Zika virus: sample testing advice
Guidance on who to test for Zika virus infection and which samples to collect.
Access to Zika virus testing services
This guidance on the laboratory diagnosis of Zika virus infection is intended for healthcare professionals in the UK. Patients concerned about possible Zika virus infection should consult an appropriate healthcare professional, for example their GP, in the first instance.
Health professionals wishing to discuss a possible case, or to ascertain local arrangements, should contact a local Infection specialist (such as a consultant in infectious diseases, microbiology or virology).
NHS testing for Zika virus is available only through the Rare and Imported Pathogens Laboratory (RIPL) at PHE Porton. RIPL provides medical and laboratory specialist services to the NHS and other healthcare providers, covering advice and diagnosis of a wide range of unusual bacterial and viral infections, including Zika virus infection.
Zika virus infection is not a notifiable disease. Therefore there is no statutory requirement to test for this infection if the test result will have no impact on clinical management.
Management of asymptomatic returned travellers
The Zika virus testing service is currently not available for individuals who have had no symptoms suggestive of Zika infection, including private patients. Such individuals include:
- asymptomatic pregnant women who have travelled from Zika-affected countries
- asymptomatic returned male travellers whose partners are currently pregnant
- asymptomatic returned male and female travellers who are trying to conceive
These individuals should be advised to follow PHE guidance on prevention of pregnancy and avoidance of sexual transmission as appropriate.
Note that symptoms such as coryza, cough and/or sore throat suggest upper respiratory tract infection and not Zika virus infection.
RIPL does not provide a Zika screening service (NHS or private) for returning holidaymakers without symptoms suggestive of Zika virus infection but who would like to be tested for family planning reasons. They should be advised to follow PHE guidance on contraception after travel to countries or areas with risk for Zika virus transmission.
Couples planning conception should be strongly advised to follow PHE guidance and delay conception for 3 months after return from a country or area with risk for Zika, rather then pay for private Zika tests that may not be fully validated for testing people who don’t have symptoms.
Investigation of patients with current or previous symptoms
Clinicians should consider Zika virus infection for:
- any patient who has, or has had, a rash illness or other symptoms suggestive of Zika virus infection, that began whilst in any country or area with risk of Zika virus transmission, or within 2 weeks of leaving that country
- any patient presenting with typical Zika-like symptoms apparently due to sexual transmission; that is, there is no history of travel a Zika-affected country or the symptoms began more than 2 weeks after travel to a Zika-affected country, and their male sexual partner had travelled within the last 3 months from a country or area with risk of Zika virus transmission
When assessing patients presenting with acute symptoms, doctors should also consider other travel-associated infections in the differential diagnosis, including:
- dengue and chikungunya virus infections
- malaria
- common infections not associated with travel, for example, influenza
- non-infectious diseases
Clinicians should also read PHE guidance on viral rash in pregnancy for other possible causes of a rash.
Which samples to collect from patients concerned about Zika virus infection
Submit serum sample (and urine if indicated) with the appropriate request form, as shown in the algorithm below. Inappropriate samples (for example, EDTA plasma in addition to serum, or urine samples taken more than 21 days after symptom onset) and those submitted with inadequately completed forms will be stored without testing. A sample handling and storage fee is charged in such cases (see charges).
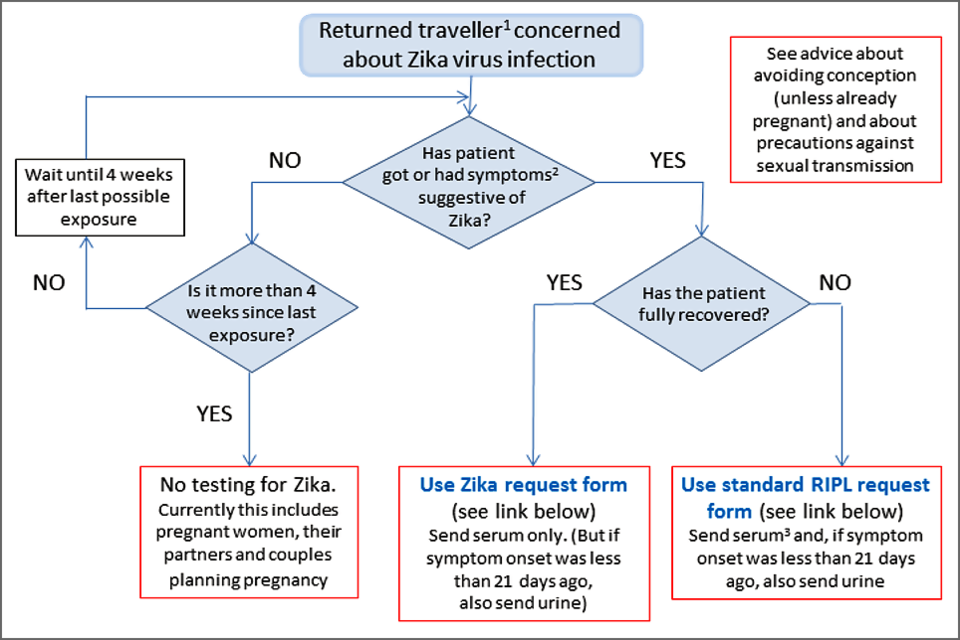
Zika testing algorithm
- Zika virus infection should also be considered in any non-traveller whose male sexual partner has travelled within the last 3 months from a country or area with risk of Zika virus transmission, particularly if this partner is male.
- Rash or at least 2 other symptoms suggestive of Zika virus infection that began whilst in a country or area with risk of Zika virus transmission or within 2 weeks of leaving or within 2 weeks of last possible sexual exposure.
- Last possible exposure is defined as the later of either the date of leaving a country or area with risk for Zika virus transmission, or the date on which last unprotected sexual contact with a potentially infectious partner took place.
- An acute serum sample should be obtained and submitted promptly. However, a follow-up sample may be required subsequently; see Interpretation of Zika virus test results.
Completion of RIPL Request Forms
Diagnostic samples must be submitted with a completed request form providing details of the patient’s travel history, symptoms and, if relevant, pregnancy. There is no need to send a separate form with each sample. Samples submitted with inadequate clinical information usually will be stored without testing. A sample handling and storage fee is charged in such cases (see charges).
The appropriate request form should always be selected using the algorithm above. For any returning traveller who is significantly unwell with acute febrile illness or who requires admission to hospital, provided detailed information about symptoms and travel to Latin America, the Caribbean or South East Asia is included, RIPL will perform Zika testing as part of a geographic panel of PCR and serology tests, without Zika having to be specifically requested.
Ideally, the appropriate request form should be printed out and completed (except for the sender’s information at the top) by the clinician who sees the patient. Send the request to the local laboratory with the clinical sample(s) along with a local laboratory request form, whether this is paper or electronic.
The local laboratory should complete the sender’s information on the request form and then forward the completed form and sample(s) to RIPL. However, local arrangements may vary, so clinicians are advised to liaise with their local laboratory first, before sending samples.
Standard sample types for routine Zika virus testing
Where serum is indicated in the algorithm above, clinicians should submit a clotted blood sample (“plain” or “serum separator gel” tube) to their local laboratory. The local laboratory should submit a minimum of 0.8 mL serum to RIPL.
Please do not submit EDTA whole blood or plasma samples in addition to serum as these sample types are no longer required for routine Zika testing. Such unnecessary samples may be stored without testing, incurring a sample handling and storage fee.
Where urine is indicated in the algorithm above, clinicians should submit urine (5 mL is sufficient, minimum volume 1 mL) in a Universal tube (without any preservative) to their local laboratory for forwarding to RIPL.
Other samples (including obstetric and neonatal) suitable for Zika virus testing
Semen
Semen can be tested for Zika RNA, but only if prior arrangement with RIPL has been made. Returned male travellers with current or previous symptoms should be investigated as indicated in the algorithm in the first instance.
A local infection specialist or senior member of the obstetric team must contact RIPL to discuss the case, before sending samples in any of the following scenarios:
Fetal abnormality (with or without amniocentesis)
Any case where an abnormal fetal ultrasound scan in a returned pregnant traveller is being investigated: maternal blood samples will be required for testing in addition to any amniotic fluid.
Termination of pregnancy
Any case where termination of pregnancy is performed for known or presumptive congenital Zika: appropriate samples for diagnostic testing will include maternal serum, placenta, cord tissue and fetal (brain) tissue.
Miscarriage or stillbirth
Any case of miscarriage or stillbirth in a woman with known or possible Zika infection (having travelled to a country or area with risk of Zika virus transmission earlier in the pregnancy): appropriate samples for diagnostic testing will include maternal serum, placenta, cord tissue and fetal (brain) tissue.
Anticipated live delivery following known maternal Zika infection
The pregnancy of any woman with laboratory-diagnosed Zika infection should be followed up in a Fetal Medicine Unit.
Whether antenatal fetal ultrasound scans have been normal or not, contact RIPL a few weeks before the anticipated live delivery to make arrangements to submit appropriate diagnostic samples for Zika virus testing. These will include placenta, cord tissue, neonatal serum, contemporaneous maternal serum, and neonatal urine. Neonatal CSF will only be appropriate if a lumbar puncture is clinically indicated.
Live delivery following undiagnosed Zika-like maternal illness
Any neonate delivered to a woman who travelled to a country or area with risk of Zika virus transmission earlier in the pregnancy and who had an illness that might have been Zika but who was not subsequently tested for Zika antibodies (even if Zika PCR tests performed in the acute phase were negative): appropriate samples for diagnostic testing will be advised on a case-by-case basis.
Note that no Zika virus testing will be required for a normal neonate born to a woman who travelled to a country or area with risk of Zika virus transmission earlier in her pregnancy but who either:
- had a negative Zika antibody test 4 weeks or more after her last possible exposure to Zika virus, or
- had no symptoms suggestive of Zika virus infection during travel or within 2 weeks of leaving and who did not have an antenatal Zika virus antibody test performed; if maternal testing for Zika antibodies was not performed antenatally, it is not necessary to perform it postnatally
Zika virus laboratory tests available at RIPL
PCR testing for detection of Zika virus RNA
- the in-house real-time PCR used at RIPL is based on a published assay. It has undergone full technical validation at RIPL for use on serum/plasma, urine and semen, and further in-service quality assurance continues
- the assay can be performed on blood, urine, saliva (mouth swabs), semen, amniotic fluid and tissue such as placenta or umbilical cord (see Other samples suitable for Zika virus testing)
Positive results are always telephoned to the referring laboratory and clinical significance discussed.
- Zika virus PCRs are performed daily, Monday to Friday, at RIPL and results are reported within 5 working days of sample receipt
- the cost of testing is as indicated for any real-time PCR in the RIPL User Manual. Note that where both serology and PCR are performed on a patient’s sample(s), as is often required, the total charge will be limited to the RIPL panel flat fee
Serological testing for the detection of Zika antibodies
- tests for Zika IgG and IgM currently in use at RIPL are commercial ELISAs that have undergone full technical validation at RIPL, for use on blood samples from patients with symptoms suggestive of Zika virus infection. Further in-service quality assurance is ongoing
- the preferred sample for serological testing is serum
Positive results are always telephoned to the referring laboratory and likely clinical significance discussed.
- Zika virus antibody tests are performed daily, Monday to Friday, at RIPL and so results are usually reported within 5 working days of sample receipt
- the cost of testing is as indicated in the RIPL User Manual. Note that where both serology and PCR are performed on a patient’s sample(s), as is often required, the total charge will be limited to the RIPL panel flat fee
Sample handling and storage without testing
Inappropriate samples (such as unnecessary EDTA plasma, or urine samples taken more than 21 days after symptom onset) and those submitted with inadequate clinical information will usually be stored without testing. The charge for this sample handling and storage is as indicated in the RIPL user manual.
Interpretation of Zika virus test results
Detection of Zika virus RNA in any sample is diagnostic of infection with this virus. If Zika virus RNA is not detected in a patient’s samples, this does not exclude previous infection with this virus.
Detection of Zika virus IgM, together with Zika virus IgG, in a serum sample from an individual who has had recent symptoms, will usually suggest recent Zika virus infection.
Detection of Zika virus IgG without IgM, in a serum sample from an individual who has had recent symptoms, will often reflect recent Zika virus infection. This is because Zika virus IgM is frequently not detectable in individuals who have previously had dengue virus infection. Sometimes it is not possible to determine whether a positive Zika virus IgG result (without IgM) is due to recent Zika virus infection, past Zika virus infection, cross-reactivity from another flavivirus infection or non-specific reactivity. In such cases, it is usually appropriate to manage the patient as if they may have had recent Zika virus infection.
If Zika virus antibodies are not detected in a serum sample collected at least 2 weeks after the onset of an acute viral illness featuring fever, rash, arthralgia or conjunctivitis in a pregnant woman, appropriate investigations for alternative causes including parvovirus, rubella, CMV, dengue and chikungunya infections must be carried out, if not already performed. If no firm diagnosis is made, the patient should be individually discussed with a local Infection specialist and contact with RIPL for further advice considered.
If Zika virus antibodies are not detected in a serum sample collected 4 or more weeks* after the last possible travel-associated or sexual exposure, then recent Zika virus infection is highly unlikely.
Therefore, pregnant women with negative antibody results for such samples do not require further extra fetal ultrasound follow-up, unless there are additional concerns.
*This period of 4 or more weeks is derived by adding together 14 days, representing the estimated upper limit of the incubation period for Zika virus, and a further 14 days representing a maximum time for the appearance of Zika antibodies after symptom onset (although available evidence indicates that antibodies usually appear much earlier than this).
Contact details and testing laboratory address
Main RIPL number
01980 612 348. Service available 9am to 5pm, Monday to Friday.
Imported Fever Service
0844 77 88 990. This service is available, 24 hours a day, 7 days a week, for urgent discussion of any acutely unwell febrile patient in whom severe travel-associated infection is suspected. Please do not use this number for routine Zika enquiries.
Testing laboratory address
Rare and Imported Pathogens Laboratory
PHE Porton
Manor Farm Road
Porton Down
Wiltshire
SP4 0JG
DX address
DX 6930400, Salisbury 92 SP
Updates to this page
-
Updated guidance on managing asymptomic patients, and investigating patients with current or previous symptoms.
-
Updated guidance on Zika testing.
-
Updated in line with changes in travel and sexual transmission advice and revised Zika virus risk ratings, and changes to sample submission process.
-
Clarification of testing arrangements.
-
Revised substantially to reflect the use of serological testing.
-
Updated symptom list.
-
Updated Zika testing advice.
-
First published.