Veterinary Pharmacovigilance in the UK Annual Review 2020 – a summary of veterinary adverse events
Published 31 August 2022
1. Annual Review 2020 – a summary of veterinary adverse events
Pet owners, farmers, animal carers, vets and anyone else interested in animal welfare should read this review. It gives information about the reports of side effects we received during the year. These reports were usually about animals, but sometimes people had a side effect after giving a medicine to an animal, or after accidentally touching or taking an animal medicine.
The annex to this review lists the changes made to medicine information leaflets during the year.
2. Highlights
During 2020 we improved 88 of the medicine information leaflets included with animal medicines. The changes made were based on adverse event reports received. They ensured that the benefits of using these medicines continued to outweigh any risks. They also made users better informed about the medicines they are using.
The VMD received 6,139 adverse event reports during 2020, almost a 14% decrease from the previous year. The Covid-19 pandemic and associated lock-down contributed to this dramatic decrease: face-to-face consultations with vets were not possible for a time and consequently regular vaccination schedules were disrupted.
The VMD would like to thank owners and veterinary professionals for continuing to report adverse events as without these reports, this valuable work would not be possible.
Detailed information about the reports received can be accessed through VMD’s adverse events dashboard.
3. Introduction
Veterinary pharmacovigilance is the monitoring of all adverse event (AE) reports, both adverse reactions and lack of expected efficacy (LEE), for emerging patterns of undesirable effects, following the use of veterinary medicines.
Without the information submitted by reporters, we would not be able to continually make users better informed about the medicines they are using.
Within the UK, vets, animal owners and other people who work with animals, administer many millions of doses of different types of veterinary medicine to animals every year. In a small number of cases, an AE occurs. This may occur during, or sometime after, the use of a medicine.
The reports described in this review are those received after everyday use of veterinary products in the United Kingdom, by animal owners, vet practices and farm workers; this review does not include reports associated with scientific studies.
During 2020, VMD received and assessed 6,139 adverse event reports. This was a significant decrease on the previous year, compared to a 0.5% decrease from 2018 to 2019.
Acting on the adverse event information received; the product information leaflet enclosed with medicines was improved for 88 products.
Most of the reports received described events that occurred in animals during or after the use of authorised veterinary or human medicines. Many reports involved the use of multiple products, some of which may not have been authorised medicines.
Some reports described reactions experienced by humans exposed to products used to treat animals. Others involved the detection of the residues of veterinary medicines in a food product intended for human consumption, usually milk, before it entered the food chain.
This Summary provides an overview of the adverse events received in 2020, and a list of the product information leaflet changes (See Annex).
4. Number and types of reports received
4.1 The overall number of adverse event reports received in 2020 was significantly lower than 2019.
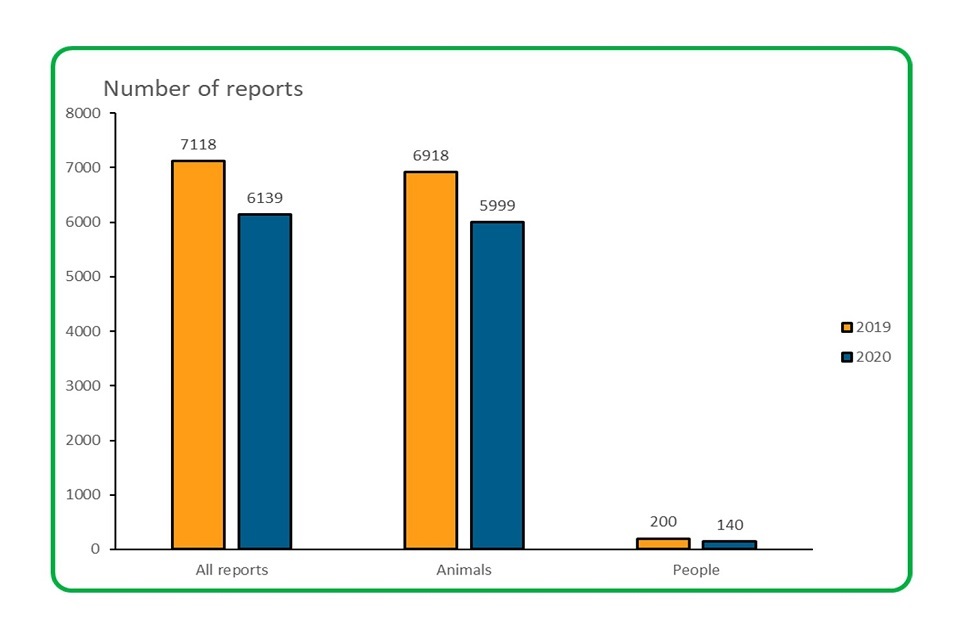
Bar Chart showing the overall number of adverse event reports received in 2019 and 2020
- The total number of reports received in 2020 decreased from 7,118 in 2019 to 6,139; a decrease of almost 14%
- Animal reports in 2020 decreased from 6,918 in 2019 to 5,999; a decrease of 13.3%)
- Reports involving people decreased from 200 in 2020 to 140; a decrease of 30%
4.2 The number of adverse event reports received for the major species decreased, except for sheep.
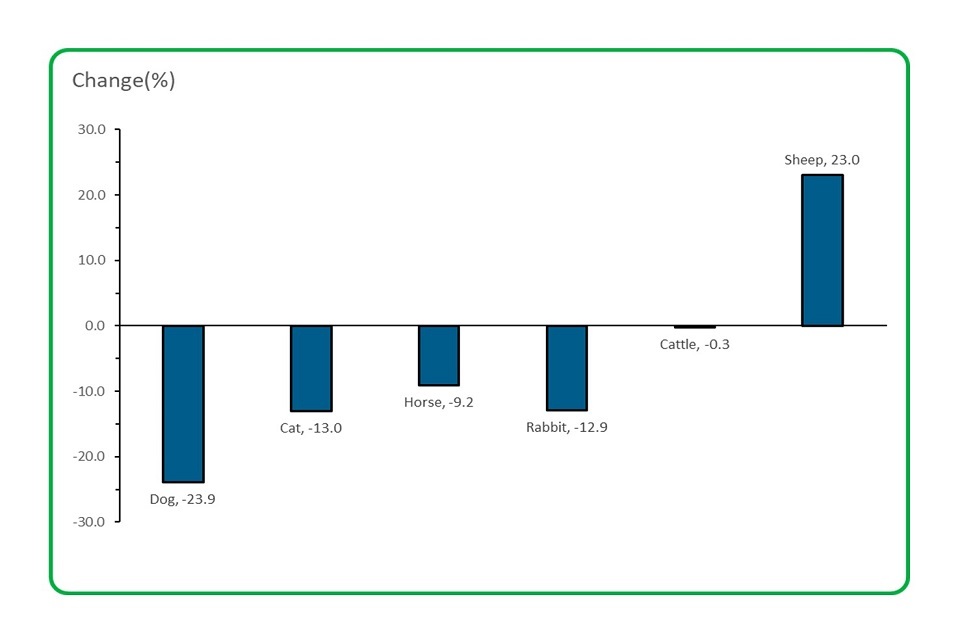
Bar chart depicting the number of adverse event reports received for the major species decreased, except for sheep
Number of reports received in 2020 for:
- dogs decreased by almost 24%
- cats decreased by 13%
- horses decreased by over 9%
- rabbits decreased by almost 13%
- cattle was almost unchanged, decreasing by 0.3%
- sheep increased by 23%
4.3 For the species most often reported, the number of lack of expected efficacy (LEE, medicine not working) reports decreased, but not for sheep.
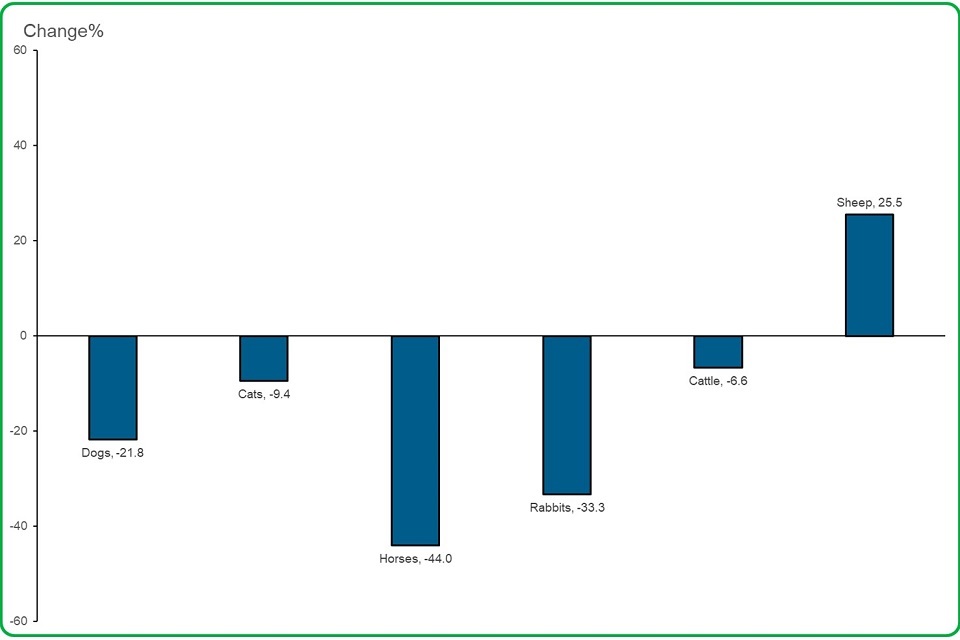
bar chart depicting For the species most often reported, the number of lack of expected efficacy (LEE, medicine not working) reports decreased, but not for sheep
- 22% decrease in the number of LEE reports involving dogs compared to 2019
- 9% decrease in LEE reports for cats
- 44% decrease in LEE reports for horses
- 33% decrease in LEE reports for rabbits
- 6.6% decrease in LEE reports for cattle
- 25.5% increase in LEE reports for sheep
For sheep, we received many lack of efficacy reports against clostridium and pasteurella vaccines between April and October. There was also a peak of lack of efficacy reports received against blowfly strike in August and September.
4.4 The number of safety (adverse reaction) reports decreased in companion animal species most often reported but increased in production species.
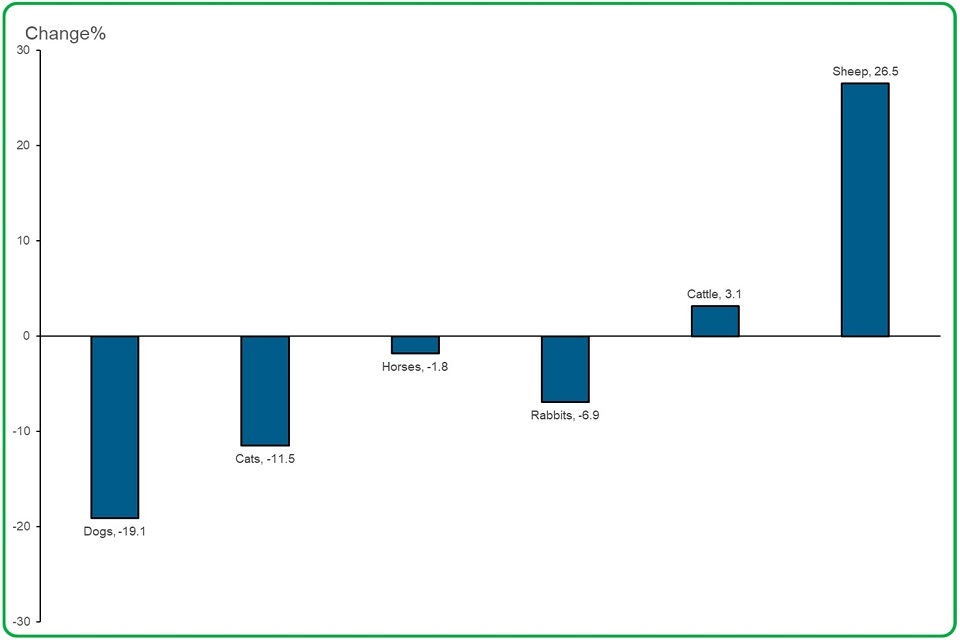
Bar Chart depicting The number of safety (adverse reaction) reports decreased in companion animal species most often reported, but increased in production species.
safety reports received for:
- dogs decreased by 19%
- cats decreased by 11.5%
- horses decreased by almost 2%
- rabbits decreased by almost 7%
- cattle increased by 3.1%
- sheep increased by 26.5%
The most safety reports in connection with sheep involved vaccines, either to prevent or reduce clostridium and pasteurella or footrot. The next most often reported products for sheep were endectocides containing moxidectin.
4.5 The number of reports of lack of expected efficacy (LEE) was much smaller for horses and pets than the number of reports of adverse reaction. For food animals, such as cattle and sheep, lack of efficacy was more often reported than problems with safety.
| Animal | Number of Adverse Reaction reports (species %) | Number of LEE reports (species %) |
|---|---|---|
| Dogs | 2894 (92.2%) | 244 (7.8%) |
| Cats | 1391 (96.7%) | 48 (3.3%) |
| Horses | 267 (90.5%) | 28 (9.5%) |
| Rabbits | 256 (87.1%) | 38 (12.9%) |
| Cattle | 131 (38.3%) | 211 (61.7%) |
| Sheep | 105 (33.7%) | 207 (66.3%) |
5. Products reported and animals affected
5.1 Most products involved in animal adverse event reports were authorised veterinary medicines.
This is to be expected as non-authorised products should only be used where a suitable authorised product is not available and VMD is only responsible for monitoring adverse events involving authorised veterinary medicines. Vets are required to use veterinary medicines authorised in the UK to treat animals, unless there is no suitable medicine available. Using their clinical judgement, they can decide to use a:
- medicine authorised in the UK for use in people
- veterinary medicine not authorised in the UK
- specially prepared (extemporaneous) medicine
In 2020 94.75% of the products mentioned in animal adverse event reports were UK authorised veterinary medicines.
Of the remaining 5.25% of products:
- 2.6% were UK medicines authorised for use in people
- 0.4% were compounded medicines, previously known as extemporaneous products
- 0.4% were imported medicines
- 0.2% were veterinary medicines that are exempt from marketing authorisation requirements
- the remaining products were either unidentified products or those without medicinal value, such as microchips or sutures, household pesticide sprays or disinfectants
5.2 Immunological products were the veterinary medicines most often associated with animal adverse event reports.
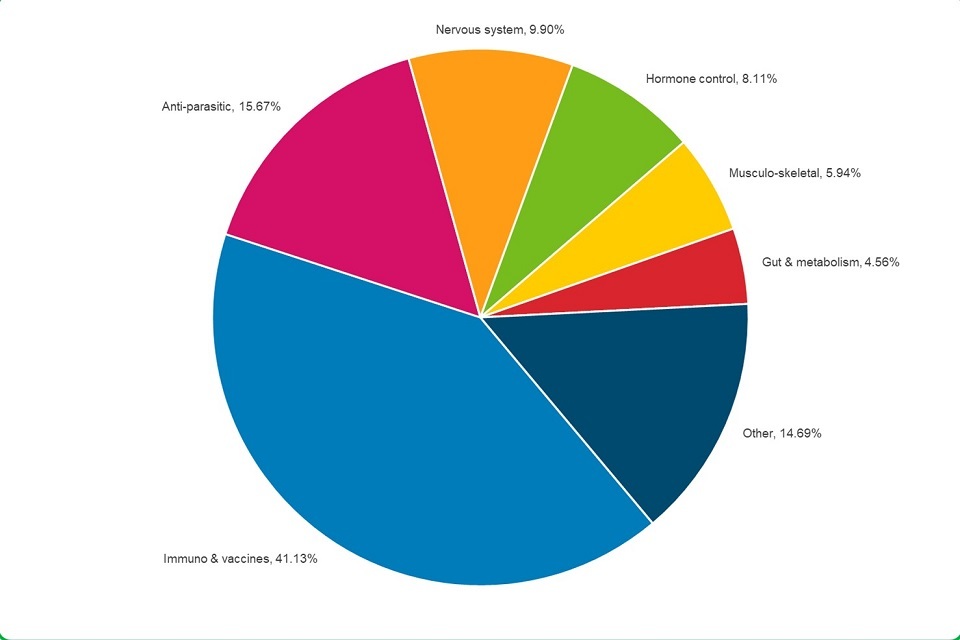
Pie Chart depicting Immunological products were the veterinary medicines most often associated with animal adverse event reports.
- 41.1% of medicines were for aiding the immune system
- 15.7% were for the control of parasites
- 9.9% were for the nervous system, mostly anaesthetics
- 8.1% were for controlling hormone balance
- 5.9% were for treatment of bone and joint problems
- 4.6% were for treating digestive and metabolic problems
Other products included:
- 3.6% treatments to prevent or treat infection, other than vaccines
- 2.4% treatments for skin problems
- 1.4% for cancer treatments, fluids, vitamins and treatments for breathing problems
- 1.3% for eye or ear problems
- 1.1% treatments for heart or circulation problems
- 0.6% for bladder problems
6. Important messages
6.1 For anyone administering veterinary medicines
- Obtain veterinary medicines from a reputable source. Look for the VMD Accredited Internet Retailer Scheme logo if you are buying medicines online.
- Always follow the instructions for use provided in the package leaflet, including any advice to avoid harm coming to you whilst administering the medicine, or in the time after administration.
- Report a problem with an authorised veterinary medicine to the Marketing Authorisation Holder or to us. We cannot take regulatory action in relation to a medicine without sufficient evidence of a problem. Social media does not provide the evidence we need.
- Report a problem with a medicine especially prepared for your animal (a compounded or extemporaneous product) to the pharmacist/vet/manufacturer of that medicine or to us. Let us know if you were given insufficient information to allow you to use the medicine safely.
- Use appropriate safety equipment when administering medicines that may be harmful to your own health.
- Use appropriate personal protective safety equipment or animal restraints when administering medicines to animals that may harm you or cause you to injure yourself with a needle if they move unexpectedly.
- Seek immediate medical attention if you accidentally inject yourself with an oil-based vaccine. Also report the incident to us, with as much information as possible.
- Be aware of the hazards posed by some surgery-only medicines, for example, inhaled anaesthetics are a risk to unborn children.
- If you are planning to euthanase a horse, follow the product leaflet guidance and have a secondary plan available, in case the original method does not achieve the required outcome. Wear head protection.
- Reduce the chance of accidental exposure by keeping animal medicines out-of-sight and reach of children (and animals) and separate from personal medicines.
- Clean and dry any dosing equipment thoroughly between uses, particularly if used with different medicines.
- Dispose of empty medicine containers promptly, in accordance with labelling instructions. Oral horse medicine syringes are attractive to dogs. Discarded ‘empties’ ingested by dogs can have serious or fatal consequences.
- If you suspect your animal has been poisoned by a veterinary medicine, seek advice from your vet or the Veterinary Poisons Information Service, then report the adverse event to us. If you have been affected, seek medical advice first and then report to us.
6.2 For people who work or play with treated animals
- Never allow animals recently treated with topical medicines e.g. spot-ons, collars, to share a bed with people.
- Always ensure that spot-on anti-parasitic products are completely dry before allowing anyone, including other pets, to kiss, cuddle or groom the treated animal.
- Do not allow your dog to run free in areas inhabited by farm animals or horses. These animals can excrete medicine residues that could be harmful to your dog, if ingested.
- Do not allow animals recently treated with topical medicines to enter streams, rivers or other water courses, as these medicines can be fatal to water life.
7. ANNEX
7.1 Product information changes relating to pharmacovigilance
The following table lists the changes made to product literature resulting from the assessment of Adverse Event report information. Without the information submitted by reporters we would not be able to continually make users better informed about the medicines they are using, and reduce some of the risks associated with the use of those medicines.
We thank all reporters for their continuing support in providing us with the essential information we need to monitor medicine safety.
This table lists all pharmacovigilance-related regulatory actions taken during 2020. Information received prior to 2020 will have contributed to the evidence leading to the initiation of these actions.
This table shows the changes made to section 4.6 of the Summary of Product Characteristics. Equivalent changes were also made to the Product Information Leaflet that is enclosed with each medicine.
7.2 For food-producing and other large animals
| Product name | Marketing Authorisation Holder | Active ingredient(s) | Updated text |
|---|---|---|---|
| Alonate-P 400 mg/g oral paste for horses and ponies | Bimeda | Pyrantel embonate | Impaction of the small intestine may occur very rarely, in foals infected with high numbers of Parascaris equorum. Symptoms (colic) may be seen as soon as 30 minutes after treatment. |
| AMOXICILLIN GLOBAL VET HEALTH 500 mg/g, powder for use in drinking water for chickens, turkeys, ducks and pigs | Global Vet Health | Amoxicillin | Penicillins and cephalosporins may cause hypersensitivity reactions which may occasionally be serious. Rarely, gastro-intestinal tract signs associated with alteration of the intestinal flora (for example, loose stools, diarrhoea) may occur. In the case of allergic reactions, treatment should be discontinued, and symptomatic treatment should be initiated. |
| Cevac Chlamydia | Ceva | Chlamydophila abortus | A transient temperature rise may be observed after vaccination (average of 1.5°C for a maximum of 3 days). In very rare cases abortions may occur where the vaccine strain can be identified. Molecular investigations revealed the possible presence of the vaccine strain 1B in placentas collected in cases of enzootic abortion of ewes vaccinated with Cevac Chlamydophila. Although the causal relationship has not been firmly established (differential diagnosis incomplete), and the representative sampling should be specified in the domestic context, the possibility that the vaccine strain can be at the origin of abortions cannot however be excluded in the present state of knowledge. This should be considered in the benefit / risk analysis conducted by the vet before prescribing the vaccine, especially in farms where the infection pressure related to Chlamydophila abortus is low. |
| Coxevac suspension for injection for cattle and goats | Ceva | Coxiella burnetii | Cattle: It was very common to see in laboratory trials a palpable reaction of maximum diameter of 9 to 10 cm at the injection site, which may last for 17 days. The reaction gradually reduces and disappears without need for treatment. Systemic signs as lethargy, hyperthermia and/or anorexia have been observed rarely in post marketing safety experience. |
| Cydectin TriclaMox 5 mg/ml + 200 mg/ml pour-on solution for cattle | Zoetis | Moxidectin, Triclabendazole | Digestive tract disorders such as diarrhoea, neurological disorders such as ataxia, hypersensitivity/allergic reactions and skin irritation at application site may be observed very rarely. |
| Hexasol LA | Norbrook | Oxytetracycline, Flunixin | The use of tetracyclines during the period of tooth and bone development, including late pregnancy, may lead to discolouration. Although the product is well tolerated, occasionally a local reaction of a transient nature may be observed. Hypersensitivity reactions (collapse) may occur very rarely. Such reactions may evolve to a more severe condition (anaphylaxis), which may be life-threatening. |
| Levafas Diamond oral suspension | Norbrook | Oxyclozanide, Levamisole hydrochloride | At normal oxyclozanide dose levels, cattle may show slight softening of the faeces with the occasional animal showing increased frequency of defecation and transient inappetence. Rarely, animals may also display signs of ataxia, incoordination, recumbency and depression. Additionally, rarely, sheep may show an allergic reaction such as submandibular oedema, ear flap oedema and swelling of the head. If such a reaction occurs, appropriate treatment should be administered without delay. Such reactions may evolve to a more severe condition (anaphylaxis), which may be life-threatening. |
| Nobilis ND C2 | MSD | Newcastle disease virus | Blinking or headshaking may be observed when ice-cold vaccine is administered via the eye/nose drop method. |
| Permacyl 236.3 mg/ml powder and solvent for injection for cattle | Divasa-Farmavic | Penethamate hydriodide | In very rare cases the symptoms of adverse reactions range from mild skin reactions such as urticaria and dermatitis to severe reactions such as anaphylactic shock with tremors, vomiting, salivation, gastrointestinal disorders and laryngeal oedema. |
| Resflor 300/16.5 mg/ml solution for injection for cattle | MSD | Florfenicol, flunixin | Anaphylactic reactions were reported in very rare cases during post-marketing surveillance. Those reactions might be fatal. Subcutaneous administration of the product may result in injection site swellings that become palpable 2-3 days after injection. The duration of the injection site swellings ranged from 15-36 days post-injection. Grossly, this is associated with minimal to mild irritation of the subcutis. Extension into the underlying muscle was noted in only a few instances. By 56 days post-dosing, no gross lesions were observed that would require any trim-out at slaughter. |
| Veteglan 0.075 mg/ml solution for injection for cows, sows and mares | Labatorios Calier | d-cloprostenol | Adverse reactions in horses including sweating (occurring within 20 minutes of treatment), increased respiratory and cardiac rates, signs of abdominal discomfort, watery diarrhoea and depression may occur when exceptionally high doses are given. However, adverse reactions are usually mild and transient. |
7.3 For pet animals
| Product name | Marketing Authorisation Holder | Active ingredient(s) | Updated text |
|---|---|---|---|
| Activyl Tick Plus spot-on solution for dogs: 75 + 240 mg, very small; 150 + 480 mg, small; 300 + 960 mg, medium; 600 + 1920 mg, large; 900 + 2880 mg, extra large | MSD | Permethrin, Indoxacarb | If adverse reactions occur bathe the animal with mild soap and rinse with large amounts of water. |
| Alfaxan/Alfaxan Multidose 10 mg/ml solution for injection for dogs, cats and pet rabbits | Jurox | Alfaxalone | Based on post marketing safety experience, neurological signs (convulsions, myoclonus, tremor, prolonged anaesthesia), cardio respiratory signs (cardiac arrests, bradycardia, bradypnea) and behavioural signs (hyperactivity, vocalisation) have been reported very rarely. |
| Amoxibactin tablets: 50 mg for dogs and cats; 250 mg for dogs; 500 mg for dogs | Le Vet Beheer | Amoxicillin | Mild gastrointestinal symptoms (diarrhoea and vomiting) may occur very rarely (less than 1 animal in 10,000 animals treated, including isolated reports) after administration of the product. Hypersensitivity reactions (allergic skin reactions, anaphylaxis) may occasionally occur very rarely. In these cases, administration should be discontinued, and a symptomatic treatment given. |
| Bravecto Chewable tablets for dogs: 112.5 mg, extra small; 250 mg, small; 500 mg, medium; 1000 mg, large; 1400 mg, extra large | Intervet | Fluralaner | Lethargy, muscle tremor, ataxia and convulsions have been reported very rarely in spontaneous reports. Most reported adverse reactions were self-limiting and of short duration. |
| Bravecto spot-on solution for dogs: 112.5 mg, extra small; 250 mg, small; 500 mg, medium; 1000 mg, large; 1400 mg, extra large | Intervet | Fluralaner | Mild and transient skin reactions such as erythema or alopecia at the application site were commonly observed in clinical trials (1.2% of treated dogs). Emesis, lethargy and anorexia have been reported very rarely in spontaneous reports after the use of this product. |
| Caniphedrine tablets for dogs: 20 mg; 50 mg | Richter Pharma | Ephedrine | In rare cases increased pulse frequency, ventricular arrhythmia and central nervous excitation have been observed. These symptoms disappear following dose reduction or termination of treatment. Due to the pharmacological properties of ephedrine the following effects can occur at the recommended therapeutic dose: Cardiovascular effects (like tachycardia, atrial fibrillation, stimulation of the heart activity and vasoconstriction). Stimulation of the central nervous system (leading to sleeplessness, excitation, anxiety and muscle tremors). Mydriasis. Bronchodilatation and decrease of mucus release in the respiratory mucosal membranes. Reduction of the motility and tone of the intestinal wall. |
| Cardalis chewable tablets for dogs: 2.5 mg/20 mg; 5 mg/40 mg; 10 mg/80 mg | Ceva | Benazepril | Vomiting, diarrhoea and pruritus have been reported very rarely in spontaneous reports. |
| Carporal tablets for dogs: 40 mg; 160 mg | Le Vet Beheer | Carprofen | Typical undesirable effects associated with NSAIDs such as vomiting, soft faeces/diarrhoea, faecal occult blood, loss of appetite and lethargy have been reported very rarely. These adverse reactions occur generally within the first treatment week and are in most cases transient and disappear following termination of the treatment but in very rare cases may be serious or fatal. |
| Cestem flavoured tablets for dogs: medium and small; large | Ceva | Febantel, praziquantel, pyrantel | Gastro-intestinal signs (vomiting, diarrhoea), possibly associated with lethargy, have been observed very rarely in spontaneous reports. |
| Ectoline Duo Spot-on solution for cats & dogs: 50 mg/ 60 mg, cats; 100 mg/ 120 mg, very large cats; 67 mg/20 mg, small dogs; 134 mg/40 mg, medium dogs; 268 mg/80 mg, large dogs; 402 mg/ 120 mg, very large dogs | Virbac | Fipronil, Pyriproxyfen | Transient cosmetic effects such as wet appearance or slight scaling can occur very rarely at the application site. According to the accumulated experience on these active ingredients within spot on pharmaceutical forms, transient cutaneous reactions at the application site as squamosis (scaling of the skin), local alopecia (hair loss), pruritus (itchiness), erythema (redness of the skin), skin discolouration) and general pruritus or alopecia may be very rarely observed after use. Hypersalivation, reversible neurologic symptoms as hyperesthesia (increased sensitivity to stimuli), depression and nervous symptoms may very rarely be observed. Respiratory signs or vomiting might occur in very rare cases. |
| Endectrid spot-on solution for dogs: 40 + 10 mg, small; 100 + 25 mg, medium; 250 + 62.5 mg, large; 400 + 100 mg, extra large | Elanco | Imidacloprid, moxidectin | Use of the product may result in transient pruritus in dogs. Vomiting can occur on rare occasions. Transient local skin sensitivity reactions including increased itching, hair loss, greasy fur and redness at application site have been reported in very rare cases in spontaneous (pharmacovigilance) reports. These signs disappear without further treatment. If the animal licks the application site after treatment, neurological signs (most of which are transient) may be observed in very rare cases. |
| Enurace 50, 50 mg tablets for dogs | Ecuphar | Ephedrine | Cardiovascular effects like tachycardia, atrial fibrillation, stimulation of the heart activity; and vasoconstriction. Stimulation of the central nervous system leading to sleeplessness, excitation, anxiety and muscle tremors; Panting; Mydriasis; Cystitis; Bronchodilation and decrease of mucus release in the respiratory mucosal membranes; Reduction of the motility and tone of the intestinal wall. Due to the nature of ephedrine the mentioned effects can occur at the recommended therapeutic dose, with anxiety and cardiovascular effects being the most prevalent. In 10% of the treatments, side effects have been observed in efficacy studies. Vomiting has been reported very rarely in spontaneous reports. |
| ERAVAC emulsion for injection for rabbits | Laboratorios Hipra SA | Rabbit haemorrhagic disease virus | Lethargy and/or inappetence may be observed very rarely in the first 48 hours after injection based on post-authorisation pharmacovigilance reporting. |
| Eurican Lmulti suspension for injection | Boehringer Ingelheim | Leptospira canicola, L. icterohaemorrhagiae, L. grippotyphosa | A slight swelling (≤ 2 cm) at the injection site may commonly be observed after injection. It usually regresses within 1-6 days. This can, on some occasions, be accompanied by slight pruritus, heat and pain at the injection site. Transient lethargy and emesis may also commonly be observed. Anorexia, polydipsia, hyperthermia, diarrhoea, muscle tremor, muscle weakness and injection site cutaneous lesions may uncommonly be observed. Hypersensitivity reactions (facial oedema, anaphylactic shock, urticaria) may rarely occur, some of which are life-threatening. Appropriate symptomatic treatment should promptly be provided. |
| FILAVAC VHD K C+V suspension for injection for rabbits | Filavie | Rabbit haemorrhagic disease (RHD) virus, RHD virus type 2 | A temporary increase in body temperature of up to 1.6°C has been observed very commonly one day after vaccination in clinical studies. A limited local reaction (subcutaneous nodule, the size of which was up to 10 mm in diameter in the double dose study) which may be palpable for at least 52 days and which disappears without treatment has been observed very commonly in clinical studies.Serious hypersensitivity reactions which may be fatal have been reported very rarely from post marketing pharmacovigilance reporting. Lethargy and/or inappetence have been reported very rarely in the first 48 hours after injection, from post marketing pharmacovigilance reporting. |
| Fipralone Duo spot-on solution for dogs: 67 mg/20 mg, small; 134 mg/40 mg, medium; 268 mg/80 mg, large; 402 mg/120 mg, very large | Alfamed | Fipronil | Transient cosmetic effects such as wet appearance or slight scaling may occur at the application site. According to the accumulated experience on these active ingredients within spot on pharmaceutical forms, transient cutaneous reactions at the application site (squamosis, local alopecia, pruritus, erythema, skin discolouration) and general pruritus or alopecia may be observed after use. Hypersalivation, reversible neurologic symptoms (hyperesthesia, depression, nervous symptoms), respiratory signs or vomiting might occur. These effects occur in very rare cases. |
| Fipronil – (S) - Methoprene Ceva spot-on solution: cats 0.5 – 5 kg; cats >5 kg and dogs 2 – 10 kg; dogs 10 – 20 kg; dogs 20 – 40 kg; dogs 40 – 60 kg | Virbac | Fipronil, pyriproxyfen | Transient cosmetic effects at the application site such as spiking of the hair, wet appearance, dry residue or slight scaling were very rarely observed in spontaneous reports. These changes do not affect the safety or the efficacy of the product. Transient hypersalivation (mainly due to the excipients of the product) after licking the product and vomiting after swallowing were observed very rarely in spontaneous reports. Alopecia and pruritus at application site have been reported very rarely based on post marketing safety experience. |
| Pro-Tel Flavoured Tablets for dogs: medium and small; large | Ceva | Febantel, praziquantel, pyrantel | Gastro-intestinal signs (vomiting, diarrhoea), possibly associated with lethargy, have been observed very rarely in spontaneous reports. |
| Purevax FeLV | Boehringer Ingelheim | Feline leukaemia virus | A temporary small (< 2 cm) nodule, which regresses within 1 to 4 weeks, was very commonly observed at the site of injection during safety and field studies. Transient lethargy and hyperthermia were very commonly observed during safety and field studies and lasted usually for 1 day, exceptionally for 2 days. Anorexia and emesis have been reported very rarely based on post marketing safety experience. A hypersensitivity reaction may occur in very rare cases. Such reactions may evolve to a more severe condition (anaphylaxis). If such reactions occur, appropriate treatment is recommended. |
| Rimadyl Palatable tablets for dogs: 20 mg; 50 mg;100 mg | Zoetis | Carprofen | Typical undesirable effects associated with NSAIDs, such as vomiting, soft faeces/diarrhoea, faecal occult blood, loss of appetite and lethargy have been reported. These adverse reactions generally occur within the first treatment week and are in most cases transient and disappear following termination of the treatment but in very rare cases may be serious or fatal. If adverse reactions occur, use of the product should be stopped, and the advice of a veterinarian should be sought. As with other NSAIDs there is a risk of rare renal or idiosyncratic hepatic adverse events. |
| Scalibor Protectorband medicated collar: 0.76 g 48 cm, small and medium dogs; 1.0 g 65 cm, large dogs | MSD | Deltamethrin | Local skin reactions (e.g. pruritus/scratching, erythema/rash, hair loss) involving the neck or the skin in general have been observed which might indicate a local or general hypersensitivity reaction in rare cases. Altered behaviour (e.g. lethargy or hyperactivity) often associated with skin irritation has been reported in very rare cases. Gastrointestinal symptoms such as vomiting, diarrhoea and hypersalivation have been observed in very rare cases. Neurological problems such as ataxia and muscle tremor have been observed in very rare cases. The symptoms usually subside within 48 hours after removal of the collar. If any of these symptoms occur, the collar should be removed. Treatment should be symptomatic as no specific antidote is known. |
| Seresto Collar for cats; Seresto Collar for dogs; ≤ 8 kg, small; > 8 kg, large | Elanco | Imidacloprid, flumethrin | In rare cases behavioural disorders that may include hiding, vocalization, hyperactivity, excessive licking and/or grooming or scratching at the application site may be observed in animals that are not used to wearing collars on the first few days after fitting. Aggression after collar application was reported in very rare cases. Ensure that the collar is fitted correctly. Application site reactions such as pruritus, erythema and hair loss may occur. These have been reported as uncommon and usually resolve within 1 to 2 weeks.In single cases, a temporary collar removal may be recommended until the symptoms have disappeared. In rare cases, application site reactions such as dermatitis, inflammation, eczema, lesions or haemorrhage may occur and in these instances, collar removal is recommended. Also in rare cases, slight and transient reactions as depression, change of food intake, salivation, vomiting and diarrhoea might occur initially. As in other topical applications, allergic contact dermatitis might occur in hypersensitive animals. |
| Simparica tablets for oral use in dogs: 5 mg; 10 mg; 20 mg; 40 mg; 80 mg; 120 mg | Zoetis | Sarolaner | Mild and transient gastrointestinal signs such as vomiting and diarrhoea and systemic disorders such as lethargy, anorexia/inappetence may occur in very rare cases based on post marketing experience. These signs typically resolve without treatment. Neurological disorders such as tremor, ataxia or convulsion may occur in vary rare cases based on post marketing experience. In most cases these signs are transient. |
| Thyforon Flavoured Tablets for dogs: 200 microgram; 400 microgram; 600 microgram; 800 microgram | Eurovet | L thyroxine | Restoration of physical activity may unmask or intensify other health-related problems, such as osteoarthrosis. Adverse reactions of thyroid hormones are generally associated with excessive dosage and correspond to the symptoms of hyperthyroidism, including weight loss without loss of appetite, hyperactivity, excitability, panting, tachycardia, polydipsia, polyuria and polyphagia. Hypersensitivity reactions (pruritus) have been reported very rarely. |
| X-spectra flavoured tablets for dogs: large; medium and small | Ceva Animal Health | Febantel, praziquantel, pyrantel | Gastro-intestinal signs (vomiting, diarrhoea), possibly associated with lethargy, have been observed very rarely in spontaneous reports. |
| Zoletil 100 (50 mg/ml+50 mg/ml) lyophilisate and solvent for solution for injection for dogs and cats | Virbac | Zolazepam, tiletamine | Pain upon injection has been reported very rarely. This is most prevalent in cats. The following signs have been reported very rarely, mainly during the awakening phase in the dog, and during surgery and the awakening phase in the cat; Neurological signs – prostration, convulsions, coma. Cardio-respiratory signs – dyspnoea, tachypnoea, bradypnoea, tachycardia, cyanosis, have been noted at doses of 20 mg/kg and above. Certain systemic signs – hypothermia, hyperthermia, pupillary disorder, hypersalivation, hypersensitivity to external stimuli, agitation, vocalisation. Prolonged anaesthesia and difficulties when awakening (with myoclonus, restlessness, ataxia, paresis, etc.) have been observed in the recovery phase. All reactions are reversible and disappear once the active substance is eliminated from the body. |
| Zycortal 25 mg/ml prolonged release suspension for injection for dogs | Dechra | Desoxycortone pivalate | Polydipsia and polyuria were very common adverse reactions in a clinical trial. Inappropriate urination, lethargy, alopecia, panting, vomiting, decreased appetite, anorexia, decreased activity, depression, diarrhoea, polyphagia, shaking, tiredness and urinary tract infections were common adverse reactions in a clinical trial. Injection site pain has been reported uncommonly in post-authorisation spontaneous reports following the administration of Zycortal. Pancreas disorders have been reported rarely in post-authorisation spontaneous reports following use of Zycortal. The concurrent administration of glucocorticoids may contribute to these signs. |
