Life Sciences Vision (HTML)
Published 6 July 2021
Forewords
Foreword 1
From Rt Hon Boris Johnson MP, Prime Minister
I cannot think of a time when we have been more indebted to the astonishing power of Life Sciences, so much of it pioneered here in our United Kingdom.
From the discovery of dexamethasone to the global reach of the Oxford AstraZeneca vaccine, British genius and ingenuity is saving millions of lives around the world and allowing us to restore our liberties and livelihoods without spreading this lethal disease to our loved ones.
Yet these extraordinary achievements are not merely the product of brilliant science, they have also required a radically different way of supporting it. Driven by an urgency for results and a willingness to take risks, the Vaccine Task Force used government funding to mobilise private sector investment and inspire a seamless collaboration between our scientists, pharmaceutical companies, regulators, and NHS.
The great opportunity before us now is to learn the lessons of this success and make this exception the new norm, bottling the formula we have developed to tackle Covid and applying it to the search for life-changing breakthroughs against other diseases.
This document sets out our vision for doing so, helping to regain our status as a Science Superpower by making our United Kingdom the leading global hub for Life Sciences. Modelled on the approach of the Vaccine Task Force, we will direct our record investment in scientific research towards new missions – uniting our world leading academic base, the power of our capital markets and the amazing data resource of our NHS to forge ground-breaking advances against diseases such as cancer, dementia, and obesity.
We will utilise the full breadth of our regulatory freedoms from Brexit to make the UK the best place in Europe to invest in a life-science business, helping to create high-skilled and high-paid jobs that will level up communities right across the country.
Our new approach will also help to level up our United Kingdom in another vital way – perhaps the most important of all – because these diseases that are the target of our new missions are underlying causes of gross inequities in life expectancy between different parts of our country. By pioneering a new focus on prevention and early diagnosis, and by harnessing the transformative power of treatments such as cell and gene therapies, we can go further than ever before in meeting the economic, social, and moral imperative of levelling up world class health outcomes across the land.
Over hundreds of years, the work of British pioneers like Edward Jenner, Ronald Ross and Alexander Fleming have saved countless lives around the world. With this new vision, we will give their 21st century successors – like Dame Sarah Gilbert – all the support they need to do the same. There could be no more fitting legacy from all that we have been through – and no greater way to build back better.
Foreword 2
Foreword from:
- Rt Hon Sajid Javid MP, Secretary of State for Health and Social Care
- Rt Hon Kwasi Kwarteng MP, Secretary of State for Business, Energy and Industrial Strategy
- Professor Sir John Bell, GBE, FRS; Regius Professor of Medicine, The University of Oxford
- Sir Jonathan Symonds, CBE
- Lord David Prior, Chair of NHS England
Life Sciences will be one of the great drivers of growth in the twenty-first century.
Through innovation and technological advances, we will diagnose, treat, cure and prevent a much wider range of disease than is currently possible.
The research and development that leads to innovative new healthcare products will underpin a globally successful industry. There is a race on to determine which countries will lead this sector over the next decade and the prize both in terms of economic growth and human health is large. The UK is ideally positioned to compete successfully in this field with outstanding science, and globally successful pharma, biotechnology and medtech sectors.
The UK’s research and innovation response to COVID-19 demonstrates how the country can act as a global centre for innovation when government, the sector and NHS work together. For this reason, our life sciences vision has been co-developed by these same partners to ensure we have shared goals that can be delivered by working and innovating together.
Our opportunity now is to apply this approach and mindset to 7 great healthcare challenges: Cancer, Dementia, Mental Health, Obesity, Ageing, Respiratory Disease and Vaccines – and use the drive and ingenuity of the private sector, skill and intellect of UK academia and scale and expertise of the NHS to make meaningful process.
This focus on healthcare challenges will be complemented by simultaneously seeking to improve the effectiveness and attractiveness of every element of the UK Life Science ecosystem. In particular, we will collectively work to:
- build on the UK’s world class science and research capabilities – making the UK the best place in the world to trial and test products at scale, underpinned by an ever improving genomic and health data infrastructure
- make the NHS the country’s most powerful driver of innovation – through the development, testing and adoption of new technologies at a population-scale, using new technology to get diagnosis and treatment right first time, and building genuine trust between the NHS and the sector about what can be achieved by working closely together
- create an outstanding business environment for Life Science companies – in which incentives and structures are aligned to support company growth, innovation and investment – underpinned by a world class regulatory environment and bringing to bear the full financial firepower of the City of London to support companies to grow
Throughout this work, our other key partners will be patients and the public. Their trust and buy-in will be critical to success in many areas – and their response to COVID-19 – whether partaking in clinical trials at an unprecedented scale or supporting a diagnostic revolution in the UK – demonstrate just how ready they are to support the research and innovation agenda.
Delivery of all the proposed policies and programmes contained in this vision will be challenging. However, in developing this vision we have been struck by the extraordinary degree of alignment between all parties involved – both in terms of what should be delivered, and how we can work together most effectively to execute our plans. The pandemic response, and in particular the Vaccine Taskforce, demonstrates how we can fuse together the very best elements of industry, the NHS, government, and philanthropy to deliver remarkable results.
Collectively, we will work together tirelessly to ensure that this Life Science Vision delivers on its full potential.
Introduction
The human Life Sciences sector is among the most valuable[footnote 1] and strategically important in the UK economy, and critical to the country’s health, wealth, and resilience. In recent decades, advances in the Life Sciences have fundamentally improved the length and quality of life in the UK and globally, and we stand on the cusp of an era of cures, in which new technologies make previously terminal disease treatable or curable.
The sector has also been integral to the response to the COVID-19 pandemic – the greatest challenge of the post-war era. From the development of the Oxford/AstraZeneca vaccine and the partnerships between industry and the Vaccine Taskforce (VTF) that have underpinned the UK’s vaccination programme, to the RECOVERY trial identifying safe and effective therapeutics,[footnote 2] to the growth of a diagnostics industry that is sequencing emerging COVID-19 variants – UK Life Sciences have played a significant role in the global fight against COVID-19. The UK was able to have a leading Life Sciences response to COVID-19 through combining a set of existing strengths with new ways of working.
The existing strengths included:
- a world class science base, with deep expertise from basic science through to clinical research – allowing innovation to move rapidly from bench to bedside
- the National Health Service (NHS[footnote 3]), which had the research focus, capacity, and expertise to run enormous COVID-19 trials while under unprecedented pressure and the genomic capabilities to track the spread of, and variations in, the virus.
- the Medicines and Healthcare Products Regulatory Agency (MHRA), as an independent, sovereign regulator able to act with great agility and a focus on getting vaccines, drugs, and technologies to patients as safely and quickly as possible
- a thriving Life Sciences sector, with 2 of the world’s largest pharmaceutical companies, a rich array of SMEs and a wide range of medical research charities – which were willing to work together and partner to support the national and global response to the pandemic
- a highly successful Life Sciences Strategy, that ensured the UK had the infrastructure and connections to mount a rapid, multifaceted response to the many Life Sciences challenges created by the pandemic[footnote 4]
These existing capabilities were allied with a set of new ways of working:
- an at-risk mindset – accelerating and investing in projects with a clear understanding that the outcome was uncertain, and that it was possible that none of the projects would work
- integration of procurement – research and development (R&D) investments and risk were linked to procurement from the start, providing the incentive and structure for business to seriously engage, and included a concerted effort to ensure small and medium-sized enterprises (SMEs) could act as key partners
- clear and measurable objectives and metrics – so progress could be tracked and managed, and government could set clear asks of the Life Sciences sector
- private sector engagement – was fundamental and underpinned by deep regulatory engagement, cooperation on infrastructure to support trials and manufacturing, and the appropriate sharing of risk
- clear accountability and leadership – with the senior sponsorship and industrial experience required to deliver, and all unnecessary bureaucracy removed to support delivery
- long term legacy planning was central from the start – which made investments more impactful and provided stability and certainty to companies when co-investing with government
The collective ambition of the government and the sector is for the UK to build on the scientific successes and ways of working from COVID-19 to tackle future disease challenges – silent pandemics – including cancer, obesity, dementia, ageing; securing jobs and investment and becoming the leading global hub for Life Sciences. The opportunity for the life sciences sector is to work collaboratively with the UK’s best academics, the National Health Service (NHS)[footnote 5], and regulators to accelerate the development of new drugs, diagnostics, medtech and digital tools to bring life-changing innovations to patients more quickly.
This vision builds on the success of the 2017 Life Sciences Industrial Strategy and Life Sciences Sector Deals, while recognising that the context has changed significantly since those publications – with the UK’s departure from the European Union; the impact of the pandemic; and the organisational transformation of the NHS in England.
It is the changes in the NHS – of both organisational structure in England and service delivery post-pandemic across the UK – that offer the greatest opportunity to deepen collaboration and trust between government, the NHS, and the Sector.
To be sustainable, the NHS needs to focus on the right interventions early in the course of disease, with a reinvigorated approach to deliver innovations for the major diseases that drive most morbidity and mortality, with predictive and monitoring technologies, genomics and data used to prevent, detect, diagnose, and treat disease early, rather than concentrating on late-stage disease – in line with the commitments in the NHS Long Term Plan.
Delivering this change requires an approach in which new technologies can be tested rapidly and at scale, assessed and appraised using rich data and genomics-driven insights, and then adopted and spread across the NHS more quickly than is currently the case. This will require the NHS to accelerate its transition to population health management, with the system focused on the total cost of care and the long-term benefits of innovation.
This creates significant opportunities. First, to drive value creation for the sector, with the government, Medical Research Charities and NHS taking the same mission-orientated approach to innovation seen in the pandemic across a range of diseases with patients benefiting from new ways of working, the NHS operating as a data-driven test bed for new technologies, and government making the UK a hospitable commercial and operating environment for companies to innovate, grow and invest. Second, to use this approach to level-up the UK’s deep inequalities of health, which the pandemic has highlighted and worsened. Third, to use the UK’s unique Life Sciences, engineering, and health capabilities to help define and set evidence-based global standards and rules.
The vision also reflects the richness and diversity of the sector. This includes a deep heritage in Life Sciences and the modern centres of excellence distributed across the United Kingdom; the ecosystem of entrepreneurs, small and large companies, supported by a world class academic base; and broad expertise across all the key subsectors, including medtech, pharmaceuticals, diagnostics and digital.
In developing this vision, government and the sector are mindful that the UK is only one part of a much larger – and exceptionally and increasingly competitive – global ecosystem.
To remain competitive and to deliver on the ambition set out in this vision, the UK will focus relentlessly on areas in which it has, or can gain, competitive advantage – such as Genomics and Health Data; and be rigorous in addressing areas of weakness such as the fast and comprehensive adoption of new products across NHS England and the incentives available to manufacture in the UK. Through deeper end-to-end collaboration and more coordinated co-investment between government, the NHS, and the sector we will collectively deliver impact that is significantly greater than the sum of its parts.
Following the conclusion of the next Spending Review, the government will outline its next steps in delivering the shared ambitions contained in this document.
The vision
The vision focuses on what government, the NHS, regulators, companies, medical research charities, academia and philanthropy must do to create the environment in which industry can grow and succeed in the UK, and patients and the NHS can receive a real benefit.
This is focused on 4 themes:
- building on the new ways of working from COVID-19 to tackle future disease missions
- building on the UK’s science and clinical research infrastructure and harnessing the UK’s unique genomic and health data
- supporting the NHS to test, purchase and spread innovative technologies more effectively, so that cutting-edge science and innovations can be embedded widely across the NHS as early as possible, and rapidly adopted in the rest of the world
- creating the right business environment in the UK in which companies can access the finance to grow, be regulated in an agile and efficient way, and manufacture and commercialise their products in the UK
Healthcare Missions
The vision will also focus on specific ‘Missions’ that are technology or disease specific.
In each, there is an opportunity to take a VTF-type approach, with a single empowered decision maker to mobilise private and public sector science and investment. These missions will also help the NHS to solve some of the biggest healthcare problems of our generation.
These missions are:
- Improving translational capabilities in neurodegeneration and dementia.
- Enabling early diagnosis and treatments, including immune therapies such as cancer vaccines.
- Sustaining the UK position in novel vaccine discovery development and manufacturing.
- Treatment and prevention of cardiovascular diseases and its major risk factors, including obesity.
- Reducing mortality and morbidity from respiratory disease in the UK and globally.
- Addressing the underlying biology of ageing.
- Increasing the understanding of mental health conditions, including work to redefine diseases and develop translational tools to address them.
These missions have been developed by government, NHS England, Industry, academia, and the medical research charities with a common goal of creating the right conditions for industry, academia, and the NHS to come together to focus on the major causes of morbidity. These areas have often been ignored due to the cost and complexity of developing products for these indications, particularly in the late stage of the disease; the regulatory environment, which must be more enabling of activity in these areas; and the access and uptake of successful products. Success will have a disproportionately positive impact on people living in poorer areas of the UK, where the prevalence of long-term conditions and cancer is highest.
Reflecting the NHS Long Term Plan, these missions will focus more on preventing, diagnosing, monitoring, and treating disease early rather than providing late-stage interventions at the end of life. This will include more disease prediction, public health prevention based on a range of innovative tools including digital tools, diagnostics therapeutics and technologies, and collaboration between the NHS and public health and social care systems. This shift will create large opportunities for industry but will also be essential for the sustainability of the NHS and all healthcare systems worldwide.
Preconditions for success
Through developing this vision, and engaging widely with the sector, we have identified 4 preconditions for success which must be met over the next decade for the potential of the vision to be fulfilled. Each of these preconditions is critical, and the ambitions in the vision will not be deliverable if they are not met.
These preconditions are set out below.
NHS as an Innovation Partner
The NHS is critical to the delivery of nearly every element of the vision, at both a national and operational level. COVID-19 has demonstrated the NHS’s ability to trial, embrace and deliver innovation to patients at unprecedented speed and scale – and this momentum must now be seized and applied across the breadth of innovation and disease areas. We want to deliver the medicines, technologies and tools that enable NHS staff to care and treat more effectively, with ‘right first time’ diagnosis and treatment and the capacity to partake in research and innovation – in line with the ambitious vision for the Future of UK Clinical Research Delivery. This vision sets out proposals for immediate action in NHS England alongside an ambition to work closely with NHS Scotland and Wales, and Health and Social Care Northern Ireland to achieve these aims across the UK.
Investment in science and research in Life Sciences must be maintained and grown over the next decade.
The UK’s competitiveness as a globally leading location for Life Sciences is heavily reliant upon the UK’s science and research ecosystem. The government has set an ambition for the UK to be a Science Superpower, and for the UK (across government, Industry and Philanthropy) to invest 2.4% of Gross Domestic Product (GDP) in Research and Development (R&D) by 2027 – delivering this will be critical for the success of the vision.
The governance and oversight of NHS health data must be simplified to drive research and innovation, supported by ongoing public engagement, transparent use and the highest standards in data protection maintained to build public trust.
Over the next decade, high quality health data will be one of the primary drivers of global Life Sciences research and innovation and improved health outcomes. The NHS has potentially the richest longitudinal health data in the world – but the governance of, and access to, this data must be radically simplified, while simultaneously being made more secure and research-ready, to unlock its full research and innovation potential. We can only achieve this vision with the full support of patients, the public and NHS, and must build trust into its delivery.
Access to finance
Innovative UK Life Sciences companies need to be able to access capital to grow and innovate. Drawing on the City of London’s position as one of the world’s leading financial centres, private finance needs to be available to support the growth of promising companies and ideas, so that they realise their economic potential in the UK.
UK competitiveness in life sciences
Science and research
Times Higher Education World University Rankings 2021 for life sciences
- Harvard University
- University of Cambridge
- University of Oxford
- Massachusetts Institute of Technology
- Stanford University
The UK’s science and research offering is amongst the best in the world. Our universities feature prominently in global rankings for Life Sciences teaching and research, with 2 in the Times Higher Education World University Rankings 2021 top 5.[footnote 6]
The UK also punches well above its weight globally in its share of research publications. UK researchers produce the third highest number of Life Sciences papers in high quality journals worldwide, after the USA and China,[footnote 7] and since 2007, the UK’s field-weighted citation impact has been the highest in the G7.[footnote 8]
Field-weighted citation impact
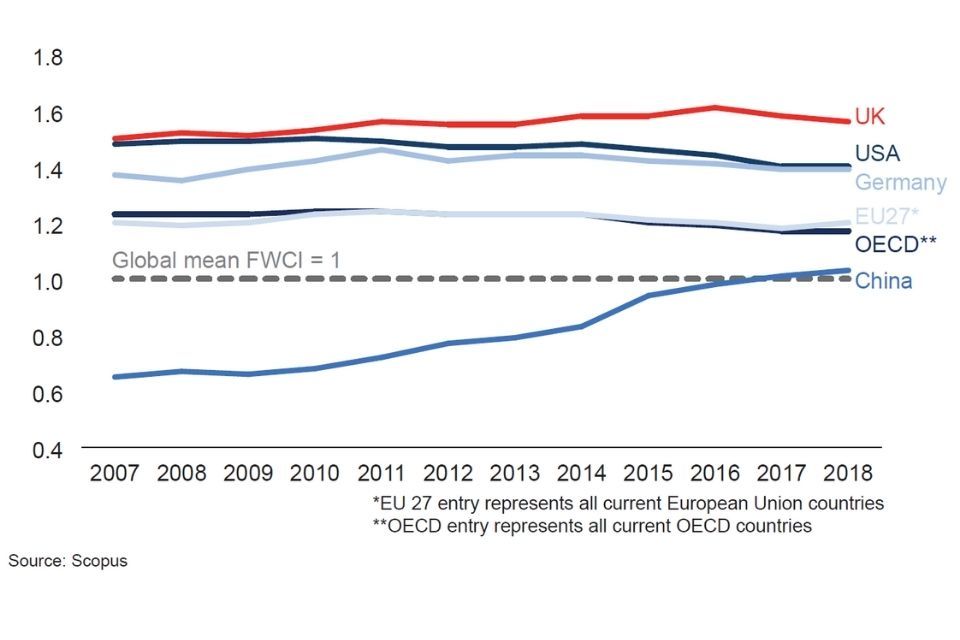
Chart - field-weighted citation impact
The UK Life Sciences sector is highly collaborative, with a recent survey of 15 companies by ABPI identifying over 1,000 links between academia and the pharmaceutical industry.[footnote 9] Partnerships were present at every career stage, from undergraduate placements to full-blown academic posts.
On Clinical Research the UK is one of the top 3 destinations for delivery of commercial early phase trials and delivered 12% of all global trials for innovative cell and gene therapies in 2019.[footnote 10] Over 1 million people from across the UK have taken part during the last year,[footnote 11] with over 40,000 participants enrolling in the RECOVERY trial,[footnote 12] which is significantly larger than other comparative COVID-19 trials.
The UK government provides significant funding for health R&D, spending $3.4 billion in 2019.[footnote 13] Total and per capita government funding for health research ranks second only to the US among OECD countries, with 21% of the UK government’s R&D spending focused on health in 2019.[footnote 14]
Government per capita spend on health R&D ($)
| Country | Per capita spend ($) |
|---|---|
| USA | 121 |
| UK | 51 |
| France | 31 |
| Germany | 25 |
| Japan | 20 |
Source: OECD GBARD, 2015 constant prices & PPPs, World Bank, 2019
Operating and business environment
The UK is seen as an attractive market for investment in the Life Sciences, ranking second only to the United States in the number of Foreign Direct Investment projects financed in 2019. The attractiveness of the UK business environment is backed up by strong rankings in both the Ease of Doing Business and Global Innovation Index rankings (globally 8th and 4th respectively).[footnote 15][footnote 16]
Life sciences inward foreign direct investment projects
| Country | Number of life sciences inward foreign direct investment projects |
|---|---|
| USA | 222 |
| UK | 82 |
| China | 64 |
| India | 44 |
| Russia | 27 |
| Germany | 25 |
| France | 23 |
| Australia | 22 |
| Ireland | 21 |
| Switzerland | 15 |
| Italy | 14 |
| Japan | 12 |
| Canada | 11 |
| Republic of Korea | 6 |
| Sweden | 2 |
Source: FDI Markets, 2019
However, the UK lags behind comparator countries in terms of the strength of its public markets, reducing the options for companies seeking to access late-stage capital in the UK. Initial Public Offerings (IPOs) by life sciences companies on UK exchanges tend to be fewer and smaller in size than comparator countries, reflecting a tendency for these companies to seek to list on exchanges with greater liquidity and valuations such as the US’s NASDAQ. The UK ranked 7th globally in 2020 by amount raised through IPOs, and 5th by the total number of IPOs, with both figures being a fraction of those for the USA and China.[footnote 17]
Life sciences IPOs
| Country | Amount raised (£ million) |
|---|---|
| USA (95) | 17,268 |
| China, including Hong Kong (57) | 8,816 |
| Republic of Korea (24) | 1,467 |
| India (2) | 671 |
| Italy (1) | 445 |
| Australia (10) | 235 |
| UK (5) | 133 |
| Belgium (2) | 123 |
| Norway (3) | 123 |
| Sweden (3) | 104 |
(x) indicates number of IPOs.
Source: S&P Capital, 2020.
Access to new medicines and technologies
The UK aspires to be the world leader for development, testing, access, and uptake of new and innovative treatments and technologies. Yet, industry partners have in the past expressed concerns about the speed of uptake of proven products, and the barriers that prevent the timely spread of new technologies.
The latest edition of the EFPIA Patients WAIT (Waiting to Access Innovative Therapies) Indicator shows that in 2020, 72% of medicines approved by the European Medicines Agency between 2016 and 2019 received a positive National Institute for Health and Care Excellence (NICE) appraisal.[footnote 18] This places England behind 5 other European countries, some of which operate very different health systems, making access and uptake comparisons difficult. Rates of access to more recently licensed medicines will be even higher in England as the (NICE) has recommended 100% of new active substances for use in the NHS in England since 2019.
Rate of availability of medicines approved by the EMA between 2016-2019 to patients in 2020
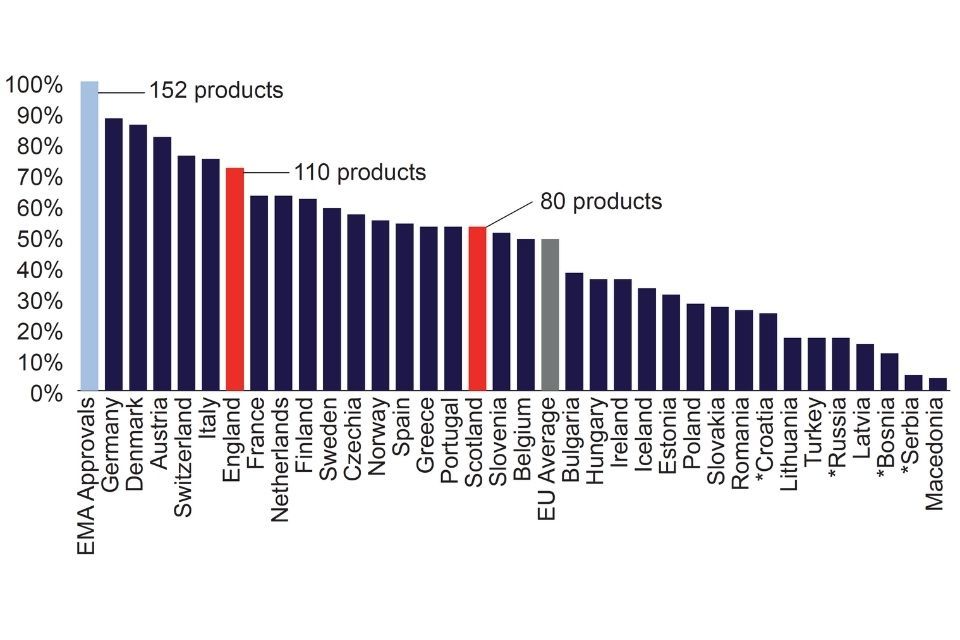
Chart - rate of availability of medicines approved by the EMA between 2016 to 2019 to patients in 2020
Availability is however only one element of the access process. The 2021 WAIT Indicator publication also reports the time taken between marketing authorisation and the date of availability to patients in European countries for the same cohort of novel medicines. England performs well with respect to comparator countries, ranking 7th with median time to availability of 297 days.
New initiatives have sped up the time for some treatments to reach patients, from the launch of the Innovative Licensing and Access Pathway, improvements in NICE assessment timelines, the establishment of the Cancer Drugs Fund and opportunities for new and innovative commercial deals with NHSE/I under the Commercial Framework.
NICE is now among the fastest health technology assessment bodies in the world and the average time from marketing authorisation to first NICE output was 1.3 months in 2019 to 2020.[footnote 19] Further changes to NICE’s methods and processes will streamline the approvals process and ensure patients benefit from innovative treatments.
There is less quantitative evidence to understand and compare internationally the NHS’s service-wide uptake of innovative, cost-effective medtech, diagnostics and digital products. However, there are product specific examples in which the uptake of cost-effective innovations has been lower than in comparator countries.[footnote 20]
As set out in the Uptake and Access section of this vision, it will be critical that there is a continued focus on improving the speed and scale with which new medicines and technologies are utilised in the UK over the coming years.
Section 1: Building on the UK’s science and research infrastructure and harnessing the UK’s unique genomic and health data
Strategic goal
Build on the UK’s clinical research, genomic and health Data capabilities to make the UK a highly effective and efficient place in which to test and trial new technologies for the most important healthcare challenges – creating value for industry and early access for NHS patients.
The UK has a rich history as a centre for science and innovation in Life Sciences. This is underpinned by long-standing infrastructure investments by the National Institute for Health Research (NIHR), UK Research and Innovation (UKRI) and the medical research charities; the deep science skills base across industry, academia, and the NHS; and the ingenuity and drive of industry – from the world’s largest Life Sciences companies to the smallest SMEs.
Government and the sector are committed to building on this rich history and making the UK one of the very best places in the world in which to develop, test and trial new technologies.
This requires a focus on 3 related areas:
- Clinical research: recover performance post-pandemic and making it as easy and economic as possible to run innovative, efficient, and high-quality clinical research across the UK.
- Genomics: harness the UK’s prior investments to fully integrate genomics into health service delivery through the Genomic Medicine Service, and deliver significant advancements in the understanding, diagnosis, and treatment of disease.
- Health data: in a secure and transparent manner, harness the NHS’s unique health data to understand and tackle population health challenges, and drive advances in Life Science research and innovation – generating real patient benefit through better care today, as well as public understanding, enthusiasm, and support for its use in research to deliver future improvements in healthcare.
The UK’s intention is to create a genuinely unique operating environment through integrating these 3 capabilities, underpinned by the world’s most agile and responsive regulatory environment. This will allow new medicines and technologies to be tested and trialled at scale in the most rigorous of environments, with Genomics and Health Data used to create much deeper, real world evidence into product safety and effectiveness.
Clinicial research
Overarching ambition
Drive value creation for industry and patients through faster, cheaper, better-quality and more diverse clinical research, delivered through a digitally enabled and pro-innovation clinical research environment, with research embedded across the NHS as a core part of effective patient care. The UK will be ambitious in bolstering the delivery of clinical research across all phases, treatment types, conditions and technologies, and supporting the generation of real world evidence.
Clinical research is fundamental to healthcare innovation. It is the single most important way we turn cutting-edge science into more effective diagnosis, treatment and prevention – which improves care quality and patient outcomes,[footnote 21] bolsters the efficiency of health service delivery[footnote 22] and improves NHS staff retention and wellbeing.[footnote 23] It is a precondition of the success of this Life Sciences Vision that the sector continues to support and invest in the UK’s clinical research infrastructure and that the vision for the Future of UK Clinical Research Delivery is delivered in full, as part of the overarching ambition for 2.4% of GDP to be spent on R&D by 2027.
The success of the UK’s COVID-19 research response relied heavily on ongoing investment in the foundations of the UK’s clinical research infrastructure. The cornerstone of which is the NIHR, which provides the expertise, facilities, training and technology to enable research and innovation to thrive.
However, COVID-19 has exposed areas that we must improve to bolster the resilience of UK clinical research, secure future investment and deliver improved healthcare for all patients. The pandemic recovery provides the opportunity to reset and cut bureaucracy to better enable and support rapid research delivery. And to embed clinical research across the NHS – nationally, regionally and across all clinical teams to support this agenda.
The UK government and devolved administrations, the NHS, and the entire sector will therefore work together through the UK Clinical Research Recovery Resilience and Growth programme to address these concerns and make the NHS fully research ready by delivering the following.
Embed clinical research across the NHS, bolstering capacity and creating a research-positive culture in which all staff are supported and expected to participate
This will be done through:
- creating the system capacity, incentives, and enablers in the NHS to support all staff to actively participate in research and innovation programmes
- making it a core expectation of the incoming Chief Executive of NHS England, as well as national, regional, and local NHS leadership and the Department of Health and Social Care that they actively support the research, innovation and uptake agenda. Forthcoming legislation will create specific duties for Integrated Care Systems in England to promote and support research and innovations
- monitoring and reporting research and innovation activity across the NHS, to increase transparency and allow for constructive, evidence-based improvement in places where focus on research and innovation could be increased
- government, working with the professional regulators, will embed research and innovation in standards for registered professionals and provide the necessary support and development resources to help healthcare professionals be research active
- fully delivering the UK Clinical Research Recovery Resilience and Growth Programme in response to the challenges created by the pandemic, including restarting non-COVID-19 research to recover to pre-pandemic levels as soon as possible and delivering a broad suite of actions to make the UK clinical research environment faster, more efficient, and more resilient
Make the UK the leading global centre for innovative research design and delivery, across all types and phases of trials
This will be done through:
- the NIHR, MHRA and NICE and the NHS will work with universities and research sponsors to ensure studies are delivered in the most innovative and effective ways that support rapid integration into routine care pathways
- supporting virtual and decentralised trial delivery; and building on the momentum of COVID-19 research, the Vaccines Registry, and advances in digital infrastructure to increase access and involvement of NHS patients and service users
- harnessing the NHS’s potential as a source of real world evidence and the use of patient registers and registries to support the development, uptake, and demonstration of the outcomes of medtech and pharmaceuticals
- further enhancing expert early advice for researchers via the Health Research Authority (HRA) and the MHRA to support efficient trial design, approval, and start-up
- establishing a new find, recruit and follow-up service to expedite clinical trial set up and delivery, by supporting partner site selection, securing access to the data needed for delivery, and by identifying the most appropriate recruitment approaches
- building on the progress made by the G7 on Clinical Trial Protocols to actively champion and utilise novel clinical trial designs and regimens in the UK and internationally, to reach clinical endpoints more quickly. This will build on the UK’s established leadership in areas such as Human Challenge Studies and other novel trial methodologies
- the MHRA will also continue to advocate for and champion innovation and research friendly global regulatory standards through global regulatory fora and bilateral relationships – as well as the use of novel biomarkers or surrogate markers where the impact of treatment on disease is not well understood
- maintain the UK’s strong Intellectual Property regime, recognising the important role this plays in securing the value of new technologies that are trialled and tested in the UK
Cut bureaucracy and red tape to create a more efficient and effective research environment
- in line with the recommendation of the Taskforce for Innovation, Growth and Regulatory Reform (TIGRR), use the recently passed Medicines and Medical Devices Act 2021 to radically improve existing legislation on clinical trials, so that it is no longer reflects the EU’s Clinical Trial Directive
- build on the work already started by the CEOs of UK regulators to remove unnecessary or burdensome bureaucracy associated with research approvals. For example, by actively expediting research set-up through initiatives such as the HRA’s Rapid Research Ethics Committee Review
- transform set-up times through expedited and standardised costing and contracting across the NHS
- learn and apply the lessons from COVID-19, in terms of rapid trial start up, enrolment and delivery – and the role of government, the NHS and NIHR in prioritising the most strategically important and impactful studies
Ensure the UK remains a financially attractive location for R&D
This will be done through the UK’s competitive tax environment and generous system of tax reliefs benefitting the life sciences industry, including R&D tax credits.
Reflect the diversity of the UK’s population in future clinical research
System partners, including the medical research charities, will work together to proactively increase the racial, age, gender, and geographic diversity of clinical trial participants and those in real world data sets. This will include the development of novel processes and guidance to increase uptake among traditionally underserved communities, including those in rural or small-town settings; ethnic minority groups; women; as well as children and the elderly.
Genomics at scale
Overarching ambition
To create the most advanced and integrated genomic research healthcare ecosystem in the world, underpinned by the latest science and technology, in order to drive better health outcomes, early detection, diagnosis and treatment of disease, and innovative research – in line with the Genome UK plan.
The UK has a proud history in genomics: from the discovery of the double helix, through to research on the sequencing of DNA and discovery of DNA arrays, to the discovery and development of most of the world’s next generation sequencing tools.
The UK now has internationally leading research cohorts, such as UK Biobank, underpinned by strengths in clinical practice, with the UK home to the world’s first national genomic medicine service, where patients can access whole genome sequencing, delivered by the NHS Genomics Medicine Service and Genomics England. This capability has been particularly critical in the response to COVID-19.
The UK’s investment and expertise in genomics means that it now has an unparalleled opportunity to use genomic research assets to drive the next generation of Life Sciences discoveries, deliver genomics-enabled clinical trials and support the growth and R&D of innovative genomics-focused companies.
This will be done through the following.
Continuing to support and enhance our genomic research infrastructure
The combination of world class ‘omics assets contained in longitudinal cohorts, such as UK Biobank, Our Future Health, NIHR BioResource and Genomics England, alongside a clear route to patient impact is globally unique and continues to be strongly supported by industry. These will continue to be used to enhance our research cohort genomic infrastructure – with a joined-up “front door” and interoperable research environments bringing scale benefits to researchers and industry.
Evaluating variants and their role in prediction and public health
The availability of polygenic risk scores from the UK Biobank cohort will allow these tools to be used to better predict most common and some rare diseases. Combining novel arrays with these prevention tools will allow for large pilot studies in the NHS and in Our Future Health, which can then be applied more widely to define and address individual risks.
Utilising new genomic tools to improve prediction and early diagnosis capabilities
As seen with the partnership between NHS England and GRAIL to undertake GRAIL’s pivotal studies of asymptomatic cancer detection in the UK. Future expansion of the prediction and early diagnosis agenda, such as the new-born sequencing pilots that are currently in public dialogue, will bring more sequencing capacity to the UK, create a substantial opportunity for novel gene therapies to transform the lives of patients with genetic conditions, and deepen insight into many common and rare disease areas.
Bringing the best emerging science and technology to bear on cancer diagnosis and treatment
Alongside early detection and prediction, we have an opportunity to build on early pilots and deploy emerging technologies (such as long read sequencing, methylation, transcriptomics, proteomics and other medtech) to improve diagnosis, stratification of patients, referrals to clinical trials, and personalised therapeutics for patients.
Our ambition is to have the closest link between clinical research, iterative innovation and patient care of any country in the world.
Delivering a world class offer on functional genomics
here are now thousands of genetic variants known to be implicated in disease pathogenesis. The challenge now is to understand how these variants mediate their effects. A new set of tools including single cell sequencing, dynamic gene expression profiling, and systematic CRISPR screens will, when allied with insights from genomics datasets and advanced imaging and pathology, open up high throughput approaches to understanding the role of variants and hence identifying novel drug targets. As part of the implementation of our healthcare genomics strategy, Genome UK, MRC is already leading work to scope the offer.
Health data
Overarching ambition
Unleash the potential of the UK’s health data to make the UK the best place in the world to undertake ground-breaking R&D; to start and grow Life Sciences and AI companies; and bring to market new medicines, medtech and diagnostics, transforming the NHS with efficient, patient-centred, and personalised care.
To support our vision of a healthcare system that is able to focus more on early diagnosis, treatment and prevention of disease and harnesses cutting edge innovation, we need to make data accessible, in a trustworthy and transparent way. This is a significant opportunity, recognised by other countries, that will underpin transformative improvements in health outcomes and service delivery, and provide profound insights to support the development of new medicines and technologies. It is a precondition to the success of this vision that the UK seizes the opportunity provided by Health Data.
However, much must be done to unlock the potential of health data and to enable integration with the UK’s broader research and genomic capabilities.
COVID-19 has highlighted long-standing problems in the landscape. As is the case in other countries, UK health data is mainly focused on managing patient records in the support of clinical processes and is not structured to facilitate population-wide research and analysis. It is fragmented across a complex institutional landscape, is of variable quality, and is often difficult and slow to access.
The pandemic has, however, also provided a glimpse of the enormous potential of health data, in enabling partnerships with researchers and industry to rapidly and safely develop, trial and evaluate new vaccines and treatments, as well as to test the effectiveness of deploying technologies at scale. The ambition is to make this type of population-wide, big-data capability available to support the development, trialling, and evaluation of a much wider range of innovations.
Routinely, we must ensure that data from multiple sources can be linked to create a consolidated ‘picture’ of the whole person and continuum of care pathway, identify the most suitable patients for clinical research, and continue efforts to improve quality and standardisation. This will take effective, coordinated action – bringing together partners in England and working closely with the devolved administrations – as set out in the draft Data Saves Lives Strategy published on 23 June 2021, in a way that secures and retains public trust and consent around who has access to their data and for what reason.
Government and the sector’s top priorities are the following.
Take concerted action, across DHSC, NHS England, NHSX and NHS Digital, working with key partners in the health data landscape such as Genomics England as well as the devolved administrations, to continue the development of unrivalled ‘at-scale’ data infrastructure to deliver top R&D opportunities
Make all types of data available, linkable and ‘research-ready’, in a streamlined secure and privacy-protected way. This includes co-ordinated, strategic action and improvements to data access systems at the national and regional levels.
Provide innovators with smoother and quicker access to reliable, high quality ‘real world’ data alongside clinical and genomic data
This will support more effective and efficient clinical trials; ease robust regulatory approval through the rapid accumulation of high quality and holistic data; and allow more accurate assessment and evaluation of new innovations and technologies.
We will do this through:
- accrediting a handful of Trusted Research Environments to become the default route for accessing large-scale NHS data, built to be interoperable and highly secure, to increase access to ‘at-scale’ data while protecting the public interest
- overhauling the governance on data access to ensure that patients, NHS organisations and registries have the confidence and clarity they need to engage with innovators, bringing more consistency and efficiency in decision-making whilst adhering to the highest data protection standards
Ensure the UK is positioned at the forefront of a new era of computational biology, with at-scale genomics, imaging, pathology, and citizen-generated remote monitoring data assets
We will create a vibrant hub in which to develop and deploy AI-enabled tools and technologies. By identifying and targeting new treatment targets, we can help transform the NHS with predictive, personalised prevention, diagnosis and care. If we can consolidate our rich genome sequencing and imaging data, we will have unique scale and diversity to train AI, and support fast, safe deployment; as well as support developments in areas such as Functional Genomics.
Seize opportunities to support the NHS and patients through innovative NHS data partnerships
These partnerships will be ones that fundamentally drive improvements in health outcomes and/or reduce health inequalities – whether this is through clinical research, validating AI using the UK’s uniquely diverse population, or continuously surveying the uptake, safety and efficacy of diagnostics, treatments, and care pathways. In line with the public’s expectations, it is critical that, where patient data is used to support the development of new treatments and technologies, patients and the NHS receive a fair share of the benefits.
Working with all elements of the sector, take concerted action to develop and recruit the data and analytical skills in the NHS and wider ecosystem
These skills will be critical for utilising and delivering the full potential of the UK’s health data – in particular to support population-wide measures that support the early detection, diagnosis and treatment of disease at scale.
Co-develop work with the NHS, patients, the public and medical research charities across the UK
This will safeguard trust and transparency in how health data can be accessed to support R&D. This includes reviewing patient consent models, incorporating clear standards on the use of privacy-enhancing technologies, enforcing clear expectations and ‘red-lines’ around the use of data, transparency, and public benefit, and actively promoting diversity in the use of NHS’s data- including to tackle health inequalities.
Ensure alignment with other data-driven programmes such as Getting It Right First Time
This drives improvements in treatment and patient care through in-depth review of services, benchmarking and presenting a data-driven evidence base to support change.
Across all the work outlined in this vision on research, genomics and data, government, the NHS, and partners will be confident in making the positive case for how the UK’s unique strengths and expertise can be used to drive the development of technologies and insights to benefit patients and public in the UK and globally.
It will be critical when delivering the policies outlined above that they secure support from patients, the public, NHS staff, academic and industrial researchers, software developers and data analysts. In particular, it is essential we continue to build support from patients and the public for their data to be used for research and innovation, and there must be a clear onus on demonstrating the public benefits, such as addressing health inequalities, while ensuring research provide robust safeguards against possible bias in the data.
Life sciences and levelling up
The Life Sciences sector is an important driver of levelling up the wealth and health of the UK.
The sector has a wide geographic distribution. Over 66% of employment is outside of London and the Southeast, and roles tend to be high wage with a high gross value add.
Regional disparities in health also play an important role in entrenching other forms of inequality, and diminishing local economic growth
The policies in this vision are designed to maintain and build upon the rich geographic diversity of the sector in the UK, and to address some of the key drivers of health inequality.
The vision will do this by:
- driving economic growth and self-sustaining clusters of excellence across the UK, such as supporting Manchester to become a world leading centre for Genomics and Data; building on the unique medtech heritage in Yorkshire and Humber; and developing Liverpool’s reputation as a leading centre for Infection and Immunology
- tackling the most pressing healthcare challenges such as cancer, cardiovascular disease and obesity, which disproportionately impact those from the most deprived areas and minority backgrounds
- creating a competitive environment to incentivise and onshore high-value manufacturing capabilities in the UK, including in regions outside of London and the South-East, where there is deep expertise in pharmaceutical and medtech manufacturing
- developing a high-skilled workforce and strong pipeline of talent across industry, academia and the NHS, including through improving uptake of apprentices, to support the development of new and existing manufacturing centres across the UK
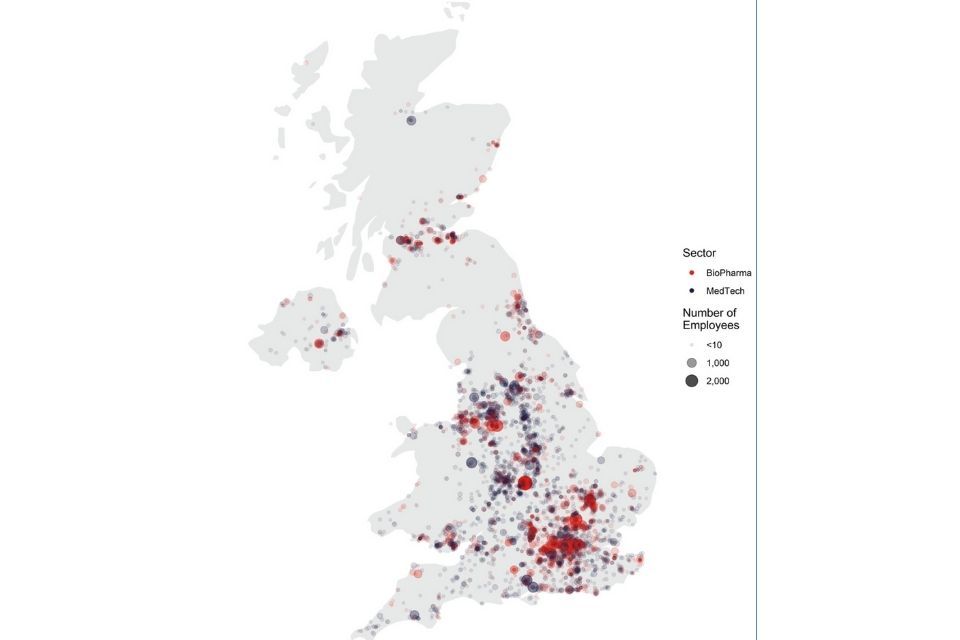
Map - distribution of life sciences employment across the UK, 2019
Distribution of life sciences employment (BioPharma and MedTech) across the UK, 2019.
Section 2: Access and uptake
Strategic goal
Make the UK the best place in the world to discover, develop, test, trial, launch and adopt new treatments and technologies, by creating a forward-thinking commercial environment where the NHS can strike flagship deals and where proven, clinically and cost-effective innovations are rapidly adopted and spread across the country to bolster the health of the nation, deliver greater value for the taxpayer and stimulate economic growth.
The NHS is central to every element of this vision, and the NHS’s partnership across the breadth of the work outlined in this document is a precondition to its success.
Since the publication of the Accelerated Access Review, major steps have been taken to improve the access, adoption and spread of innovation across the NHS and to support NHS England to be a more effective innovation partner.
For example:
- NHS England has established the Commercial Medicines Directorate, Transformation Directorate, and launched the Commercial Framework to create new flexibilities and partnerships with industry. This has led to several globally leading deals for cutting-edge innovations, including for GRAIL’s Gallier blood test, an innovative cancer diagnostic, and Novartis’ Inclisiran, a cholesterol-lowering drug
- the Accelerated Access Collaborative (AAC) has been established to tackle barriers to adoption and spread of the most transformative treatments and technologies. In 2019-2020 alone the AAC helped over 700,000 patients access proven health and care innovations, resulting in patients spending 125,000 fewer days in hospital and over£50m of savings for the NHS
- NHSX has been created to drive digital transformation across the NHS in England, supporting the rollout of digital technologies and ensuring the health system is equipped to support and foster innovation
The NHS has also showcased its potential as an innovation partner during the UK’s response to COVID-19. It played a fundamental role in supporting many of the most globally important trials of novel vaccines, drugs and medtech, deploying them rapidly through national guidance and clinical leadership, which saved many thousands of lives in the UK and globally.
However, while the NHS has significantly increased its ability to deliver innovative treatment and technologies, there remain concerns about the uptake and spread of proven products. We must seize opportunities to expedite the adoption of proven innovation and to address the barriers that prevent the timely spread of new technologies. For this vision to be a success, the NHS must go further to tackle the barriers that stand in the way of patients accessing clinically and cost-effective innovations. Collaboration will be central to success and the NHS will need to work in close partnership with other key bodies across the system, such as NICE and MHRA, to drive uptake.
In doing so, the NHS can help to support a reduction in health inequalities and further improve outcomes for all patients across the UK. At the same time, through providing a testbed in which medicines and technologies can be trialled and evaluated at scale, with those proven to be effective utilised at pace, the NHS can act as a significant driver of Life Sciences value creation.
The vision commitments on Uptake and Access are underpinned by the 2019 Voluntary Scheme for Branded Medicine Pricing (VPAS), which demonstrated government, NHS and Industry’s shared commitment to help patients access new treatments while managing affordability. Future work on Uptake and Access will embody the spirit that allowed the comprehensive VPAS to be negotiated with trust and good faith, balancing affordability, and patient access with support for a thriving Life Sciences industry.
To deliver on the ambitions above, we will work with the devolved administrations and the NHS in Scotland and Wales and Health and Social Care Northern Ireland to broaden best practice across the UK alongside strengthening the ability of the MHRA and NICE – liaising with the Scottish Medicines Consortium (SMC) – to facilitate patient-focussed, innovative, deals in all parts of the UK.
To improve the access to, and spread of, innovation the NHS will do the following.
Make the NHS the best place in the world to strike innovative commercial partnerships to address the most pressing healthcare challenges
The NHS will work with industry, academia and NIHR to trial ground-breaking innovations at scale and support their subsequent adoption and spread across the NHS:
- increase NHS England’s commercial capacity to deliver innovative deals for medicines, digital, diagnostics and medtech and incentivise the development in areas of comparative strategic importance or need; while continuing to embed and operationalise the principles of the Commercial Framework
- strengthen collaboration between NICE, NHS England, MHRA and NIHR and devolved administrations, providing dedicated resources and processes to deliver core functions to drive access and uptake, as well as innovative deals that work for patients, taxpayers, and industry. This means ensuring products in areas of comparative strategic importance or need can be evaluated at scale in the NHS and then deployed across the health system at pace
- bolster the AAC’s strategic planning, horizon scanning and demand signalling capabilities, to proactively identify and triage commercial opportunities for new treatments and technologies which address NHS England’s priority demands
- deliver a high ambition NICE Methods Review that ensures NICE retains its global leadership in the evaluation and appraisal of new medicines and technologies
- develop a new framework for reimbursement of digital health technologies and digital therapeutics to create clarity for innovators and investors on the pathway for safety approvals by the MHRA, technology approvals by NHSX, efficacy approvals by NICE, and commissioning decisions by the NHS in England
- explore the potential to develop a UK Medtech Gateway – to support market entry of international firms in the UK, and help them to develop proof of concept and then scale in the NHS – in alignment with the work of the Accelerated Access Collaborative
Make accessing the NHS clearer and simpler, with support for the real-world evaluation of innovation and the spread of proven products across the system
- explore opportunities for commercial innovation offered by the introduction of Integrated Care Systems (ICS) and reforms to the National Tariff System; and ensuring that new payment systems and incentive schemes are clear how individual innovative treatments and technologies are to be funded by ICS commissioners, how providers are to be reimbursed and how incentives for purchasing, pathway redesign and adoption are aligned. We will align NHS England’s national scale and purchasing power with the ability of ICS to act as local testbeds and launch sites for innovation
- make it as easy for clinicians to use MedTech and Digital Health Products, including AI, as it is for them to prescribe medicines. This will include embedding both MedTech and digital health properly in clinical workflows and patient self-care toolkits
- strengthen innovation metrics, especially for medtech and highly innovative medicines, to identify and address unwarranted variation and allow for more accurate international comparisons. This will inform and further enhance the AAC’s crucial work to spread proven innovations across the NHS in England –through taking targeted, tangible action to address unwarranted variations in uptake
- explore opportunities to boost capability and coordination of regional infrastructure, data and people to support real world evaluation of late-stage medtech, building on existing programmes, including the £140m NHS AI Health and Care Award delivered by the NHSX AI Lab in partnership with the AAC
- launch the Innovation Service as a clear point of entry for innovators to find information and support from system partners and access the NHS and ensure its unique role and alignment with existing organisations and entities is clear to the sector
Focus NHS support for innovation on areas that will have the greatest benefit to the UK, making it the most forward-thinking and prevention focused healthcare system in the world
- improve demand signalling and bolster horizon scanning, to clearly articulate research and innovation needs in priority areas and better understand and enable the NHS to prepare for the rapid adoption of new clinically and cost-effective products as soon as they are available
- create new managed access arrangements which support UK patient access to innovations with high levels of inherent uncertainty whilst further evidence is collected to determine their efficacy. This includes the launch of the Innovative Medicines Fund by NHS England, continuing to improve access to multi-indication treatments, exploring new access schemes for medtech and ensuring joined-up regulatory and access arrangements
- aligning and simplifying funding streams across system partners to support adoption and care pathway transformation for clinically and cost-effective innovations which address the most pressing needs of the NHS in England. Including through initiatives such as the MedTech Funding Mandate
- support the innovative use of off-patent drugs and technologies where no approved treatment exists, to address unmet patient need, as, for example, was done with Dexamethasone during the pandemic, without undermining the regulatory system and incentives to innovate
Establish a frontline culture of research and innovation across the NHS
- make greater use of clinical leaders and champions in the work of the AAC, while identifying and supporting more NHS innovators to pursue their innovation without having to leave the health service through proven initiatives like the Clinical Entrepreneurs Programme
- embed research and innovation as priorities within key strategic documents, such as the NHS England People Plan as well as in local and regional structures, including ICSs and NHS Trusts. Alongside this, it will also include working with bodies including Care Quality Commission, NIHR and UKRI to create new ways to encourage and support improvement and innovation; and support for regional, local and frontline partners in the adoption and spread of innovation including through the continued work of the Academic Health Science Networks (AHSN) and in the context of wider system reform
What this vision means for patients and the public
COVID-19 has made health – and the systems that underpin it – an everyday topic in public discourse. As a result there is an understanding of the nature of disease and the impact of genomic variants, how vaccines and diagnostics are being used to prevent it, how large trials are run to find medicines to treat it, how data defines the next steps in the strategy to tackle it, and the phenomenal contribution that the NHS and regulatory bodies such as the MHRA have made to defeat it.
Realising this vision means bringing together these elements of the life sciences ecosystem so that we can tackle not just the COVID-19 pandemic, but the equally devastating causes of much of the death and illness in the UK and globally. By using the R&D opportunities afforded by capabilities in genomics and health data we can support innovative companies to develop predictive tools and early diagnostics that can stop members of the public from becoming patients in the first place. We can use our clinical trials and research infrastructure to understand the most effective innovative treatments, so that we can tackle both common and rare diseases. Through advanced manufacturing, we can develop ways of producing those innovative medicines to allow swift delivery and deployment to patients, and support high quality, well paid jobs in the UK. And in these areas, regulatory authorities and the NHS can act as innovative delivery partners, helping safe and effective treatments reach more patients quickly.
COVID-19 has also underlined that health is also a matter of social policy. Progress in these areas will help patients around the UK and particularly in areas where the healthcare challenges identified in this vision are linked with lower socio-economic status.
Section 3: Create an outstanding environment for life sciences business to start, grow and invest
Strategic goal
Make the UK the most attractive location in Europe to start and grow a life sciences business, with an internationally competitive offer on manufacturing and the world’s leading regulatory environment.
The UK has one of the most amenable business environments in the world.[footnote 24] This is underpinned by an internationally competitive tax offer, high quality skills and infrastructure, and a commitment to global free trade and open supply chains.
In March 2021, the government published the Build Back Better: our plan for growth, which outlined the government’s plans to support economic growth through investment in infrastructure, skills, and innovation.
Building on these commitments, the Life Sciences Vision will outline what the government and sector will do collectively to continue to improve the UK’s competitiveness in 5, interrelated areas, which are critical to the UK’s attractiveness as a location for life sciences businesses:
- Access to Finance
- Regulation
- Skills
- Manufacturing
- Trade and Investment
Without making every effort to deliver an outstanding commercial environment, we risk missing the opportunity to commercialise the technologies developed through the UK’s science and research infrastructure, reaping the economic benefit of the sector’s significant investment in the UK, and losing the sector’s tax contributions that help fund UK public services.
Access to finance
Overarching ambition
Develop a globally competitive Life Sciences investment ecosystem where private and public Life Sciences companies can access long-term capital within the UK from investors committed to building successful companies in the UK at every stage of their growth, so that they have a genuine choice to stay and grow here.
Innovation in the Life Sciences is often a long-term, capital-intensive process. As a result, the sector is particularly reliant on long-term investment to finance growth. HM Treasury’s Patient Capital Review[footnote 25] identified that there is a shortage of this type of capital, particularly at the later stages. While the UK has a strong pipeline of SMEs and entrepreneurs emerging from its science and engineering base, there are challenges for these companies to scale in the UK, and these companies often turn to overseas (typically US) investment.
Supply of capital to support Life Sciences typically comes from venture capital (VC) funds and public markets. Despite strong overall investment in the sector, there are clear issues and challenges with both areas in the UK. Since 2016 there has been an increase of 201% in the average size of private capital raised in the US VC markets (from £6.8 million to £20.5 million). This increase has not been replicated in the UK, where the increase was 61% for the same period from £5.3 million in 2016 to £8.8 million in 2020.[footnote 26] This funding gap between the UK and US is not distributed equally, with particular issues at the later ‘growth’ stages.
The difference in supply of VC funding means that promising UK companies are looking to US investors when raising funding to reach their potential. This can be problematic, as companies will then sometimes move or relocate a significant proportion of their operations to the US to be closer to investors.
There are also weaknesses in the UK’s public markets, which limit the funding available for the Life Sciences sector. This, in part, is down to valuations, where Life Sciences companies can expect to achieve between 20-30% higher on Nasdaq compared to the LSE. Further, the level of share trading for Life Sciences companies on the LSE is often limited, and lower than equivalent companies listed on Nasdaq.
Government has recently made a series of significant interventions to improve the availability of capital. It is providing £200 million in funding through the Life Sciences Investment Programme. In addition, through the UK-UAE Sovereign Investment Partnership, Mubadala has made an £800m commitment to investment in the UK Life Science sector. On top of this £1 billion, work is underway to support greater investment into the Life Sciences by Institutional Investors and Pension Funds.
We must build on these initiatives and create a Life Sciences investment ecosystem whereby private Life Sciences companies have greater access to later stage capital at scale within the UK from investors committed to building UK-based companies; and UK public markets are able to offer a genuine alternative to the Nasdaq, with growing Life Sciences firms choosing to keep and locate as many staff as possible in the UK. It is a precondition to the success of this vision that Access to Finance for Life Sciences companies is substantively improved.
Therefore, government will work with the sector to do the following.
Establish a Life Sciences Scale Up Taskforce
This will drive progress on the ease with which life science companies can start, grow, and scale up in the UK. This will consider and develop recommendations on issues that inhibit scale-up and growth, identified through the development of the vision, as well as the recommendations of the Productive Finance Working Group (which is due to report later this year) in the context of the life sciences industry.
Support the development of a world leading UK life sciences VC ecosystem
We will ensure it has the skills, knowledge, and experience to analyse and assess the life sciences sector for big opportunities. This will include focused work to attract specialist talent from Boston and San Francisco and to support the upskill of generalist investors.
Successfully launch the £200 million Life Sciences Investment Programme (LSIP) in Summer 2021
The LSIP will deliver around £600 million long-term capital to unlock the potential of the UK’s best health and life science innovations, allowing companies to grow and ensure the UK remains a world-leader in life sciences innovation. Attract large and specialist international capital funds – through the government’s Office for Investment, including new sovereign partnerships – building on the success of the UK-UAE Sovereign Investment Partnership.
Strengthen the public markets ecosystem, building on Lord Hill’s UK Listing Review, to support more of the UK’s leading Life Sciences companies to list here
While the Review and its recommendations are cross-sectoral, their delivery would encourage more Life Sciences companies and founding entrepreneurs to list on the LSE and secure competitive valuations.
Collectively, these initiatives will bring new capital alongside an evolution in our domestic capital markets, which are crucial to retaining high growth life sciences businesses, enabling them to choose to stay in the UK, provide high value jobs across the country, generate tax revenue and support the UK’s ambition to become a world-leading science superpower.
Regulation
Overarching ambition
Use the opportunity of Brexit to deliver a progressive UK regulatory offer with the capacity to unleash innovation in regulatory processes; use real world evidence and novel biomarkers and surrogate markers to support early patient access to novel and preventative treatments; and provide an aligned, integrated and simple regulatory journey for companies to engage with.
The UK regulatory environment is already strong. The MHRA is recognised as a global leader, playing a fundamental role in shaping global standards, and the regulatory response to COVID-19 demonstrated the speed and agility with which the UK system can act.
The UK needs to continue to act with pace to realise the opportunity to set regulatory standards in areas of rapid innovation – an ambition emphasised by the recent TIGRR report. It must also be simple for companies to navigate the regulatory system, with processes that are integrated and aligned with key partners, such as NICE and NHS England.
With regulation the area in which the UK’s departure from the European Union creates greatest change for the sector, there is a real opportunity to develop and deliver a world class sovereign regulatory environment, recognising the implications of EU Exit in relation to Northern Ireland and the importance of equity of patient access across the UK.
There are 3 core areas that will be prioritised over the coming years:
- processes, systems and people
- partnership, working and system join-up
- regulation in a global context
Processes, systems and people
For medicines, the MHRA will work with NHS partners and international regulators to deliver the fastest regulatory assessments and decisions. This will involve innovative regulatory models, building on the approaches developed for the Early Access to Medicines Scheme (EAMS) and the Innovative Licensing and Access Pathway (ILAP). There is a particular opportunity to support early treatment and prevention through developing innovative regulatory models for the treatment of individuals who are pre disease or have nascent disease, and for diseases (such as dementia) where there are limited or no biomarkers, and a need for surrogate markers or where impact on outcomes will not be seen for many years.
For Medical Devices and In-Vitro Diagnostics, the MHRA will consult with the sector on the proposed new regulatory framework later in 2021. The UK’s aim is to have a best-in-class regulatory environment for both Devices and Diagnostics. This will build on those elements of the EU’s Medical Device Regulations 2017 and In-Vitro Diagnostics Regulations 2017 that work, but also aggressively explore and execute improvements that support innovation and drive patient safety. In particular, the MHRA will deliver the world’s leading regulatory model for Digital Health products, which will be a key driver of innovation in the next decade and are not well regulated anywhere in the world currently – reflecting the recommendation from TIGRR and industry feedback. Delivery of the new regulatory regime for Devices and In-Vitro Diagnostics will also recognise and respond to the structure of the sector, in which over 95% of companies are SMEs.
The UK’s approach to regulation will be underpinned by the use of real world evidence, and the deployment of novel analytics and data tools to speed up and add rigour to regulatory processes. This will allow a step change in the UK’s approach to Vigilance and significantly enhance patient safety.
The MHRA will also build on learnings from COVID-19 to refine and improve existing regulatory processes and systems. This will include early access to expertise and advice, digitisation, virtual regulatory inspections, integrated systems and use of real world evidence.
Partnership working and system join-up
The work of the MHRA will be underpinned by an integrated, multiagency approach in which it works in complete alignment with NICE, NHS England, NHSX and the NIHR.
As part of a new Life Sciences Regulatory Commitment, the CEOs of the respective organisations are committed to providing companies a simplified, bespoke journey from regulatory approval to NICE appraisal through to discussions with NHS England on Access and Uptake.
This integrated, multiagency approach will be particularly important when supporting the most innovative new medicines and technologies, such as with:
- highly innovative commercial deals, such as the partnership collaboration on Inclisiran, which rely upon simultaneous engagement with empowered and aligned staff from across NIHR, MHRA, NICE and NHS England
- software as a medical device, where the technology is rapidly evolving and intensive collaboration between NHSX and NICE is essential to maintain a joined up regulatory and access environment
As with many areas of the vision, health data will be a critical enabler of this system working more effectively. Work will continue across the Health System to ensure that data can be safely and appropriately accessed by regulators, so that real world evidence and advanced analytical tools can be used to continuously assess safety and effectiveness.
Regulation in a global context
The MHRA has played an important role in setting and championing global regulatory standards for medicines and medical devices in recent years, and that will continue over the next decade.
In particular, the MHRA will:
- take opportunities to cooperate and form partnerships with like minded regulators globally. The MHRA has already joined the US Food and Drug Administration (FDA) Project Orbis, which has already allowed rapid access to new cancer medicines for NHS patients, such as Tagrisso for early stage lung cancer. In addition, the Access Consortium, will see the MHRA working together with Australia, Canada, Switzerland and Singapore to provide access to high quality, safe and effective therapeutic products across the 5 countries
- play an enthusiastic role in global standard setting forums – shaping, driving, and promoting international best practice. The UK has joined the International Council for Harmonisation of Technical Requirements for Pharmaceutical of Human Use, the International Medical Device Regulators Forum, and the Medical Device Single Audit Programme
- deepen cooperation between regulators globally through new free trade agreements and regulator to regulator agreements, including with partners such as the US Food and Drug Administration. These offer opportunities to deepen cooperation, exchange information and encourage adoption of international standards and best practice
Innovative licensing and access pathway
The ambition of this new licensing and access pathway is to reduce the time to market for innovative medicines. The ILAP combines the MHRA’s globally recognised strengths of independence and high standards of quality, safety, and efficacy, with improved efficiency and flexibility, readying the MHRA for a new era in medicines approvals in the UK.
Central to realising this ambition is how the ILAP provides a single integrated platform for sustained collaborative working between the MHRA, partners and the medicine developer.
By harnessing expertise at the right time from the MHRA’s partners, the National Institute for Health and Care Excellence (NICE), the Scottish Medicines Consortium (SMC) and NHS England and NHS Improvement (NHSE&I), the ILAP allows for enhanced coordination and monitoring of important product development activities. The “Innovation Passport”, a new medicine designation, acts as the gateway to entry into the pathway and will be awarded to innovative products submitted to the ILAP.
A successful Innovation Passport designation then triggers the MHRA and partners to create the “Target Development Profile” (TDP). This “living document” will set out a unique product-specific roadmap towards patient access in the UK healthcare system.
The ILAP has been well received by the sector with the first innovation passport designation being issued at the end of February 2021 for a treatment for adults with von Hippel Lindau disease (a rare genetic disorder that causes cancer).
Skills
Overarching ambition
To develop a strong talent pool across industry, academia, and the NHS, ensuring the Life Sciences sector has access to the variety of skills it needs to support innovation, and entrepreneurs feel they can access the human capital required to grow their companies in the UK.
Talent is paramount for the UK’s competitiveness as a location for Life Sciences innovation and investment. The provision of skills is essential to delivering better health outcomes, building the UK’s health resilience, as well as boosting productivity and driving forward areas of UK strength in Life Sciences including genomics, clinical trials, and artificial intelligence.
According to the Science Industry Partnership, by 2030 the sector has the potential to create around 133,000 jobs, through replacement and growth.[footnote 27] This demand covers digital, computational, and statistical literacy; translation and commercial skills to ensure the sector is able to make the most of the UK’s world-leading science and research base; and a variety of other skills needs including bioinformatics, clinical pharmacology, regulatory science, engineering and material science, complex manufacturing and advanced therapies.
To meet this demand, government, working in partnership with industry will ensure the sector has the tools it needs to recruit, reskill and develop employees in both specialist and non-specialist roles.
Our ambition is to develop the highly skilled workforce needed to position the UK as the global hub for Life Sciences, ensuring the sector has access to the skills, talent and people it needs to innovate and grow and are able to capitalise on emerging opportunities.
To meet this ambition, over the coming years, government and the sector will:
- welcome and advocate the free flow of Life Sciences talent globally, underpinned by the UK’s new immigration system, with specialist routes such as the Global Talent visa
- build links between Life Sciences university courses and industry, so that new graduates are educated in the cutting-edge skills and techniques that industry needs, and are ‘industry-ready’ when starting life sciences jobs
- support the sector to meet emerging skills demands by exploring with DfE how the Skills Value Chain approach, currently being piloted in the Manufacturing sector, could be used to stimulate adoption of emerging skills in the Life Sciences sector. Such emerging skills demands would likely encompass current areas of shortage, such as bioinformatics, data analytics, computational biology and visualisation technology
- support the growth and development of UK-based entrepreneurs with the skills and expertise to run and grow successful Life Sciences businesses. This includes through taking advantage of schemes such as HMT’s Help to Grow MBA-style training offer, continuing to deliver the unique NHS Clinical Entrepreneur programme, and taking full advantage of the role the private sector (both companies and investors) can play in nurturing and developing Life Sciences leaders
- support the movement of academics into industry and back again to support the development of a more rounded scientific skills-base and support the expansion of clinical academics to support Life Sciences growth, particularly in areas such as clinical pharmacology. For medtech, support clinicians to collaborate on the development and iteration of new technologies
- drive up the provision of Life Sciences apprenticeship training across level 2-7, through better industry co-ordinated engagement with Life Sciences employers. We will seek to enhance the quality and quantity of skills provisions; improve SME engagement in skills provision, including the apprenticeship levy, building sustainable cohorts for specialist subjects
- boost the proportion of the apprenticeship levy recovered by the Life Sciences sector from 24% to surpass the national average of 31% by working with industry to ensure the apprenticeship system works for the Life Sciences companies, and in particular life sciences SMEs
- support the development of a world leading UK Life Sciences VC ecosystem, ensuring it has the skills, knowledge and experience to analyse and assess the Life Sciences sector for investment opportunities
Transforming the UK’s diagnostic capabilities post-COVID
As the UK emerges from the COVID-19 pandemic, government will look to establish the UK as a world leader in diagnostic services and innovation. This will include changing the model of diagnostics services to be more proactive, taking advantage of new technologies and changed public behaviour, and using the NHS Test & Trace infrastructure to support the Life Sciences industry.
The model for UK diagnostics will change, moving towards earlier diagnosis, with a greater emphasis on prevention and prediction. Government and the NHS want to bring testing closer to patients using innovative diagnostic techniques to improve patient access and outcomes, as well as expanding capacity in existing diagnostic modalities. Government will also use the pandemic legacy to improve testing for other infectious diseases, as well as ensuring that we have the capacity to respond to future pandemics.
The infrastructure built up by Test & Trace will also be used to support the Life Sciences sector. The network of laboratories developed to support the testing effort will, once the pandemic is over, be able to support the expansion of research and clinical trials capacity in the UK.
Later this year, the Department of Health and Social Care will set out its strategy for the future of diagnostics in the UK, and the Life Science sector will play a critical role in developing this strategy.
Manufacturing
Overarching ambition
Create a globally competitive environment for Life Science manufacturing investments, building on the strengths of our manufacturing R&D, our network of innovation centres, the manufacturing response to COVID-19 and delivery of the Medicines and Diagnostics Manufacturing Transformation Fund.
The UK has a proud history of Life Sciences manufacturing, across pharmaceuticals and medtech. There are over 2,000 life science manufacturing sites across the UK.[footnote 28] However, this represents a significant reduction over the last 25 years, and since 2009, production volumes have fallen by 29% and over 7,000 jobs have been lost.[footnote 29]
This decline has national, regional and local economic impact, and has meant that technologies and products which were originally developed in the UK have not been commercialised or manufactured in the UK, and that globally mobile inward investments have tended to go to competitor countries.
As well as an economic impact, the reduction in manufacturing capacity also has health resilience and innovation implications. The COVID-19 pandemic demonstrated the vulnerability created by the UK’s reduced manufacturing base, while the reduction of proximal manufacturing and R&D in the UK lessens the speed of innovation, particularly in medtech where the development process is highly iterative.
As manufacturing technology develops, and the underpinning materials become more complex and valuable, it is important that government and the sector work to attract key elements of the supply chain into the UK. This can ensure that a greater share of the end-to-end manufacturing process is in the UK, capturing the return on the government’s investments in R&D in manufacturing and improving UK health resilience.
The government and sector’s ambition is to drive a renaissance in UK manufacturing over the next decade. This will look to target areas in which the UK has, or could develop, a meaningful competitive advantage (such as Cell and Gene Therapies, oligonucleotides, viral vectors, advanced diagnostics or Wound Care) – as opposed to making generalist offers which are not competitive internationally. Given the geographical spread of the UK’s manufacturing base, improving the UK’s competitiveness in this area will especially support the creation and retention of jobs in the North and Midlands.
There will be 7 core elements of the UK’s offer.
Build on the UK’s Pandemic Manufacturing Infrastructure and deliver the Medicines and Diagnostics Transformation Fund
The VTF has invested over £350 million to increase vaccine manufacturing capacity in the UK and NHS Test and Trace will have created the capacity to manufacture 2 million Lateral Flow Devices per day by Autumn 2021. These investments will support the UK’s pandemic preparedness, but many of the assets have extensive commercial value in non-pandemic settings that should be utilised – and will be complemented by delivery of the £20m Fund, which is providing grants to support UK Life Science manufacturing.
Continue to support the UK’s manufacturing innovation ecosystem
The UK has developed a series of world class manufacturing innovation centres such as the Cell and Gene Therapy Catapult, CPI Medicines Manufacturing Innovation Centre and the Nucleic Acid Therapy Accelerator underpinned by R&D hubs. Support for medicines manufacturing innovation, including through these Centres, must continue to assist growth companies to scale-up and make initial manufacturing investments in the UK, driving the growth of a vibrant and economically powerful innovative system and sector. We recognise the importance of attracting investment in R&D Pilot Lines as a means to then securing commercial scale manufacturing for both medtech and medicines manufacturing.
The UK’s competitive tax environment
Underpinned by the lowest headline rate of Corporation Tax in the G7, and a generous system of tax reliefs benefitting the Life Sciences industry, including R&D tax credits, the Patent Box and Super Deduction, which provides a first-year relief of 130% on new plant and machinery.
Support the formation and expansion of Manufacturing Clusters
International evidence suggests the development of clusters is important for sustaining and attracting manufacturing investments in particular geographies. Government will therefore work with UK-based manufacturers and local partners to support cluster formation around existing sites, as well as interventions, such as Freeports and access to shared technical facilities and advice, that will support cluster formation in new areas of the country.
Enhance the Manufacturing Skills Base
To ensure there is a sustainable flow of qualified staff available to undertake Life Sciences manufacturing roles and support the formation and expansion of clusters. This should reflect the extensive work that the Medicines Manufacturing Industry Partnership have already done on this area, the distinct skills requirements of medtech, and the Advanced Therapy Skills Training Network National Training Centres.
Support the Transition to Net Zero
The Life Sciences sector is committed to playing an enthusiastic role in the transition to Net Zero. Government will work with the sector to support this process, including through improving the sustainability and carbon footprint of manufacturing processes, removing plastics and other non-renewables from products, and, where this is not feasible, work with the sector on new materials science.
Trade and investment
Overarching ambition
Develop a modern, forward-thinking international trade strategy that utilises a variety of policy levers domestically through export promotion, bilaterally through Free Trade Agreements (FTAs), and multilaterally at international forums, to support the growth of, and investment in, the UK’s life science sector; underpinned by a deep understanding of the importance of Intellectual Property (IP) and Contract Law.
The UK has a long history as an advocate for free trade, open supply chains and inward investment. We were the most prominent advocate of free trade while a member of the European Union, and post-Exit, will continue to champion the importance of free trade bilaterally, multilaterally and in other relevant fora. As demonstrated in the pandemic, the UK will also always respect the sanctity of Contract Law.
Over the next decade, the UK will unequivocally champion open, competitive free trade and work to support the Life Sciences sector, through tariff elimination, a strong rules-based approach to tackling non-tariff barriers, promoting IP regimes that are more closely aligned with the UK’s gold standard globally and through creating the most hospitable environment possible for inward investment.
The UK will deliver this through an ambitious programme of future trade negotiations, including the recent signing of an agreement in principle with Australia, negotiations with the US and New Zealand, while looking to new opportunities in the Indo-Pacific region, such as a future Free Trade Agreement with India and accession to the Comprehensive and Progressive Trans-Pacific Partnership. The UK will also use multilateral fora, such as the World Trade Organization, World Health Organization and the G7 presidency to seek agreement on trade policy commitments to facilitate global trade and support the Life Science sector.
In addition to core trade policy, the UK will be unrelenting in supporting both inward investment and export promotion, and proactively develop more and better mechanisms to support this.
To deliver on this ambition, the UK will:
- use Trade Policy to grow the UK’s reputation as the leading global supporter of the Life Sciences sector. This includes reducing regulatory barriers to trade through increasing international recognition of UK conformity assessment and approvals, agreeing or expanding mutual recognition agreements, ensuring the MHRA’s participation within international Life Sciences forums, and ensuring a level playing field for UK Life Science companies operating abroad. We will expand partnerships with other countries and encourage cooperation through the promotion of internationally agreed standards to reduce barriers to trade and encourage regulatory cohesion
- demonstrate the UK’s global leadership and bring in growing markets by developing comprehensive and futureproof multilateral agreements for the elimination of tariff and non-tariff barriers to trade in Life Sciences. We will strike new agreements and improve existing trade relationships with the UK’s top trade partners
- continue to promote and expand export and investment opportunities for UK Life Sciences through providing bespoke advice, support and opportunities through the Department for International Trade’s extensive trade promotion network, both in the UK and globally. This will continue to include account management for investors through UK regional and global networks and linking export promotion to trade policy and integrating life science suppliers into healthcare infrastructure projects supported by the UK
- provide a smoother, clearer, more efficient and effective system to better support new and existing inward investors to invest into the UK. This will build on existing “first points of contact” such as the INVEST in GREAT website, the extensive account management service delivered by the Office for Life Sciences and Department for International Trade, and the other “Front Doors” that have been developed by key organisations. We will consult widely with industry to ensure the enhanced “Investment Front Door” is targeted to needs of investors and offers a world-leading service, clearly articulating the UK offer, incorporating the most successful elements of industry engagement conducted by the UK’s Vaccine Taskforce
Life sciences pledge
The UK has a proud history as a centre for Life Science innovation, and a profound appreciation of the importance of the rule of law.
Government has repeatedly demonstrated, especially in the pandemic, the importance of free trade and its commitment to strongly uphold the rule of law.
Following publication of the vision, government and the sector will explore the development of a pledge that reflects the UK’s intention to support the laws, practices and principles which underpin the operation of the Life Sciences sector.
Life sciences and net zero
The Life Sciences sector has an important role to play in supporting the government’s net zero target, as healthcare currently contributes around 5% of the UK’s carbon emissions.[footnote 30]
There is already intensive work being undertaken across the public and private sector, including:
- life sciences companies have committed to reduce their environmental footprint. For example, GSK is aiming to have a net zero impact on climate and a net positive impact on nature by 2030, AstraZeneca has announced a $1 billion ambition to deliver a carbon negative value chain by 2030, and J&J aim to source 100% of their electricity needs from renewable sources by 2025
- the Greener NHS Initiative is supporting the NHS and its supply chain to reach net zero by 2045
The Life Sciences Vision will further support government and industry targets on net zero through:
- supporting the development of preventative healthcare and early diagnosis technologies that reduce the need for prolonged treatment of advanced illness and frequent visits to healthcare settings
- optimisation and digitisation of the UK’s regulatory system, allowing for a reduction in paper use and enabling virtual inspections of manufacturing and clinical trials sites
- supporting the development and deployment of green manufacturing technologies and working with the medtech sector to explore advances in materials science and the opportunities to utilise sustainable, reusable and recyclable materials in new products -recognising that company size will impact the time companies require to adapt
- ensuring that the Life Sciences can play a prominent role in cross-sectoral forums and work on net zero
Section 4: Addressing the great healthcare challenges
Much of the content of this vision is disease agnostic, recognising that it would be ineffective for government and the sector to attempt to develop a central plan for research and innovation across the many thousands of diseases that afflict people in the UK and globally.
However, there are a series of diseases or conditions that drive very significant mortality and morbidity in the UK and globally, where government and the sector could work together to address more effectively, learning from the impact of deep collaboration in combating COVID-19. These are population health disorders, and the prediction, early diagnosis, treatment and prevention of these diseases will be the core of this vision.
The 7 areas below each share 2 characteristics: the scale of their impact on the NHS and health systems globally and the size of the commercial opportunity for industry in addressing them. These are the prerequisites for government, industry, philanthropy, medical research charities, devolved administrations, and the NHS to work together to deliver mission-oriented approaches for particular diseases or conditions.
Improving translational capabilities in neurodegeneration and dementia
Overarching ambition
Build on the existing dementia research ecosystem and partner with industry and academics to accelerate the pace of translational studies into novel dementia treatments, focussing on filling the dementia knowledge gap, identifying new therapeutic opportunities, target validation, new diagnostic, prognostic, and treatment biomarkers, and supported by novel trial design and adaptive license strategies.
Dementia and Alzheimer’s Disease is the leading cause of death in the UK, with an economic cost of over £25 billion per annum.[footnote 31][footnote 32] This cost will grow significantly in the coming decades as the population ages, a trend that will be replicated globally, and particularly in large middle-income countries whose demographic profiles are rapidly changing.
Despite the FDA licensing the first new treatment for Alzheimer’s Disease in nearly 20 years in June 2021, there has only been incremental progress in our understanding of the disease and other common dementias in recent decades, with only supportive care and symptomatic treatment available for those with the condition. Over the last decade, there have been a range of UK-led initiatives to increase the volume and quality of research into dementia and other forms of neurodegeneration, including:
- the UK Dementia Research Institute, supported by £190 million of funding from the Medical Research Council, focuses on basic and translational science, early-stage development of diagnostics and treatments, experimental medicine, and the development of a new generation of technology to support those living with dementia
- the NIHR Dementia Translational Research Collaboration, which supports Biomedical Research Centres and NHS-university partnerships to conduct dementia research
- the Dementia Discovery Fund, a £250 million international VC programme that invests in, and creates, new biotech companies to deliver high impact therapeutics for age-related dementias
These and other initiatives have played an important role in coalescing government, industry and philanthropic investment. However, there are important opportunities to build upon this foundation and complement these capabilities with a particular focus on getting more new products into the clinic, and ultimately, into patients who have no other treatment options.
The core components of this Mission would be the following.
Translational science capabilities
Development of a hub and spoke translational research model to complement the successful hub and spoke UK Dementia Research Institute (UK DRI) structure, focused solely on target validation, taking novel molecules into the clinic. This model would allow companies, the UK DRI and other academic groups to more efficiently and effectively take products into early-stage trials, in a fast and efficient manner that allowed them to reach their clinical endpoint more quickly. Delivery of this capability would also be underpinned by developing innovative diagnostic and digital technologies (including genomic, cardionomics and imaging) to develop novel clinical endpoints that could then be used in future trials. There is also an opportunity to utilise the Dementia Platform UK and Our Future Health infrastructure together with the biosensor, AI, biomarker, and genetics expertise to deliver supportive studies into early determinants of dementia, in the context of genetic and environmental risk-factors.
Regulation
The MHRA will work to be the most innovation-friendly regulator in the world, including on diseases such as Dementia where the regulatory challenges are especially complex. This includes utilising innovative clinical trial regulation to support earlier patient access to new treatments and technologies, novel approaches for surrogate marker detection, and adaptive licensing approaches that facilitate faster, safer access to potential new treatments and diagnostics.
Ecosystem join-up
Ensuring that new Translational research capacity is fully aligned with existing programmes and funding, including the Dementia Research Institute and the Dementia Ecosystem consortium already founded by the Institute.
Enabling early diagnosis and immune therapy for cancer, including through cancer vaccines
Overarching ambition
Drive the development and commercialisation of new cancer medicines, diagnostics, and genomic and predictive technologies in the UK, acting as a testbed for oncology innovation.
There are more than 166,000 cancer deaths in the UK every year, and 1 in 2 people in the UK will develop cancer in their lifetimes.[footnote 33] There is worrying evidence that diagnosis rates have dropped significantly during the pandemic.
In recent decades there have been a very wide range of initiatives to improve cancer research and treatment. This has led to cancer being the single largest disease area funded by the NIHR, and a very consistent area of focus for the NHS in its major policy documents including the ‘NHS Plan’ (2000), ‘NHS Five Year Forward View’ (2014) and ‘NHS Long Term Plan’ (2018).
Importantly, over the last decade, there have been real improvements in the diagnosis and treatment of cancer. These include using genomic and genetic analysis to more precisely diagnose cancer and adjust treatment regimes, the use of novel data and analytical approaches, such as Polygenic Risk Scores, and significant improvements in the treatment of some types of cancer through the development of Advanced Therapies. Technological advancement supports, but does not replace, the classic cancer maxim: early diagnosis and treatment are the key determinants of survival.
With vast public, private and philanthropic investment in cancer, the UK needs to be relentless in focussing energy and attention in those areas in which it could gain competitive advantage.
For that reason, government and the sector will focus on the following.
Development and commercialisation of immuno-oncology, cancer vaccines and diagnostics in the UK
The UK has a very strong cluster of Advanced Therapy companies forming in Stevenage around the Cell and Gene Therapy Catapult, and deep expertise in genomic science and data.
To maintain and grow this, the UK will do the following.
Focus on at scale cancer diagnostics
Building on the agreement NHS England has already reached with GRAIL, there are further opportunities to form innovative commercial partnerships with cancer diagnostic companies. These will allow the NHS to have early, cost effective access to new diagnostic technologies through committing to trialling them at scale in clinical settings and provide industry with a powerful demonstration of the products’ efficacy in real world settings and complement programmes and infrastructure such as Our Future Health and UK Biobank.
Immuno-oncology and cancer vaccines
There is broad consensus on the enormous potential of immunological interventions for cancer, and the progress that is likely to grow over the next decade. The UK will build on its global leadership in this area,[footnote 34] focused on clinical trials for new therapies and using our manufacturing scale-up infrastructure to develop their manufacturing science in the UK and locate critical early-stage manufacturing investments in the UK. The UK will also marry its deep expertise in immuno-oncology and vaccines to explore the potential of cancer vaccines, in light of the advances in vaccine technology during the pandemic.
Genomic and polygenic risk scores
Genomic and genetic analysis has been crucial in driving progress in oncology in recent years. There is an opportunity to use the UK’s existing capability and capacity in this area to understand and characterise cancers in much greater detail and use this to further inform the development of novel therapeutics, diagnostics, medtech and clinical pathways.
Support commercialisation of cancer technologies in the UK
Through supporting uptake and access of products through the availability of innovative licensing and access arrangements, and creating the broader business environment (especially on areas like Access to Finance and Skills) where companies can access the financial and human resource required to grow and flourish.
UK and US bilateral cancer summit
The UK and US government, in collaboration with stakeholders from across the sector, will convene the first ever joint UK – US Cancer Summit to share ideas and identify opportunities for collaboration to accelerate advances in lifesaving approaches to cancer
Sustaining the UK position in novel vaccine discovery, development, manufacture and use of vaccines
Overarching ambition
Build on the UK’s deep expertise in vaccines and investments in the pandemic to strengthen the UK ecosystem, with a particular focus on new and novel technologies.
The UK has a rich history in vaccines. Since Edward Jenner, smallpox, and the milkmaid in the late eighteenth century the UK has played a significant role in the development and deployment of a very wide range of vaccines and related technologies.
This includes very significant academic expertise in basic, translational, and clinical immunology and vaccinology; deep experience and expertise in clinical trials – from novel first in human studies to late-stage trials in the UK and abroad; through to delivery, with among the most comprehensive domestic vaccination programmes in the world and as one of the largest single funders of GAVI, the Global Vaccine Alliance.
The UK’s vaccine response to COVID-19 has reflected this deep expertise. The UK has played a fundamental role in the development of a number of different vaccines; shown significant commercial and regulatory flexibility to get vaccines to patients quickly; and has had among the world’s fastest rollouts, while also being the fourth largest contributor to the COVAX initiative. Government and the sector have also made significant investments in vaccine manufacturing capacity, which was an acknowledged area of weakness pre-pandemic.
The opportunity now is to build on this deep heritage, and the exceptional response to COVID-19, by deepening the UK’s vaccine development capacity and continuing to improve the UK’s industrial infrastructure. Modern vaccine technology has the potential to prevent and treat a range of non-infectious diseases, so continuing to advance UK capability and capacity in this area can have wide health benefits, as well as support the G7 ambition to have vaccines developed and deployed within 100 days of a future pandemic.
To do this, the UK will do the following.
Continue to improve core immunology, vaccinology and clinical trial design and infrastructure
This will be done so that the UK maintains its academic excellence in these areas and can continue to operate as a global hub for the development of new vaccines and their trialling in first-in-human and larger, late-stage efficacy trials. This would also include a strong focus on clinical immunology, and the widespread alignment and integration of studies on natural immunity with vaccine clinical trials, by using standardised assays; and increasing the use of Human Challenge Trials, which can allow for much faster time to result and would help cement the UK’s reputation for excellence in this research method.
Deepen UK expertise in vaccine formulation and delivery
Many of the vaccines developed to address the current pandemic are at the cutting edge of science. However, they rely on traditional methods using vials and syringes that are slow to distribute and deliver to patients at scale, involve complex supply chains and specialist storage and distribution conditions. There is therefore an opportunity to focus on developing new modes of administering vaccines, including on innovating delivery through the mouth (oral), skin (transdermal or intra-dermal) or nose (intra-nasal), and tackle the barriers that exist in the market which prevent the scale-up and commercialisation of those novel vaccine formulation technologies.
Utilise the unique long term research potential of the National Vaccine Research Registry and other COVID-19 research programmes
Through the Vaccine Research Registry and research programmes such as Imperial College’s REACT study, hundreds of thousands of individuals have volunteered to take part in COVID-19 research, and the overwhelming majority (94% in the case of the Registry) have indicated a willingness to be contacted in future about non-COVID-19 research. Government and all parts of the sector should take consolidated action to keep these participants research active and invite them to join major new research cohorts such as Our Future Health.
Consider what more can be done to grow vaccine manufacturing capacity and capability
During the pandemic, the VTF has invested over £350 million in building the UK’s vaccine manufacturing capacity. This has developed the government’s existing Catapult programme and also enhanced private sector capacity. Linked to the Manufacturing section earlier in this document, there is an opportunity to seize the positive momentum created by the response to the pandemic, and secure inward investments that allow a greater range of novel vaccine platforms, as well as single use disposable equipment to be manufactured at scale in the UK.
Strengthen and maintain government-industry partnerships
Key to the VTF’s success in developing vaccine candidates has been its joint working with academia, industry, medical research charities, and the regulator. Industrial, technical and manufacturing expertise, for example, supported the VTF in making shrewd investments at a critical time. Through utilisation of pre-existing NIHR, UKRI, MHRA and Innovate UK supported expert networks, the UK proved its capability to discover, develop, manufacture, conduct clinical trials, and authorise a product for safe use faster than anywhere else in the world. Maintaining investment in these networks will sustain swifter progression of novel technologies, as the new UK Health Security Agency develops its coordination capability in the coming months.
Continue and in future expand the UK’s domestic vaccine programmes
The UK’s success in delivering the COVID-19 vaccines at enormous scale built heavily on the deep NHS and public health experience in concurrently running multiple, national vaccination campaigns every year. The rate of scientific progress in vaccine development means that a range of innovative new vaccines are likely to be placed on the market in the coming years. The Department of Health and Social Care, MHRA, Joint Committee on Vaccination and Immunisation, UK Health Security Agency and NHS England will need to be ready to assess these in an agile manner, and, if cost effective, roll these out in an agile manner – with the right surveillance and diagnostic systems in place to collect real world data and understand real world performance.
Pandemic preparedness partnership and the G7
The UK’s contribution to the coronavirus pandemic response has been vital. From the development of the Oxford/AstraZeneca vaccine, the RECOVERY trial identifying safe and effective therapeutics such as dexamethasone, to the growth of a diagnostics industry in the UK that is contributing to sequencing emerging variants, the UK has made a rapid and significant contribution to the global fight against COVID. The UK is now using the G7 Presidency to ensure the world is better prepared for future pandemics.
Global Pandemic Radar
In May, the Prime Minister launched plans for a new Global Pandemic Radar to identify emerging COVID-19 variants and track new diseases around the world.
The pathogen surveillance network will work globally to identify, track and share data on new coronavirus variants and monitor vaccine resistance in populations.
The Radar is expected is expected to be up and running with a network of surveillance hubs before the end of 2021. This renewed focus on surveillance will save lives and protect health systems by spotting diseases before they cause future pandemics and enable the rapid development of vaccines, treatments and tests.
The World Health Organization will lead the implementation group responsible for establishing the Radar, supported by the Wellcome Trust. Through the development and delivery of the Radar, the UK government, World Health Organisation and Wellcome Trust will work closely with other governments’ centres of disease control, non-governmental organisations and research organisations.
Pandemic Preparedness Partnership
The Prime Minister asked Sir Patrick Vallance to convene a group of experts from industry, international organisations and leading experts in a ‘pandemic preparedness partnership’, to advise the G7 on steps that can reduce the time to develop and deploy high quality diagnostics, therapeutics and vaccines (DTVs) to 100 days in response to a future health threat. The first 100 days after the identification of an epidemic threat are crucial to changing its course and, ideally, preventing it from becoming a pandemic.
A series of recommendations were set out in the ‘100 Days Mission’ report. These include proposals to focus on the R&D needed to create prototype DTVs against future pandemic threats and develop simplified vaccine manufacturing processes that could be deployed in an international manufacturing network. The report states that the exceptional performance of regulators and the delivery of clinical trials should be embedded into routine to enable a rapid future response. It also proposes that pandemic rules of the road should be agreed in advance to enable prioritisation in trials for novel DTVs, speed of data sharing, and financing mechanisms that allow rapid scaling for equitable access.
The UK government has always been clear that delivering these objectives would require collaboration between government, academia, industry and international bodies. The former Secretary of State for Health therefore invited CEOs and representatives from leading life sciences companies to the G7 Health event in Oxford to discuss these issues with G7 Ministers. Industry leaders backed the ambition of the 100 Days Mission and committed to joining forces with governments and international organisations to explore how we can ensure we are prepared to respond to a future pandemic threat. This led towards the roadmap being endorsed by G7 Leaders in Carbis Bay, and it will now form the basis for a public-private approach to implementation of the proposals.
Alongside Pandemic Preparedness and the 100 Days Mission, government and the sector were pleased that the 2021 G7 Leaders Communiques emphasised the importance of continued concerted global action to combat Antimicrobial Resistance. This will be critical for future pandemic preparedness and the UK government and whole Life Science sector is committed to continuing to play a prominent role in this important area.
Prevention and treatment of cardiovascular disease and its major risk factors including obesity
Overarching ambition
Deliver cutting edge research that demonstrates the impact innovative service/drug/digital/device combinations can play in addressing the major causes of cardiovascular disease, in particular obesity.
Cardiovascular diseases are responsible for 1 in 4 deaths in the UK and are a leading cause of premature disability and mortality, with a disproportionate impact on low income communities across the UK.[footnote 35]
Many of the risk factors for cardiovascular disease are preventable and yet increasingly prevalent. For example, the UK has among the highest rates of obesity in the developed world.[footnote 36] This causes profound healthcare challenges for the NHS.
Despite the increasing prevalence of obesity and other risk factors in the UK and globally, there has been little progress in recent decades in demonstrating, at a population level, the efficacy of interventions (whether behavioural or medical) that allow individuals to, for example, sustainably reduce their BMI, and in so doing, reduce their serious, related disease.
The growing burden of, and lack of progress in treating, obesity and obesity-related disease creates a significant market opportunity, and the scope to trial innovative solutions.
The 2020 Obesity Strategy committed to a range of measures to address obesity. To this end, government, NHS England, medical research charities and industry could collaborate and deliver a programme of large studies to build real world evidence, by simultaneously testing multiple different technological solutions to obesity and other risk factors to assess if and how well they work at scale, in live healthcare settings.
In practice, this could involve trialling a combination of a novel medicine or medtech that allows an individual to lose weight, with digital technologies then used to support an individual to maintain their weight loss and remain at a healthy weight.
Multiple technologies could be “mixed and matched” adaptively in the trial to examine the efficacy of different combinations, and the UK’s diversity and clinical research and data infrastructure would allow the trial to be delivered at scale (20,000+ participants) in a representative population.
If any of the technology combinations then proved effective, it would then be possible to use an innovative deal to incrementally trial in larger populations, and if efficacy is maintained, support a national rollout.
Reducing the mortality and morbidity of respiratory disease, in the UK and globally
Overarching ambition
Reduce the pressure on the NHS and improve clinical outcomes, through driving improvements in the underpinning understanding of respiratory disease, as well as its treatment and diagnosis.
Respiratory disease affects 1 in 5 people in the UK. Lung diseases account for approximately 700,000 UK hospital admissions and over 6 million inpatient bed-days each year.[footnote 37] Despite the inclusion of lung health as a clinical priority in the NHS Long Term Plan, outcomes continue to stagnate.
Asthma causes over 1 million severe attacks and 1,400 deaths per year in the UK; two-thirds of these are potentially preventable.[footnote 38] However, in the UK, there is an over-reliance on “rescue” therapies prior to or after an attack, with over 15.5 million blue rescue inhalers prescribed every year, and more than 130,000 people taking at least 3 courses of oral corticosteroids each year.
For chronic obstructive pulmonary disease (COPD), the UK has one of the highest mortality rates worldwide.[footnote 39] COPD is the second largest cause of emergency hospital admission in the UK and costs £1.9 billion per year.[footnote 40] Moreover, 5-year survival post-hospitalisation for a COPD exacerbation is lower than most cancers, at only 50%.[footnote 41]
There is a real opportunity to advance the science in this area, and through that significantly reduce the number of attacks, hospitalisations and deaths over the next decade. Therefore, government, industry and NHS England will explore options to do the following.
Create more effective treatment options for asthma, particularly children and young adults
Build on the significant improvements in the understanding of the biological processes that lead to the airway inflammation that causes asthma by identifying new biomarkers for asthma and leverage genetics to better identify populations that will respond to new treatments and support patients with uncontrolled disease.
Drive innovation in the understanding and treatment of COPD
In spite of the high unmet need for COPD, breakthroughs in innovation have been limited, and until recently, no new treatments have shown impact on mortality rates. There is an opportunity to identify disease and intervene earlier and move from symptom control towards disease modification. There are also opportunities for further research into novel targets addressing cell dysfunction, lung tissue damage and airway remodelling, and the potentially transformational role of biologics for certain COPD patients, as well as targeting cell senescence.
Improve care pathways through improving diagnostic capacity and technology
Both in the UK and globally, there are opportunities to provide earlier access to treatment for respiratory diseases including TB, asthma and COPD through better diagnostics and monitoring technologies that improve clinical understanding of respiratory disease and its underlying causes. Such technology could improve the speed with which individuals can move through clinical pathways and be critical in addressing the burden of respiratory disease in low- and middle-income countries.
Ageing: to understand the pathways associated with multi-system ageing and to utilise these to discover new diagnostics, therapeutic and medtech interventions
Overarching ambition
Through deep collaboration between government and Industry, advance the medical science and understanding of ageing, in order to begin to advance viable products towards the clinic.
It is self-evident that multiple organ systems decline and fail with age and that it is likely that there are some common underpinning mechanisms that lead to this ‘ageing’ process. This is a relatively new field but one which has progressed rapidly with much activity in the biotech and academic sectors.
Understanding the biological processes that lead to this ageing phenomenon will obviously be crucial if therapies are to be found to better manage the ageing process and to reduce the multi-morbidity that occurs in ageing individuals and is the single biggest cause of stress to Western healthcare systems.
Diseases often group together in clusters, producing particular patterns of multi-morbidity and these are likely to result from common biological mechanisms. Understanding these will help in identifying individuals at risk of multiple concurrent chronic diseases and may also provide therapeutic interventions that cross conventional disease taxonomies.
There is a wealth of emerging literature on mechanisms believed to be involved in common ageing processes in multiple organs. These include DNA repair, telomer shortening, stem cell regeneration, insulin-like growth factor (IGF) signalling, nutritional factors and chronic inflammation. Molecular pathways involving nicotinamide adenine dinucleotide (NAD) or sirtuins have been well described and there is now an abundance of literature on potential pathways and targets that might be used to address the most inevitable cause of disease in human populations.
There is now significant opportunity to invigorate this field in collaboration with industry, to discover both pathways and potential targets for therapeutic intervention. These approaches could help better manage the multimorbidity arising from chronic diseases that emerge in an increasingly ageing population.
Mental health: redefining the problem and using novel approaches to identify new therapeutic and technological opportunities
Overarching ambition
Address the significant unmet need for innovative new treatments and technologies, through deepening the understanding of mental ill health, and using this knowledge to advance the development of new therapies and products.
Mental ill health has driven an increasingly large burden of disease, with it now representing the single largest driver of disability in the UK. This is often associated with additional chronic physical co-morbidities. It is the intention of a consortium of industry and academic investigators to think about how these disorders can be redefined and therapies developed.
Most pharmaceutical companies have abandoned the field of mental health because the discovery and clinical development paradigms used in this field are very difficult to execute and that the fundamental understanding of these disorders is not readily available to allow conventional target discovery.
It is clear, however, that the classical nomenclature that underpins mental health disorders could be reconsidered based on activation of particular pathways, biomarkers and symptom complexes that will allow a different approach to be taken to develop therapies for individual disabling symptoms rather than arbitrarily defined disease entities. This will require detailed phenotypic and genotypic characterisation of patient populations and, where possible, the characterisation of pathway activation using biomarkers or imaging.
Many of the symptoms of mental health disorders are shared between different defined entities and it may prove more useful to tackle these individual symptom complexes than to treat the diseases one at a time. For example, anhedonia bridges multiple different disorders and could be targeted as an individual symptom that could be amenable to pharmacological intervention. In addition, exciting new science strongly implicates neuroimmune cascades. This consortium would greatly leverage’s the UK’s investment and world-leading expertise in Immunology.
Mental health care also offers the possibility of combining a set of tools, including psychological therapies and digital tools, alongside pharmacology, to improve patient outcomes and experience. The UK has an unprecedented opportunity to accelerate patient access to these evidence-based therapies and technologies through close collaboration among the MHRA, NICE, NHS and organisations performing late-stage clinical trials.
Our ambition is to identify a collaboration of industry partners using these tools and bringing them together to evaluate therapeutic approaches in experimental and clinical trial settings. This could provide the information necessary to define new outcomes that could be used by regulators to accelerate access to novel therapies and improve mental health.
Section 5: Delivery and implementation
This Vision is a high-level document that outlines government and the sector’s ambitions for the coming decade but does not set out the specific programmes through which these will be delivered. Following the Spending Review, government will set out, in detail, its delivery plan and governance for taking this work forward at pace, and the sector will have the opportunity to decide where it wishes to co-invest.
There is exceptionally strong alignment between government, the NHS and the sector on the priorities in the vision, but agreement that to maintain this alignment and momentum, there must be effective execution. In developing the vision, a number of factors that will be key to execution have become clear.
Delivering on the preconditions for success
Throughout the document, 4 preconditions have been highlighted: the NHS operating as an innovation partner; government and the sector continuing to invest in science and research; unlocking the potential of the UK’s health data; and increasing access to capital for growth companies. These are essential priorities for delivery and will underpin the success of the wider vision.
Clear leadership and accountability
All programmes need accountable leaders with the authority and industrial experience required to deliver complex programmes of work that will often involve multiple public and private sector partners. The VTF demonstrates that this model works and can deliver.
Governance
Effective utilisation and evolution of the structures that have been developed since the publication of the Life Science Industrial Strategy in 2017 to oversee the relationship between government and the sector, and appropriate Governance of individual programmes. In order for there to be a focus on collaborative implementation across the wide range of national bodies that will have important delivery responsibilities under this vision, we will refresh the membership and terms of reference for the Life Sciences Implementation Board, which sits under the Life Sciences Council, to collectively develop implementation plans, in partnership with sector representatives and champions.
Metrics
Every programme or project taken forward under this vision must have clear SMART objectives that set out in granular detail what will be delivered by when, with wider statistics on the UK’s competitiveness provided annually via the Life Science Competitiveness Indicators.
Cross-UK delivery
To unlock the full potential of the UK as an attractive global location for life sciences, the UK government and English NHS are committed to working closely on all aspects of implementation with the devolved administrations and the Scottish, Welsh, and Northern Irish health services.
Reflect the diversity of the sector
The UK is fortunate to have a geographically and technologically diverse life science sector. It is important that these are fully reflected when delivering the ambitions outlined in this vision.
Organisations that have engaged in the development of the Life Sciences Vision
- Abingworth
- Academic Health Science Networks Academy of Medical Science Accelerated Access Collaborative Achilles
- Ada Lovelace Institute
- Advanced Medical Solutions
- Alchemab
- Alzheimer’s Research UK
- Arctoris
- Autolus
- Association of British HealthTech Industries
- Association of the British Pharmaceutical Industry
- Association of Medical Charities AstraZeneca
- BBraun
- Benevolent AI
- Berghealth
- BioIndustry Association
- British Generic Manufacturers Association British Heart Foundation
- British In Vitro Diagnostics Association British Heart Foundation
- British Lung Foundation
- Cambridge University
- Cancer Research UK
- Care Quality Commission
- Cell Centric
- Chief Scientist Office – Scotland
- Cogent Skills
- Compass Pathways
- Convatec
- Coloplast
- DeepMind
- Dementia Industry Group
- Department for the Economy – Northern Ireland
- Earlham Institute
- Epidarex Capital
- Freeline Therapeutics
- Fujifilm
- Genetic Alliance
- Genomics England
- GlaxoSmithKline
- Health Data Research UK
- Health Research Authority
- Human Fertilisation and Embryology Authority Human Research Authority
- Human Tissue Authority
- Imperial College London
- Immunocore
- INIVATA
- Invest Northern Ireland
- IQVIA
- Johnson & Johnson
- Kyowa Kirin
- L&G
- Livanova
- Medicines and Healthcare products Regulatory Agency
- Medical Research Council
- Mediplus
- MSD
- National Health Service England
- NHS Digital and NHS Digitrials
- NHSX
- National Institute for Health and Care Excellence
- National Institute for Health Research National Voices
- Newcastle University
- Novabiotics
- Novartis
- Novo Nordisk
- Nuffield Department of Population Health NTL World
- Oncimmune
- Our Future Health
- OSI
- Oxford Nanopore
- Oxford University
- Penlon
- Pfizer
- Phillips
- Polar Capital
- Professional Standards Authority Quadram Institute
- RedX
- Resmed
- Roche
- Sanofi
- Smith & Nephew
- Stryker
- Sustainable Medicines Partnership SV Health Investors
- Synairgen
- Syncona
- Takeda
- Thermofisher Scientific
- UCB
- UK Biobank
- UK Dementia Research Institute
- UK Research & Innovation
- Understanding Patient Data
- University College London
- University of Birmingham
- University of Liverpool
- Vaccitech
- Wellcome
- Welsh Government
- 3D Life Prints
-
Office for Life Sciences, 2020, Bioscience and Health Technology Sector Statistics ↩
-
Dexamethasone, whose efficacy was confirmed by the RECOVERY trial, is estimated to have already saved over 1 million lives since its effectiveness was demonstrated https://www.england.nhs.uk/2021/03/covid-treatment-developed-in-the-nhs-saves-a-million-lives/ ↩
-
Throughout this document, ‘NHS’ is used as an umbrella term referring to all the publicly funded healthcare systems of the United Kingdom. Otherwise, the specific organisation is identified (such as ‘NHS England’). ↩
-
Successful effort on jabs attributed to development of post-Brexit life sciences strategy over past 4 years. https://www.ft.com/content/9798f0c1-1712-442e-a795-518c39a9e418 ↩
-
Throughout this document, ‘NHS’ is used as an umbrella term referring to all the publicly funded healthcare systems of the United Kingdom. Otherwise, the specific organisation is identified (such as ‘NHS England’). ↩
-
Nature Index Annual 2021 tables: countries/territories - life sciences ↩
-
BEIS, 2019, International comparison of the UK research base, 2019 ↩
-
ABPI, 2019, Industry and Academia Links Survey 2019 ↩
-
www.nihr.ac.uk/news/uk-covid-19-research-passes-one-million-participants/27215 ↩
-
Office for National Statistics, Research and development expenditure by the UK government, 2019 ↩
-
Office for Life Sciences, 2020, Life Sciences Competitiveness Indicators ↩
-
See for example Ali et al, 2020, Open Heart ↩
-
Jonker, Fisher, Dagnan, 2019, Journal of Evaluation in Clinical Practice ↩
-
Lichten et al, 2017, Health Research Policy and Systems ↩
-
Lichten et al, 2017, Health Research Policy and Systems ↩
-
World Bank, 2020, Doing Business: Measuring Business Regulation ↩
-
https://www.gov.uk/government/publications/patient-capital-review ↩
-
OLS Analysis of Capital IQ data ↩
-
https://www.scienceindustrypartnership.com/skills-issues/sip-2030-skills-strategy/ ↩
-
Office for Life Sciences, 2020 Bioscience and Health Technology Sector Statistics ↩
-
Office for National Statistics: Index of Production time series ↩
-
A study in The Lancet estimated the NHS’s carbon footprint to be 25 megatonnes per year, ~5% of the total UK carbon emissions of 551 megatonnes published by ONS. ↩
-
Office for National Statistics: Leading causes of death, UK: 2001 to 2018 ↩
-
12% of Cell and Gene Therapy trials globally happen in the UK, and the UK is, by a considerable margin, the leading centre in Europe for the development of Advanced Therapies. ↩
-
British Lung Foundation: Estimating the Economic Burden of Respiratory Illness in the UK, 2017 ↩
-
National Institute for Health and Care Excellence: COPD Clinical Knowledge Summary ↩
