Annual Statistics of Scientific Procedures on Living Animals, Great Britain, 2020
Updated 15 July 2021
1. Key results
| -15% | In 2020, 2.88 million procedures were carried out in Great Britain involving living animals. |
| This is a decrease of 15% on last year, and the lowest number of procedures since 2004. | |
| 50% | Around half of all procedures were experimental procedures (1.44 million). |
| The other half were for the creation and breeding of genetically altered (GA) animals (1.44 million). | |
| 92% | The majority (92%) of procedures (both for experimental and breeding purposes) used mice, fish, or rats. |
| These species have been the most used for more than a decade. | |
| 53% | Over half (53%) of experimental procedures were for the purpose of basic research. |
| Most commonly focusing on the the immune system, the nervous system, and cancer. |
2. Summary statistics
- 2.88 million procedures were carried out in Great Britain involving living animals in 2020
2.1 Experimental procedures
These procedures involve using animals in scientific studies for purposes such as: basic research and the development of treatments, safety testing of pharmaceuticals and other substances, specific surgical training and education, environmental research and species protection.
-
1.44 million procedures carried out for experimental purposes
-
57% of procedures used mice
-
14% of procedures used rats
-
13% of procedures used fish
-
over half of experimental procedures were for basic research, the top three areas targeted in this research were the immune system, the nervous system and oncology (cancer)
-
96% of all experimental procedures were assessed as sub-threshold, mild, non-recovery or moderate in severity, 4% were assessed as severe
2.2 Creation and breeding of GA animals
This refers to the breeding of animals whose genes have mutated or have been modified. These animals are used to produce GA offspring for use in experimental procedures but are not themselves used in experimental procedures.
-
1.44 million procedures for the creation and breeding of GA animals
-
86% were for creation and breeding of mice
-
13% were for creation and breeding of fish
-
0.7% were for creation and breeding of rats
-
most procedures in this category were for maintenance of already established GA lines, with 10% of procedures for the creation of new lines
-
98% of all procedures for creation and breeding were assessed as sub-threshold, mild, non-recovery or moderate in severity, 2% were assessed as severe
3. Introduction
3.1 Purpose of this release
This publication meets the requirements of section 2 of the 1986 Act to publish, and lay before Parliament, annual statistics on the use of protected animals in regulated procedures.
3.2 Coverage of this release
These statistics cover England, Scotland, and Wales. For Northern Ireland, the Department of Health separately collects and publishes information on NI regulated procedures under devolved arrangements.
3.3 ‘Number of procedures’ is not ‘number of animals’
The number of procedures carried out in a year does not equal the number of animals that have been used in procedures that year. This is because some animals may be used more than once i.e. ‘re-used’, in certain circumstances. These instances are counted as separate, additional, procedures. As a result, the number of procedures is usually slightly higher than the number of animals used.
The statistics in this release and the accompanying data tables relate to the number of procedures, not the number of animals used, unless specified (i.e. data tables 1.3, 2.1, 2.2 and 2.3 relate to the number of animals).
3.4 Accompanying data tables and user guide
The accompanying data tables for this report can be found on the statistics of scientific procedures webpage. The data tables have been published online only (except for Table 1.2) which can be viewed in annex A. Since the 2018 publication, the principal data tables have been expanded to include data from 2014 with filters to allow users to view and extract the data as they wish. Within the notes section for the data tables you will find further instructions on how to use the filters. The tables that have been expanded include data from 2014 as not all data pre-2014 are comparable. See the accompanying user guide for further information.
Protected animals: Any living vertebrate, other than man, and any living cephalopod. This includes embryos after two thirds of gestation (although these are not included as countable procedures), and fish and amphibian larvae after they become capable of free feeding.
Regulated procedures: Any procedure applied to a protected animal for an experimental or other scientific purpose, or for an educational purpose, that may have the effect of causing an animal pain, suffering, distress or lasting harm equivalent to, or higher than, that caused by the introduction of a needle in accordance with good veterinary practice.
4. Total procedures
4.1 Key results
-
in 2020, there were 2.88 million procedures completed on living animals in Great Britain, this is a decrease of 15% from last year and the lowest number of procedures since 2004
-
procedures for creation and breeding have decreased by 14% and experimental procedures have decreased by 17%
Figure 1. Total scientific procedures in Great Britain, 1986 to 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 1.1. See the user guide for links to all data pre-2007.
As shown in Figure 1, the number of procedures carried out decreased from 1987 until 2001, to a low of 2.62 million. This was mainly due to a reduction in the use of rodents, rabbits and birds (although there was an increase in procedures involving fish).
After 2001, procedures increased, reaching a peak of 4.14 million in 2015, but has decreased since to 2.88 million in 2020. This is the lowest number of procedures carried out in a single year since 2004.
This year’s decrease in total procedures (15%) is the largest decrease over the last 5 years. This large decrease may be partly explained by the two national lockdowns during 2020, which may have affected the activity at establishments – see the Further information section for more details.
The number of procedures carried out on living animals is determined by several factors, including the focus of scientific and medical endeavours, the economic climate and global trends in new technologies or fields of research.
Figure 2. Total scientific procedures by type, 2010 to 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 1.2 and Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2017: time series tables, Table 1
As shown in Figure 2, the total number of procedures was rising prior to 2013, mainly due to the increase in procedures for the creation and breeding of GA animals. This increase in the earlier part of the decade can mainly be attributed to the availability of new technology which led to new research opportunities. However, more recently, the number of procedures for the creation and breeding of GA animals has been decreasing, with a decrease of 14% compared to last year.
In contrast, the number of experimental procedures remained relatively stable during the earlier part of the last decade but has decreased since 2015 similarly to procedures for creation and breeding of GA animals.
Experimental procedures involve using animals in scientific studies for purposes such as: basic research and the development of treatments, safety testing of pharmaceuticals and other substances, education, specific surgical training and education, environmental research and species protection.
Procedures for creation and breeding involve the breeding of animals whose genes have mutated or have been modified. These animals are used to produce genetically altered offspring for use in experimental procedures but are not themselves used in experimental procedures.
5. Experimental procedures
5.1 Key results
-
of the 1.44 million experimental procedures, the majority (84%; 1.22 million) used mice, fish, or rats
-
over half (53%) of all experimental procedures were carried out for basic research purposes (758,000 procedures)
-
the most common areas focused on in this research were: the immune system (20%), the nervous system (18%), and cancer (oncology;14%)
-
the severity of a procedure is determined by the degree of pain, suffering, distress or lasting harm expected to be experienced by an individual animal during the course of a procedure
-
in 2020, 96% of all experimental procedures were assessed as sub-threshold, non-recovery, mild or moderate in severity, the remainder were severe
This section covers only experimental procedures. That is, procedures that involve using animals in scientific studies for purposes such as: basic biological research, medical studies and development of treatments, training and education, environmental research, preservation of species, and safety testing of pharmaceuticals and other substances. An experimental procedure may benefit people, animals, or the environment for any of the purposes stated above. The animals used in experimental procedures may be genetically altered.
5.2 Species
The proportions of species used for experimental procedures as shown in Figure 3 have remained similar since 2014.
For most species, small year-on-year variations can be attributed to technological developments and changes in the types and stages of projects being carried out in any reporting year.
Figure 3. Experimental procedures by species, 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 1.2
Notes: Specially protected species are Cats, Dogs, Horses and Primates.
Mice, fish and rats in experimental procedures
The majority of experimental procedures used mice, fish, or rats; together these species were used in 84% of experimental procedures in 2020.
Figure 4. Experimental procedures using mice, fish and rats, 2011 to 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020 data tables: Table 1.2 and Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2017: time series tables, Table 2.2
As shown in Figure 4, there was a large decrease in the use of fish and mice (32% and 21% respectively) compared to last year. With rats having a large increase in experiments (22%), it is the second most used animal group for the first time since 2009.
The use of rats has increased for the first time since 2015 and at its highest since 2018, with a 22% increase on 2019. This is still down 23% from a decade ago. In 2020, nearly two thirds of experimental procedures involving rats (64%) were for regulatory testing (e.g. tests evaluating the safety and efficacy of substances such as pharmaceuticals), which saw an increase of 38% on the previous year.
The majority of experimental procedures involving mice in 2020 (68%) were for basic research. More specifically, most of the basic research that involved the use of mice focused on the immune system, the nervous system and oncology (cancer).
The majority of experimental procedures involving fish in 2020 (70%) were also for basic research. Most basic research that involved the use of fish focused on ethology/animal behaviour/biology, the nervous system and multisystemic.
Specially protected species in experimental procedures
Specially protected species refers to cats, dogs, horses, and primates. These species were used in 1% of experimental procedures (18,000) in 2020.
Cats, dogs, horses and primates are subject to additional protection under Section 5C of the 1986 Act. Licence holders using specially protected species must demonstrate that no other species are suitable for the purposes of the licence and must adhere to additional licence conditions.
Figure 5. Experimental procedures involving specially protected species, 2011 to 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020 data tables: Table 1.2 and Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2017: time series tables, Table 2.2.
The total number of procedures involving specially protected species has increased over the past decade from 16,000 in 2011 to 18,000 in 2020. Most notably the increase in the use of horses which has increased 29% and cats which have decreased 38% over the past decade – procedures on cats accounted for just 0.8% of all experimental procedures involving specially protected species.
The number of procedures involving horses had remained relatively stable over the past decade until the increase in 2017. The figure for 2020 has remained at a similar level to 2017 and is the highest since 2008. In 2020, the majority (96%) of experimental procedures that used horses were for regulatory procedures. The main regulatory procedure carried out on horses was for the routine production of blood-based products, which are used for a variety of diagnostic purposes.
The number of experimental procedures that used cats has increased by 11% on last year. There were 150 experimental procedures that used cats in 2020, with 42% in regulatory testing. This is a large increase in the proportion of cats used for regulatory testing, as none were reported over the last 5-year period.
The species of primates that were used in experimental procedures in 2020 were cynomolgus monkeys (2,069 procedures), rhesus monkeys (220 procedures) and marmosets and tamarins (104 procedures). The total procedures (2,393 procedures) is the lowest since 2008. The only primates used in regulatory procedures were cynomolgus monkeys.
Note: these figures do not follow the rounding conventions as stated in the user guide.
In 2020, the use of dogs increased by 3% in the last year but decreased by 5% over the last 10 years. In 2020, the majority of experimental procedures that used primates and dogs were for regulatory procedures (76% and 67% respectively). These were mainly for testing the safety of products and devices for human medicine, dentistry, and veterinary medicine.
Use of endangered species
Information was collected on whether any endangered species, as listed in Annex A of Council Regulation (EC) No 338/97, were used.
Of the 3,024 returns, 2 reported the use of endangered animals in 2020; specifically one species of eel was used in the research of the conservation of the species and one species of wild bird was used in the research of the protection of the natural environment in the interests of the health or welfare of human beings or animals.
5.3 Place of birth of primates
Of the 1,700 primates used for the first time in experimental procedures in 2020, all marmosets and tamarins and rhesus monkeys were born in the UK at a licensed establishment, whereas 98% of cynomolgus monkeys were born in either Africa or Asia.
The place of birth of primates used in experimental procedures for the first time can be found in Table 2.2 of the data tables. The place of birth of all other species used in experimental procedures for the first time in each year since 2014 can be found in Table 2.1 of the data tables.
The generation of non-human primates used for the first time in experimental procedures can be found in Table 2.3 of the data tables.
Basic research: aims to expand our knowledge of the structure, functioning and behaviour of living organisms and the environment.
Applied research: attempts to address diseases through prevention and development of treatments. Within the data tables, this is shown as ‘Translational/Applied research’.
Regulatory testing: procedures carried out to satisfy legal requirements, including: ensuring substances are produced to legal specification; evaluating the safety or effectiveness of pharmaceuticals and other substances.
5.4 Genetic status
Of the 1.44 million experimental procedures completed in 2020, 63% used animals that were not genetically altered.
Figure 6. Experimental procedures by genetic status, 2011 to 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 4 and Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2017: time series tables, Table 3.2
As shown in Figure 6, and in line with the overall decrease in experimental procedures in 2020, the number of experimental procedures involving non-GA animals has decreased by 10% in the last year, and by 41% over the last decade.
The use of GA animals in experimental procedures had been relatively stable from 2015 to 2019. In 2020 there was a 26% decrease in procedures compared to 2019, with a 15% decrease over the last decade. The drop in 2020 procedures, compared to last year, mirrors the overall decrease in creation and breeding procedures, as shown in Figure 2. This is possibly related to reduced activity in some research sectors during the two national lockdowns.
Further information regarding the genetic status of GA animals used in experimental procedures in 2020 can be found in Table 4 of the data tables.
5.5 Purpose
As shown in Figure 7, just over half (53%) of the experimental procedures carried out in 2020 were for basic research. A further 33% were conducted for regulatory testing purposes, and the remainder were mostly for applied research (14%).
Figure 7. Experimental procedures by purpose, 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 1.2
Notes: Experimental procedures carried out for higher education or training, the preservation of species and for the protection of the national environment accounted for 1% and therefore are not visible.
The proportions shown in Figure 7 have remained stable since 2014 when the data was first collected using these purpose classifications. Compared to last year there has been a slight decrease in the proportion of all procedures apart from regulatory which has increased from 26% in 2019 to 33% in 2020. The experimental purpose classifications prior to 2014 are not directly comparable.
Basic Research
In 2020, 758,000 experimental procedures were carried out for basic research purposes. The most common areas focused upon in this research, as shown in Figure 8, were: the immune system (20%), nervous system (18%) and cancer (oncology; 14%).
Figure 8. Most common areas focused upon in experimental procedures for basic research, 2020
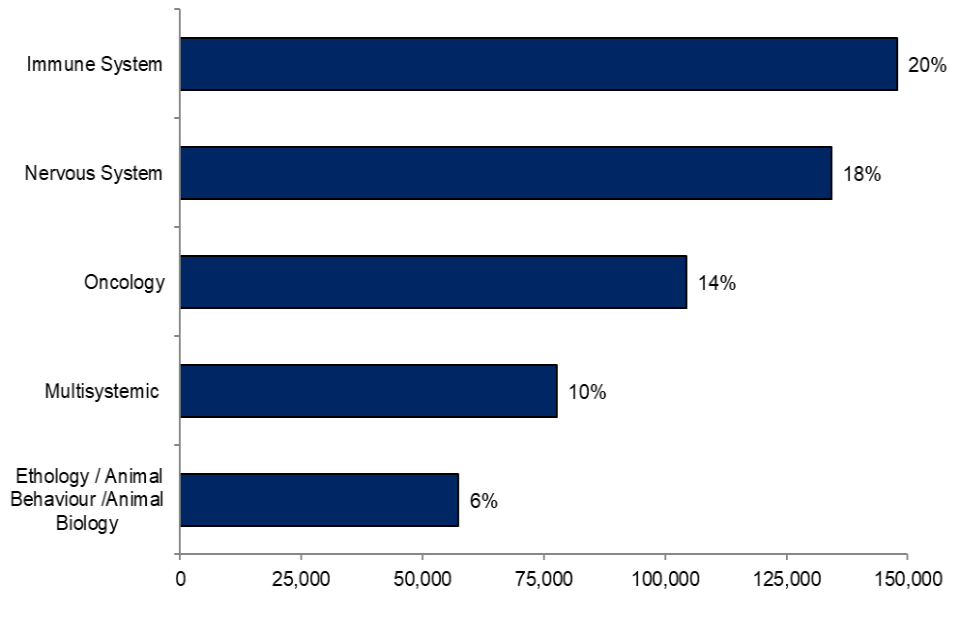
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 5
Notes: Research is classified as multisystemic when numerous body organs and systems are targeted.
The distribution of purposes for basic research has remained mostly similar since 2014. Studies into the immune system, the functioning and disease of the nervous system and cancer, including its development and control mechanisms (oncology) have been reported within the top five most common areas for basic research in each year since 2014.
For data on all purposes for basic research by species, see Table 5 of the data tables.
Applied research
There were 196,000 experimental procedures for applied research (14% of all experimental procedures) in 2020. Applied research attempts to address diseases through prevention and development of treatments and, as shown in Figure 9, the most common areas of research were human cancer (25%), human infectious disorders (19%), and human nervous and mental disorders (19%).
Almost all the experimental procedures for applied research focusing on human cancer used mice (99.5%); the remaining 0.5% involved rats, pigs and zebrafish. Almost all (99.99%) experimental procedures for applied research focusing on mental disorders used mice, rats, or fish; the remaining 0.01% of procedures involved pigs and sheep.
Figure 9. Most common areas focused upon in experimental procedures for applied research, 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 6
Notes: Non-regulatory toxicology and ecotoxicology covers toxicology, method development and investigations prior to regulatory studies.
Similar to basic research, the distribution of purposes for applied research has also remained similar since 2014, with human cancer, infectious disorders, and nervous and mental disorders consistently being within the top five most common areas of applied research in each year.
For data on all purposes for applied research by species, see Table 6 of the data tables.
Regulatory
There were 473,000 procedures carried out for regulatory purposes in 2020 (33% of all experimental procedures). Regulatory procedures are carried out to satisfy the legal requirements necessary to enable materials, products, and devices to be licensed for use. Regulatory procedures are usually carried out during the final stages of research and development and focusses on safety and efficacy. The most common procedure in 2020 was toxicity and other safety testing (40%).
Of the 473,000 regulatory procedures in 2020, the most common legislative requirements were legislation on medicinal products for human (41%) or veterinary use (28%). No procedures were carried out for cosmetics testing.
The majority (94%) of regulatory procedures were undertaken to satisfy UK and/or EU legislation.
Routine production: covers studies carried out for manufacturing processes requiring regulatory approval.
Toxicity and other safety testing: studies for safety evaluation of products and devices for human medicine, dentistry, and veterinary medicine.
Quality control: the testing of quality control parameters of a product, and any controls carried out during the manufacturing process for registration purposes, to satisfy any other national or international requirements or to satisfy the in-house policy of the manufacturer.
Other efficacy and tolerance testing: efficacy testing of biocides and pesticides is covered under this category as well as the tolerance testing of additives in animal nutrition.
Figure 10 shows the proportion of each purpose of regulatory procedures carried out in 2020, with toxicity and other safety testing, routine production, and quality control accounting for 40%, 33% and 24% respectively.
Figure 10. Experimental procedures for regulatory purposes by sub-purpose, 2020
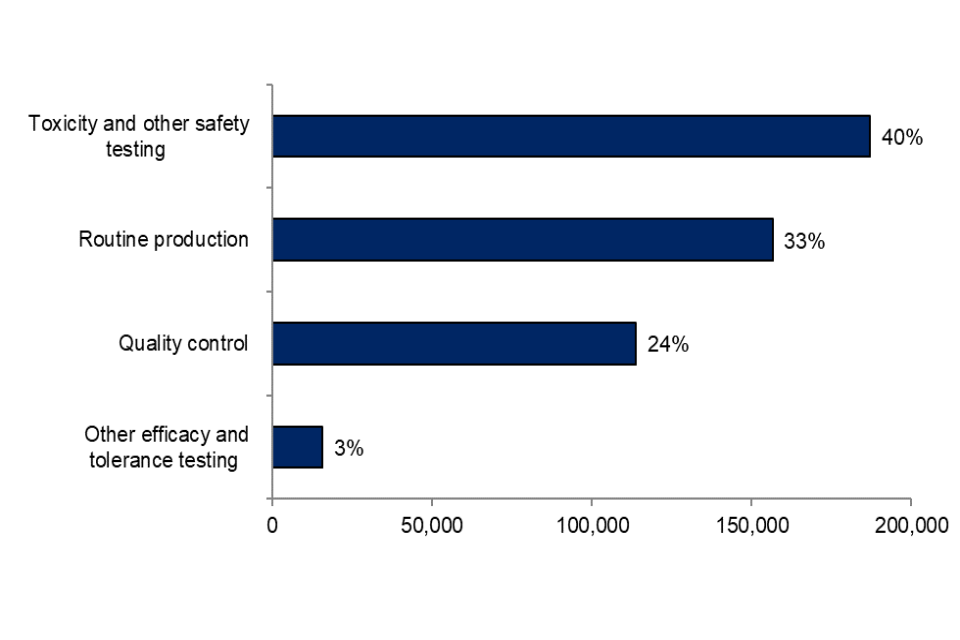
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 7.1
In contrast to their predominant use in experimental procedures for basic research and applied research, mice were used in under a third (29%) of all regulatory procedures, although they were still the most commonly used species. The second most common species used in regulatory procedures were rats (27%; 126,000). Of which, 98% were for toxicity and other safety testing including pharmacology.
Techniques of special interest
Information was collected on whether any procedures were related to techniques of interest to the Home Office (i.e. areas related to Home Office policies). The areas of interest include testing of alcohol, tobacco, and household products.
In 2020, there were 301 experimental procedures which involved the testing of household product ingredients. There was no re-use of animals for these procedures, therefore of the 301 animals; 270 minnows, 25 mice and 6 rats were used in procedures. All the rats and mice, as well as 125 minnows, were used in procedures that were assessed as mild. 145 minnows were used in procedures that were moderate.
There were 61 experimental procedures which involved the testing of products containing alcohol, in which there was no re-use of animals. All of the animals used were mice, and all of the procedures they were used in were assessed as mild.
An additional area of interest is ascites methods of monoclonal antibody production because a non-animal alternative exists. No ascites methods of monoclonal antibody production were used in 2020.
Note: these figures do not follow the rounding conventions as stated in the user guide.
Rodenticide trials
Rodenticides are a category of pest control chemicals intended to kill rodents. Rodenticide trials are field trials of such chemicals and are occasionally undertaken by commercial companies that produce them to assess how safe and effective they are when used.
Of the 3,024 returns, one reported that rodenticide trials occurred in 2020. We ask data suppliers only to indicate whether field trials of rodenticide substances occurred, as these trials are conducted in semi-field situations where the number of animals is not accurately known as the colonies are not intensively managed.
5.6 Severity
The severity (i.e. pain, distress or suffering) experienced by animals in procedures has been recorded since 2014. There are five severity assessments:
Sub-threshold: When a procedure was authorised under a project licence but did not actually cause suffering above the threshold of regulation, i.e. was less than the level of pain, suffering, distress or lasting harm that is caused by inserting a hypodermic needle according to good veterinary practice.
Non-recovery (under general anaesthesia): When the entire procedure was carried out under general anaesthesia from which the animal shall not recover consciousness.
Mild: Any pain or suffering experienced by an animal was, at worst, only slight or transitory and minor so that the animal returns to its normal state within a short period of time.
Moderate: The procedure caused a significant and easily detectable disturbance to an animal’s normal state, but this was not life threatening. Most surgical procedures carried out under general anaesthesia and with good post-operative analgesia (i.e. pain relief) would be classed as moderate.
Severe: The procedure caused a major departure from the animal’s usual state of health and well-being. This would usually include long-term disease processes where assistance with normal activities such as feeding and drinking were required, or where significant deficits in behaviours/activities persist. It includes animals found dead unless an informed decision can be made that the animal did not suffer severely prior to death.
Severity assessments measure harms to an animal during a procedure and generally reflect the peak severity of the entire procedure; they do not include harms caused to animals as a result of non-procedural events such as transport and housing.
Figure 11. Experimental procedures by severity, 2016 to 2020
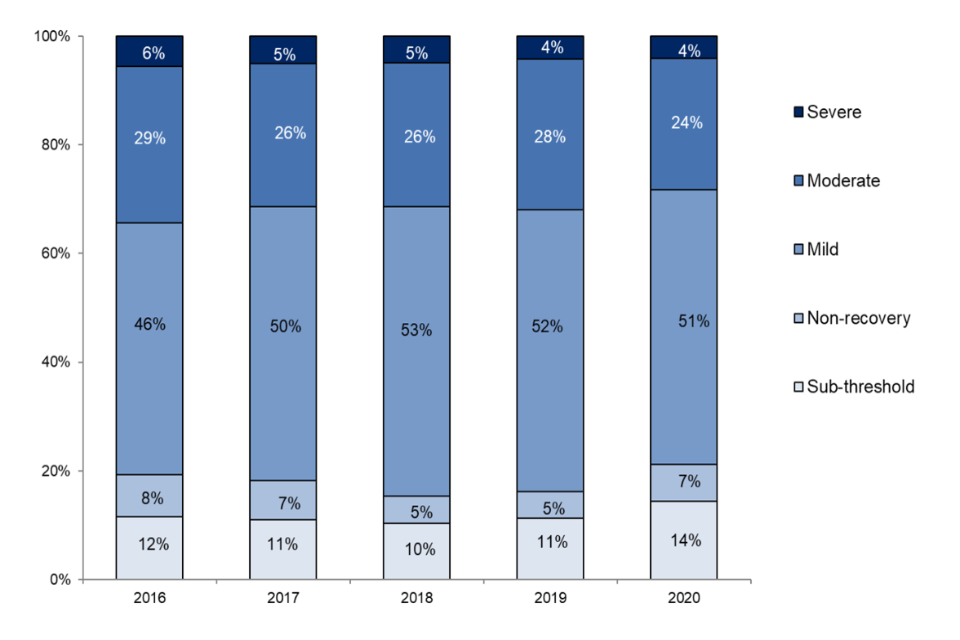
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 3.1
The proportions of severity assessments for procedures reported in 2020 were relatively similar to those seen in previous years (Figure 11), with just over half (51%) of experimental procedures in 2020 being mild. The proportion of moderate and severe severity assessments for procedures have decreased from 2019 to 2020, to 32% and 28% respectively. Whilst the proportion of sub-threshold and non-recovery have increased from 16% to 21% respectively.
The severity assessment of experimental procedures varies according to the purpose. However, as shown in Figure 12, the most common severity assessment was mild for each purpose of experimental procedure.
Figure 12. Experimental procedures by severity and purpose, 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 3.1
Neuromuscular blocking agents and anaesthesia
Neuromuscular blocking agents (NMBA) are used for muscle relaxation during some types of experimental procedure such as nerve stimulation under anaesthesia. NMBAs may be used only when given the authority to do so and with an appropriate level of anaesthesia and/or analgesia as determined in the project license.
The use of NMBA was recorded in 20 of the 3,024 returns. Of these, 18 returns reported that use of NMBA was whilst the animal was under general anaesthesia.
6. Creation and breeding of genetically altered animals
6.1 Key results
-
almost all (over 99%) of the 1.44 million procedures for the creation and breeding of GA animals involved mice, fish and rats
-
most procedures counted under creation and breeding (90%) were for the maintenance of already established GA lines
-
the majority (73%) of procedures for creation and breeding in 2020 were assessed as sub-threshold in severity
This section covers only procedures counted under the creation and breeding of GA animals. That is, the breeding of animals whose genes have mutated or have been modified and have not been subsequently used in other procedures.
6.2 Species
Almost all (over 99%) of the procedures for the creation and breeding of GA animals involved mice (86%), fish (13%), or rats (0.7%). Other species used for creation and breeding of GA animals include: amphibians, ungulates (including pigs), and birds – but together they accounted for 0.3% of these procedures.
No specially protected species (horses, dogs, cats, or primates) were used in procedures counted under creation and breeding of GA animals.
There were 10 genetically altered dogs which were subsequently used in experimental research, so are not included in creation and breeding figures.
Genetic status
Of the 1.44 million procedures for creation and breeding that used GA animals in 2020, the majority (85%) used GA animals with no harmful phenotype (i.e. the animals did not appear or behave any differently from non-GA animals).
Figure 13. Creation and breeding of GA animals by type of genetic alteration, 2016 to 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 8
As shown in Figure 13, there has been an increase in the proportion of animals used for creation and breeding that are genetically altered without a harmful phenotype (rising from 74% in 2016 to 85% in 2020).
There were some animals that were bred with the intention of producing GA animals, but resulted in non-GA animals being born (3% of animals in this category in 2020). In addition, some animals used for the creation of a new genetic line will also have been genetically normal animals (e.g. those used for superovulation).
6.3 Purpose
As shown in Figure 14, of the total 1.44 million procedures for the creation and breeding of GA animals, 90% were for the maintenance of already established GA lines, with the remainder of procedures for the creation of new lines.
Figure 14. Creation and breeding of GA animals by purpose, 2020
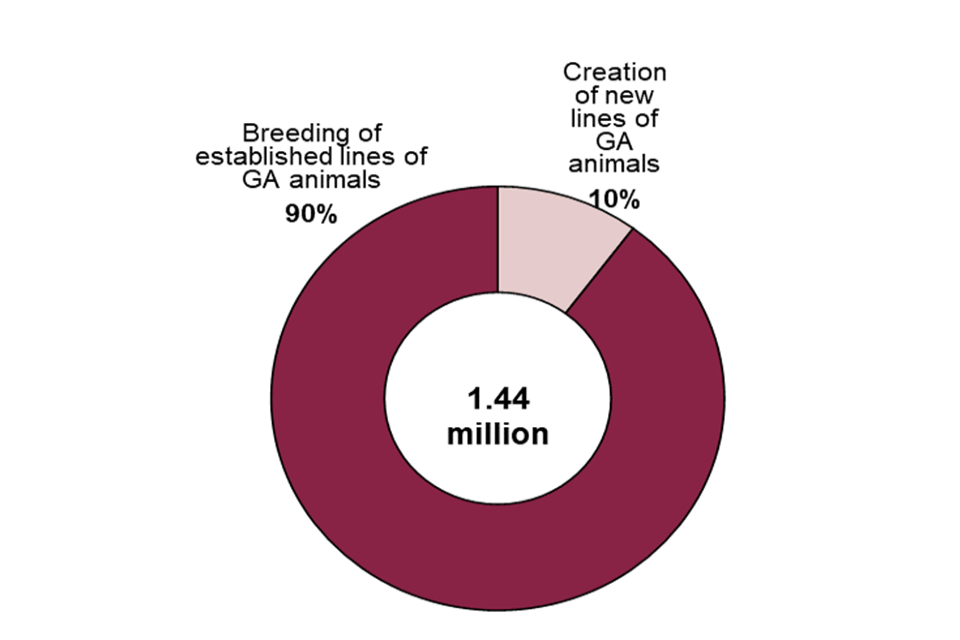
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Tables 8, 9.1 and 10
Of the 147,000 procedures that were for the creation of new GA lines, most (87%) were to create new GA lines to be used in basic research. The most common areas within basic research were multisystemic research (39,000 breeding procedures), the immune system (23,000 breeding procedures), nervous system (22,000 breeding procedures) and oncology (17,000 breeding procedures).
Creation: includes the natural breeding of different stains to produce a new strain and procedures that use standard techniques such as vasectomy for the generation of novel transgenic or mutant lines of GA animals. The birth of a GA animal counts as creation when the line is new and before is it ‘established’ (i.e. stable and characterised).
Breeding: the production of GA animals of an established line that has been bred for at least two generations. Breeding procedures also include other techniques applied to the animal after birth e.g. genotyping but not any techniques applied as part of an experiment or study.
6.4 Severity
Animals in this category were not used in regulated experimental procedures. As such, the severity experienced by GA animals created and bred are assessed as follows:
-
the observable characteristics (phenotype) of the animals, e.g. development of congenital disease (i.e. diseases present at birth) or tumours
-
in the case of animals that have no harmful phenotype but that have been biopsied specifically for genotyping (the process of taking a sample of tissue (a biopsy) and then testing it to determine the genetic make-up of an animal), the biopsy procedures will generally be assessed as mild
-
the animals assessed as severe in this category are largely animals within breeding colonies that were found dead and where the death of the animal was either a result of its phenotype or, more commonly, unexplained (all animals found dead are reported as severe unless an informed decision can be made that the animal did not suffer severely prior to death)
-
a small number of the animals used to create new lines of GA animals will have been subjected to surgical procedures (classed as moderate) or the injection of drugs (classed as mild)
Nearly three quarters (73%) of procedures counted under creation and breeding in 2020 were assessed as sub-threshold.
Figure 15. Creation and breeding of GA animals by severity, 2016 to 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 8.
Notes: For each year, non-recovery procedures have accounted for less than 1% and are therefore not visible on the chart.
The severity assessments for creation and breeding in 2020 have remained similar to 2019, where sub-threshold procedures make up the large majority (73%), and only 2% of creation and breeding procedures were assessed as severe. Mild procedures account for 23%, followed by moderate (2%) and non-recovery (0.1%).
As shown in Figure 15, in 2017 there was an increase in proportion of sub-threshold. Since 2017 the proportions across all severities has remained relatively stable.
7. Establishment and project licences
All projects and establishments seeking to conduct regulated procedures on living animals must be licensed under Animals (Scientific Procedures) Act 1986 (ASPA).
During 2020, there were 153 establishment licences 10 of which did not have any active project licences and 3,024 project licences in force.
Figure 16. Procedures and project licences by establishment, 2020

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2020: data tables, Table 11
As shown in Figure 16, the majority of project licences are held by those conducting research at universities or medical schools (81%), although just over half (54%) of all procedures took place at these universities or medical schools. In contrast, 7% of project licences are held within commercial organisations, however, they conducted 27% of all procedures carried out in 2020; this was an increase on last year with a proportion of 22% in 2019. This is due to commercial organisations conducting large programmes of work involving repetitive procedures and tests under fewer project licences. Whilst government departments proportion of licenses stayed the same compared to last year, the number of procedures dropped from 4% in 2019 to 1% in 2020.
8. Further information
Frequency of release: Annually
Forthcoming release: Home Office statistics release calendar
Home Office responsible statistician: Daniel Shaw, Chief Statistician
This report contains statistics on regulated scientific procedures performed using living animals under the Animals (Scientific Procedures) Act 1986 (ASPA).
8.1 Accompanying user guide
See the accompanying user guide for information including:
-
background information on the data collection
-
uses of the statistics, and links to related statistics
-
details on methodology and data quality issues
8.2 Covid-19 and animal procedures
The figures presented in this release relate to the number of scientific procedures on animals during the period January 2020 to the end of December 2020. In response to the coronavirus pandemic, restrictions in England and Wales started from 12 March 2020 and lockdown was applied on 23 March 2020, which imposed strict limits on daily life. This meant activity at establishments may have been affected. No extra data was collected in relation to the pandemic on its effect on the establishments.
8.3 Data quality
The UK Statistics Authority has designated these statistics as National Statistics, in accordance with the Statistics and Registration Service Act 2007, signifying compliance with the Code of Practice for Statistics.
8.4 National Statistics Status
National Statistics status means that our statistics meet the highest standards of trustworthiness, quality, and public value, and it is our responsibility to maintain compliance with these standards.
The designation of these statistics as National Statistics was confirmed in 2007 following a compliance check by the Office for Statistics Regulation. The statistics last underwent a full assessment of compliance against the Code of Practice in 2012.
Since the latest review by the Office for Statistics Regulation, we have continued to comply with the Code of Practice for Statistics, and have made the following improvements:
- easier, more intuitive data entry with pop-up messages alerting suppliers to incompatible combinations of data being entered
- each year, we consult with colleagues in the Animals in Science Regulation Unit to ensure the collection remains suitable for its purpose
8.5 Revisions
It is standard practice across all Home Office statistical releases to incorporate revisions to previous years’ data in the latest release. Corrections and revisions follow the Home Office’s statement of compliance with the Code of Practice.
The time series data tables published in the 2020 statistical report include any revisions that have been made to previously published data for the years 2014 to 2019. Since the 2019 publication the following has been revised:
| What has changed | Number of procedures affected |
|---|---|
| Purposes changed from Protection of the natural environment in the interests of the health or welfare of human being or animals to Regulatory use | 4929 |
| Severity of procedures on zebra fish changed from Severe to Sub-threshold | 211 |
| Number of Mice used increased | + 1919 |
| Number of Rats used increased | + 46 |
8.6 Changes in legislation and definitions
Prior to 1986, figures were recorded for the number of ‘experiments’ on living animals, under the Cruelty to Animals Act 1876. In 1986, the Animals (Scientific Procedures) Act was introduced, and required all ‘scientific procedures’ to be recorded. This new, broader term largely explains the increase in figures directly after 1986 (see Figure 1).
At the beginning of 2013, an EU Directive (2010/63/EU) came into effect, and as a result changed the way in which the data was collected under UK law from 2014 onwards. All figures for procedures (1986 onwards) are comparable as the definition of a procedure is unchanged. As a result of the change in methodology, the 2014 data is subject to data quality issues (see the user guide for further information).
8.7 Additional statistics for animal use in Great Britain
The annual statistics release covers regulated procedures on living animals, under the Animals (Scientific Procedures) Act (ASPA) 1986. This comprises of procedures carried out using animals for experimental purposes, and procedures counted under creation/breeding of genetically altered (GA) animals (i.e. the use of GA animals to create offspring for use in experimental procedures). The use of non-GA animals for breeding, to produce non-GA offspring for use in experimental procedures, is covered under the 1986 Act but is not included in the annual statistics. The annual statistics also do not include the use of other animals ‘used’ specifically in the support of the production and use of animals in experimental procedures or e.g. sentinel animals for the monitoring of disease within the facilities. This data on breeding an genotyping of animals for 2017 was published by the Home Office in November 2018 on GOV.UK.
8.8 Feedback and enquiries
We welcome feedback on the annual statistics release. If you have any feedback or enquiries about this publication, please contact the Statistical Transformation Team, the Home Office Unit which produced the statistics.
Public enquiries: HOAIStatisticalTransformation@homeoffice.gov.uk
Press enquiries: pressoffice@homeoffice.gov.uk
Telephone: 020 7035 3535
