for their continuing support in providing us with the essential information we need to monitor medicine safety.
These tables lists all pharmacovigilance-related regulatory actions taken during 2019. Information received prior to 2019 will have contributed to the evidence leading to the initiation of these actions. These tables show the changes made to section 4.6 of the Summary of Product Characteristics. Equivalent changes were also made to the Product Information Leaflet that is enclosed with each medicine.
6.2 For food-producing and other large animals.
| Product name | Marketing Authorisation Holder | Active ingredient(s) | Old text | New text | ||||
|---|---|---|---|---|---|---|---|---|
| Bovilis Bovipast RSP | Intervet UK Ltd | Bovine parainfluenza virus 3; Bovine respiratory syncytial virus; Mannheimia haemolytica | Immunisation may commonly result in temporary swellings at the injection site (in extreme cases narrow swellings up to 10 cm long may occur). Typically, these swellings completely disappear or reduce in size to a negligible small lump within 2 to 3 weeks after vaccination, though in individual animals very small reactions can be found for up to 3 months. Additionally, a transient slight rise in body temperature, lasting a maximum of 3 days, may commonly occur after vaccination and at the same time a slight reluctance to move may be found. In very rare cases hypersensitivity reactions, which may be fatal, may occur. | Additional text: In laboratory and field trials: Immunisation may commonly result in temporary swellings at the injection site (in extreme cases narrow swellings up to 10 cm long may occur). Typically, these swellings completely disappear or reduce in size to a negligible small lump within 2 to 3 weeks after vaccination, though in individual animals very small reactions can be found for up to 3 months. Additionally, a transient slight rise in body temperature, lasting a maximum of 3 days, may commonly occur after vaccination and at the same time a slight reluctance to move may be found. In post marketing experience: In very rare cases hypersensitivity reactions, which may be fatal, can occur. | ||||
| Buscopan Compositum solution for injection | Boehringer Ingelheim Animal Health UK Ltd | Hyoscine butylbromide; Metamizole | Additional text: In very rare cases, anaphylactic reactions and cardiovascular shock can occur. | |||||
| Eprinex Multi 5 mg/ml pour-on for beef and dairy cattle, sheep and goats | Merial Animal Health Ltd | Eprinomectin | No undesirable effects have been identified when the product is used at the recommended dose rate. | Amended text: In very rare cases, pruritus and alopecia have been observed after the use of the veterinary medicinal product. | ||||
| Equipramox 19.5 mg/g + 121.7 mg/g oral gel | Zoetis UK Limited | Moxidectin; Praziquantel | Flaccid lower lip, ataxia and swelling of the muzzle could be observed on rare occasions in young animals. Anorexia and lethargy have been reported in very rare cases. These adverse effects are transient and disappear spontaneously. In case of very high worm burdens, destruction of the parasites may cause a mild transient colic and loose faeces in the treated horse. | Amended and additional text: Mouth pain, flaccid lower lip, swelling of the muzzle, hypersalivation and anorexia have been observed in rare cases. Ataxia has been reported on rare occasions and lethargy in very rare cases. These adverse effects are transient and disappear spontaneously. In case of very high worm burdens, destruction of the parasites may cause a mild transient colic and loose faeces in the treated horse. | ||||
| Genta-Equine 100 mg/ml solution for injection for horses | Franklin Pharmaceuticals Ltd | Gentamicin | Additional text: Hypersensitivity reactions have been reported very rarely following use. | |||||
| Hyogen emulsion for injection for pigs | Ceva Animal Health Ltd | Mycoplasma hyopneumoniae | On the day of vaccination a transient mean increase in body temperature of about 1.3C is very common. In an individual pig this increase might reach 2oC, but in all cases body temperature is back to normal the next day. A local reaction at the site of injection in the form of a swelling of a diameter up to 5 cm can be very common, which can last for three days. These reactions are of transient nature and do not need further treatment. Immediate mild hypersensitivity-like reactions may occur uncommonly after vaccination, resulting in transient clinical signs such as vomiting. These clinical signs normally resolve without treatment. | Amended and additional text: On the day of vaccination a transient mean increase in body temperature of about 1.3°C is very common. In an individual pig this increase might reach 2°C, but in all cases body temperature is back to normal the next day. A local reaction at the site of injection in the form of a swelling of a diameter up to 5 cm can be very common, which can last for three days. These reactions are of transient nature and do not need further treatment. Immediate mild hypersensitivity-like reactions may occur uncommonly after vaccination, resulting in transient clinical signs such as vomiting. Serious anaphylactic-type reactions (shock, recumbency) which may be fatal have been reported very rarely in post marketing experience. Such reactions require prompt symptomatic treatment. | ||||
| K-Flor 100 mg/ml solution for use in drinking water for pigs | Laboratorios Karizoo SA | Florfenicol | Additional text: Neurological signs and death can be observed in the animals treated on rare occasions. In that case withdraw the treatment immediately. |
6.3 For pet animals
| Product name | Marketing Authorisation Holder | Active ingredient(s) | Old text | New text | ||||
|---|---|---|---|---|---|---|---|---|
| Alfaxan 10 mg/ml solution for injection for dogs, cats and pet rabbits | Jurox (UK) Limited | Alfaxalone | Additional text: Based on post marketing safety experience, neurological signs (convulsions, myoclonus, tremor, prolonged anaesthesia), cardio-respiratory signs (cardiac arrests, bradycardia, bradypnea) and behavioural signs (hyperactivity, vocalisation) have been reported very rarely. | |||||
| Animeloxan 5 mg/ml solution for injection for dogs and cats | aniMedica GmbH | Meloxicam | Additional text: In very rare cases elevated liver enzymes have been reported. In very rare cases, haemorrhagic diarrhoea, haematemesis and gastrointestinal ulceration have been reported in dogs. | |||||
| Apoquel 3.6mg / 5.4mg / 16mg film coated tablet for dogs | Zoetis Belgium | Oclacitinib | Additional text:Anaemia and lymphoma have been reported very rarely in spontaneous reports. | |||||
| Carprieve 5% w/v small animal solution for injection | Norbrook Laboratories Limited | Carprofen | None Known | Amended text: Typical undesirable effects associated with NSAIDs such as vomiting, soft faeces/diarrhoea, faecal occult blood, loss of appetite and lethargy have been reported in very rare instances. These adverse reactions are in most cases transient and disappear following termination of the treatment but in very rare cases may be serious or fatal. If adverse reactions occur, use of the product should be stopped and the advice of a veterinarian should be sought. As with other NSAIDs there is a risk of rare renal, idiosyncratic hepatic or gastro-intestinal tract adverse events. Rarely reactions at the injection site may be observed following subcutaneous injection. | ||||
| Broadline Spot-on solution for cats <2.5 kg / 2.5 – 7.5 kg | Merial | (S) Methoprene; Eprinomectin; Fipronil; Praziquantel | Additional text: Transitory blindness or impaired vision have been observed in very rare cases based on post marketing safety experience. | |||||
| Canigen KC | Intervet UK Ltd | Bordetella bronchiseptica; Canine parainfluenza virus | Additional text: In very rare cases hypersensitivity reactions may occur. Such reactions may evolve to a more severe condition (anaphylaxis), which may be life-threatening. If such reactions occur appropriate treatment is recommended. | |||||
| Cytopoint 10 mg/ml / 20 mg/ml / 30 mg/ml / 40 mg/ml solution for injection | Zoetis Belgium | Lokivetmab | Additional text: Vomiting and/or diarrhoea have also been reported in rare cases and may occur in connection with hypersensitivity reactions. Treatment should be administered as needed. | |||||
| Drontal Dog Tasty Bone 150/144/50 mg tablets | Bayer plc | Febantel; Praziquantel; Pyrantel | In very rare cases mild and transient digestive tract disorders (e.g. vomiting) may occur | Amended and additional text: In very rare cases slight and transient digestive tract disorders such as vomiting and/or diarrhoea may occur. In individual cases these signs can be accompanied by nonspecific signs such as lethargy, anorexia or hyperactivity. | ||||
| Ectoline Duo spot-on solution 67/20 mg / 134/40 mg / 268/80 mg / 402/120 mg for dogs and 50/60 mg / 100/120 mg for cats | Virbac Ltd | Fipronil; Pyriproxyfen | According to the accumulated experience on these active ingredients within spot on pharmaceutical forms, transient cutaneous reactions at the application site (squamosis, local alopecia, pruritus, erythema, skin discolouration) and general pruritus or alopecia may be observed after use. In very rare instances, hypersalivation, reversible neurologic symptoms (hyperesthesia, depression, nervous symptoms), respiratory signs or vomiting might occur. | Amended text: In very rare cases, according to the accumulated experience on these active ingredients within spot on pharmaceutical forms, transient cutaneous reactions at the application site (squamosis (scaling of the skin), local alopecia (hair loss), pruritus (itchiness), erythema (redness of the skin), skin discolouration) and general pruritus or alopecia may be observed after use. In very rare instances, hypersalivation, reversible neurologic symptoms (hyperesthesia (increased sensitivity to stimuli), depression, nervous symptoms), respiratory signs or vomiting might occur. | ||||
| Garlic sugar coated tablets | Dorwest Herbs Ltd | Garlic, Garlic Oil | Additional text: Based on post marketing experience and complementary data, emesis has been observed very rarely. | |||||
| Green Releaf tablets | Dorwest Herbs Ltd | Celery Plant, Celery Seed, Horseradish, Parsley, Watercress | Additional text: Based on post marketing experience and complementary data, diarrhoea has been observed very rarely. | |||||
| Garlic and Fenugreek sugar coated tablets | Dorwest Herbs Ltd | Fenugreek Milled, Garlic Oil | Additional text: Based on post marketing experience and complementary data on very rare occasion diarrhoea and emesis have been reported. | |||||
| Kelp Seaweed sugar coated tablets | Dorwest Herbs Ltd | Fucus, Pulv Fucus | Additional text: Based on post marketing experience and complementary data, diarrhoea has been observed very rarely. | |||||
| Loxicom 0.5 mg/ml oral suspension for dogs Meloxicam | Norbrook Laboratories Ltd | Meloxicam | Typical adverse reactions of NSAIDs such as loss of appetite, vomiting, diarrhoea, faecal occult blood, apathy and renal failure have occasionally been reported. | Amended and additional text :Typical adverse reactions of NSAIDs such as loss of appetite, vomiting, diarrhoea, faecal occult blood, lethargy and renal failure have occasionally been reported. Gastrointestinal ulceration and elevated liver enzymes were reported in very rare cases. | ||||
| MiPet Easecto 5 mg / 10 mg / 20 mg / 40 mg / 80 mg / 120 mg chewable tablets for dogs | Zoetis Belgium | Sarolaner | In very rare cases adverse reactions associated with mild and transient gastrointestinal effects such as vomiting and diarrhoea may occur. In very rare cases transient neurological disorders such as tremor, ataxia or convulsion may occur. These signs typically resolve without treatment. | Amended text: Mild and transient gastrointestinal signs such as vomiting and diarrhoea, transient neurological disorders such as tremor, ataxia or convulsion and systemic disorders such as lethargy, anorexia/inappetence may occur in very rare cases. These signs typically resolve without treatment. | ||||
| Nexgard Spectra 9.375/1.875 mg / 18.75/3.75 mg / 37.5/7.5 mg / 75/15 mg / 150/30 mg chewable tablets for dogs | Merial | Afoxolaner, Milbemycin Oxime (A3 and A4) | In clinical studies, no serious adverse reactions were attributed to the combination of afoxolaner and milbemycin oxime. Vomiting, diarrhoea, lethargy, anorexia, and pruritus were uncommonly observed. These occurrences were generally self-limiting and of short duration. | Amended and additional text: Clinical studies: No serious adverse reactions were attributed to the combination of afoxolaner and milbemycin oxime. Vomiting, diarrhoea, lethargy, anorexia, and pruritus were uncommonly observed. These occurrences were generally self-limiting and of short duration. Post-marketing safety experience: Erythema and neurological signs (convulsions, ataxia and muscle tremors) have been reported very rarely. | ||||
| Nobivac KC | Intervet International BV | Bordetella bronchiseptica; canine parainfluenza virus | Additional text: In very rare cases hypersensitivity reactions may occur. Such reactions may evolve to a more severe condition (anaphylaxis), which may be life-threatening. If such reactions occur appropriate treatment is recommended. | |||||
| Onsior 20 mg/ml solution for injection for cats and dogs | Elanco Europe Ltd | Robenacoxib | Dogs: Gastrointestinal adverse events (such as vomiting) were commonly reported but most cases were mild and recovered without treatment. Diarrhea, soft stools and dark faeces or reduced appetite were uncommon | Amended text:Dogs: Gastrointestinal adverse events (diarrhoea and vomiting) were commonly reported but most cases were mild and recovered without treatment. Soft stools and dark faeces or reduced appetite were uncommon. | ||||
| Raspberry Leaf tablets | Dorwest Herbs Ltd | Raspberry Leaf Powder | Additional text: Based on post marketing experience and complementary data, diarrhoea has been observed very rarely. | |||||
| Recicort 1.77 mg/ml + 17.7 mg/ml ear drops, solution for dogs and cats | Le Vet Beheer BV | Salicyclic acid; Triamcinolone acetoride | Additional text: In rare cases redness and skin scaling have been reported | |||||
| Scullcap and Valerian sugar coated tablets | Dorwest Herbs Ltd | Gentian Ext., Mistletoe, Scullcap, Valerian | Additional text: Based on post marketing experience and complementary data, behavioural disorders as hyperactivity, anxiety have been observed very rarely. On very rare occasion diarrhoea, lethargy and malaise have been reported. | |||||
| Simparica 5 mg / 10 mg / 20 mg / 40 mg / 80 mg / 120 mg tablets for oral use in dogs | Zoetis Belgium | Sarolaner | In very rare cases adverse reactions associated with mild and transient gastrointestinal effects such as vomiting and diarrhoea may occur. In very rare cases transient neurological disorders such as tremor, ataxia or convulsion may occur. These signs typically resolve without treatment. | Amended text: Mild and transient gastrointestinal signs such as vomiting and diarrhoea, transient neurological disorders such as tremor, ataxia or convulsion and systemic disorders such as lethargy, anorexia/inappetence may occur in very rare cases. These signs typically resolve without treatment. | ||||
| Zycortal 25mg/ml prolonged-release suspension for injection in dogs | Dechra Limited | Desoxycortone pivalate | Additional text: Pancreas disorders have been reported very rarely following use of Zycortal. The concurrent administration of glucocorticoids may contribute to these signs. |
