Annual Statistics of Scientific Procedures on Living Animals, Great Britain, 2021
Updated 17 November 2022
This version of the HTML has been corrected to amend a change in definition of non-human primate generation and colony status (see the ‘Place of birth of primates’ section), as well as a correction in the 10-year comparison for use of dogs in procedures. Specifically, under Figure 5 the text has been amended to read:
In 2021, the use of dogs in procedures decreased by 3% in the last year, and have decreased by 7% over the last 10 years.
‘Ad hoc data on non-human primates used in experimental procedures for the first time’ has also been published to support the amendment to the non-human primate generation, and colony status definitions. A corrections slip has also been added to both PDF versions.
Protected animals: Any living vertebrate, other than man, and any living cephalopod. This includes embryos after two thirds of gestation (although these are not included as countable procedures), and fish and amphibian larvae after they become capable of free feeding.
Regulated procedures: Any procedure applied to a protected animal for an experimental or other scientific purpose, or for an educational purpose, that may have the effect of causing an animal pain, suffering, distress or lasting harm equivalent to, or higher than, that caused by the introduction of a needle in accordance with good veterinary practice.
‘Number of procedures’ is not ‘number of animals’: The number of procedures carried out in a year does not equal the number of animals that have been used in procedures that year. This is because some animals may be used more than once i.e. ‘re-used’, in certain circumstances. These instances are counted as separate, additional, procedures. As a result, the number of procedures is usually slightly higher than the number of animals used.
Key results
-
3.06 million scientific procedures involving living animals were carried out in Great Britain in 2021. This is an increase of 6% on last year
-
experimental procedures have increased by 20% and procedures for creation and breeding have decreased by 8%
-
the majority (96%) of procedures (both for experimental and breeding purposes) used mice, fish, birds or rats. These species have been the most used for more than a decade
Experimental procedures
These procedures involve using animals in scientific studies for purposes such as: basic research and the development of treatments, safety testing of pharmaceuticals and other substances, specific surgical training and education, environmental research and species protection.
-
1.73 million procedures carried out for experimental purposes (57% of all procedures in 2021)
-
54% of procedures used mice
-
15% of procedures used fish
-
14% of procedures used birds
-
11% of procedures used rats
-
around half (51%) of experimental procedures were for basic research; the top three research areas were the nervous system, the immune system and cancer(oncology)
-
97% of all experimental procedures were assessed as non-recovery, sub-threshold, mild, or moderate in severity; the remaining 3% were assessed as severe
Creation and breeding of genetically altered animals
This refers to the breeding of animals whose genes have mutated or have been modified. These animals are used to produce GA offspring for use in experimental procedures but are not themselves used in experimental procedures.
-
1.33 million procedures carried out for the creation and breeding of GA animals (43% of all procedures in 2021)
-
87% were for the creation and breeding of mice
-
12% were for the creation and breeding of fish
-
0.8%, were for the creation and breeding of rats and birds
-
the majority (90%) of procedures in this category were for maintenance of already established GA lines, with 10% of procedures for the creation of new lines
-
98% of all procedures for creation and breeding were assessed as non-recovery, sub-threshold, mild, or moderate in severity; 2% were assessed as severe
Introduction
Purpose of this release
This publication meets the requirements of section 2 of the 1986 Act to publish, and lay before Parliament, annual statistics on the use of protected animals in regulated procedures.
Coverage of this release
These statistics cover England, Scotland, and Wales. For Northern Ireland, the Department of Health separately collects and publishes information on NI regulated procedures under devolved arrangements.
‘Number of procedures’ is not ‘number of animals’
The statistics in this release and the accompanying data tables relate to the number of procedures, not the number of animals used, unless specified (i.e. data tables 1.3, 2.1, 2.2 and 2.3 relate to the number of animals).
These statistics describe the nature and purpose of procedures including their actual severity. The experience of the animal at the time of death or killing is a factor in determining the actual severity and therefore the killing or death of animals is not reported separately. Further information regarding actual severity can be found here: Advisory notes on actual severity reporting
Severity of procedures
These statistics describe the nature and purpose of procedures including their actual severity. The experience of the animal at the time of death or killing is a factor in determining the actual severity and therefore the killing or death of animals is not reported separately. Further information regarding actual severity can be found here: Advisory notes on actual severity reporting (publishing.service.gov.uk).
Accompanying data tables and user guide
The accompanying data tables for this report can be found on the statistics of scientific procedures webpage. Since the 2018 publication, the principal data tables have been expanded to include data from 2014. To allow users to view and extract the data as they wish. Since the 2021 publication, tables 1.1 and 1.2 have been expanded to include country data from 2014. The tables that have been expanded include data from 2014 as not all data pre-2014 are comparable. See the accompanying user guide for further information.
Total procedures
Figure 1. Total scientific procedures in Great Britain, 1986 to 2021

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 1.1. See the user guide for links to all data pre-2007.
As shown in Figure 1, the number of procedures carried out decreased from 1987 until 2001, to a low of 2.62 million. This was mainly due to a reduction in the use of rodents, rabbits and birds (although there was an increase in procedures involving fish).
After 2001, procedures increased, reaching a peak of 4.14 million in 2015 and then started to decrease again to 2.88 million in 2020. This may be partly explained by the two national lockdowns during 2020, which may have affected the activity of the establishments.
In 2021 there was an increase in procedures to 3.06 million, this is the second lowest figure since 2007. This year’s increase in total procedures (6%) is the first increase in procedures since 2015. See the Further information section for more details.
This year there was an increase in the number of procedures in England and Scotland of 6% and 8% on last year respectively. The number of procedures undertaken in establishments in Wales decreased by 4% compared to 2020. A similar trend to Great Britain is seen in the number of procedures for England and Scotland, where the number of procedures peaks at 3.51 million and 573,000 in 2015 respectively and have lows of 2.45 million and 396,000 procedures in 2020 respectively. Whilst Wales also peaked in 2015 at 55,000 procedures, the number of procedures decreased to a low of 39,000 in 2021.
The number of procedures carried out on living animals is determined by several factors, including the focus of scientific and medical endeavours, the economic climate and global trends in new technologies or fields of research.
Figure 2. Total scientific procedures by type, 2007 to 2021
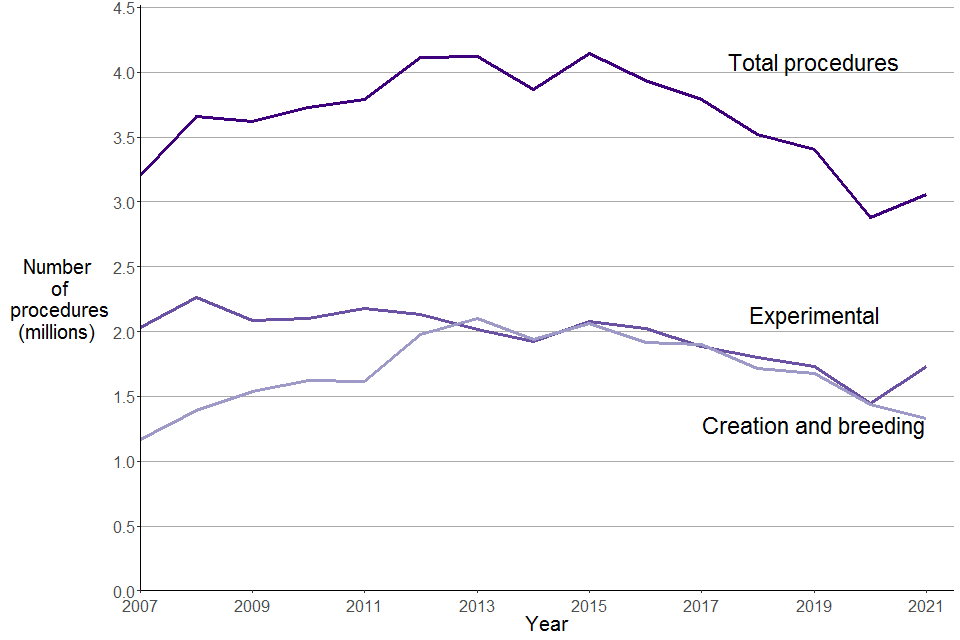
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 1.2 and Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2017: time series tables, Table 1
As shown in Figure 2, the total number of procedures was rising prior to 2013, mainly due to the increase in procedures for the creation and breeding of GA animals. This increase in the earlier part of the decade can mainly be attributed to the availability of new technology which led to new research opportunities. However, more recently, the number of procedures for the creation and breeding of GA animals has been falling, with a decrease of 8% compared to last year.
In contrast, the number of experimental procedures remained relatively stable during the earlier part of the last decade but has decreased since 2015 similarly to procedures for creation and breeding of GA animals. This year there has been an increase of 20% in experimental procedures compared to 2020. This increase may be partly explained by the two national lockdowns during 2020, which may have affected the activity at establishments compared to 2021 where there was one national lockdown– see the Further information section for more details.
Experimental procedures involve using animals in scientific studies for purposes such as: basic research and the development of treatments, safety testing of pharmaceuticals and other substances, education, specific surgical training and education, environmental research and species protection.
Procedures for creation and breeding involve the breeding of animals whose genes have mutated or have been modified. These animals are used to produce genetically altered offspring for use in experimental procedures but are not themselves used in experimental procedures.
Experimental procedures
The severity of a procedure is determined by the degree of pain, suffering, distress or lasting harm expected to be experienced by an individual animal during the course of a procedure. In 2021, 97% of all experimental procedures were assessed as non-recovery, sub-threshold, mild, or moderate in severity, the remainder were severe.
This section covers only experimental procedures. That is, procedures that involve using animals in scientific studies for purposes such as: basic biological research, medical studies and development of treatments, training and education, environmental research, preservation of species, and safety testing of pharmaceuticals and other substances. An experimental procedure may benefit people, animals, or the environment for any of the purposes stated above. The animals used in experimental procedures may be genetically altered.
Species
The proportions of species used for experimental procedures as shown in Figure 3, had remained similar from 2014 to 2020. In 2021, the proportion of birds and fish have increased from 9% to 14% and 13% to 15% respectively. Whilst the proportion of mice and rats have decreased from 57% to 54% and 14% to 11% in 2021 respectively.
For most species, small year-on-year variations can be attributed to technological developments and changes in the types and stages of projects being carried out in any reporting year.
Figure 3. Experimental procedures by species, 2021
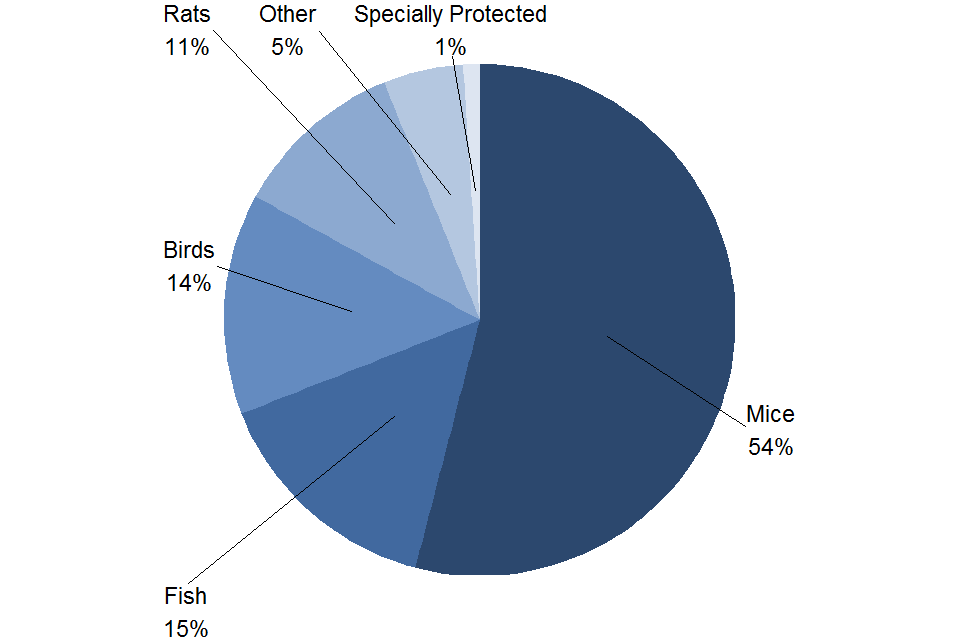
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 1.2
Notes:
- Specially protected species are cats, dogs, horses and primates.
Mice, fish, rats and birds in experimental procedures
The majority of experimental procedures used mice, fish, birds or rats; together these species were used in 94% of experimental procedures in 2021.
Figure 4. Experimental procedures using mice, fish, birds and rats, 2007 to 2021

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables: Table 1.2 and Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2017: time series tables, Table 2.2
As shown in Figure 4, there was an increase in the use of birds, fish and mice (96%, 37% and 13% respectively) compared to last year, whilst experiments on rats have decreased (5%).
The use of rats has decreased from 2007 to 2019, with an increase in procedures in 2020 (22%). This year there was a 5% decrease compared to 2020, this is still down 27% from a decade ago. In 2021, around two thirds of experimental procedures involving rats (69%) were for regulatory testing (e.g. tests evaluating the safety and efficacy of substances such as pharmaceuticals), which saw an increase of 3% on last year.
The majority of experimental procedures involving mice in 2021 (70%) were for basic research. More specifically, most of the basic research that involved mice focused on the immune system, human immune disorders and the nervous system.
Around two thirds of experimental procedures involving fish in 2021 (67%) were also for basic research. Most basic research that involved fish focused on the nervous system, human nervous and mental disorders and developmental.
The majority of experimental procedures involving birds in 2021 (90%) were for applied research. Most applied research that involved birds focused on animal diseases and disorders, other efficacy and tolerance testing and other routine production.
Specially protected species in experimental procedures
Specially protected species refers to cats, dogs, horses, and non-human primates. These species were used in 1% of experimental procedures (18,000) in 2021.
Cats, dogs, horses and primates are subject to additional protection under Section 5C of the 1986 Act. Licence holders using specially protected species must demonstrate that no other species are suitable for the purposes of the licence and must adhere to additional licence conditions.
Figure 5. Experimental procedures involving specially protected species, 2008 to 2021
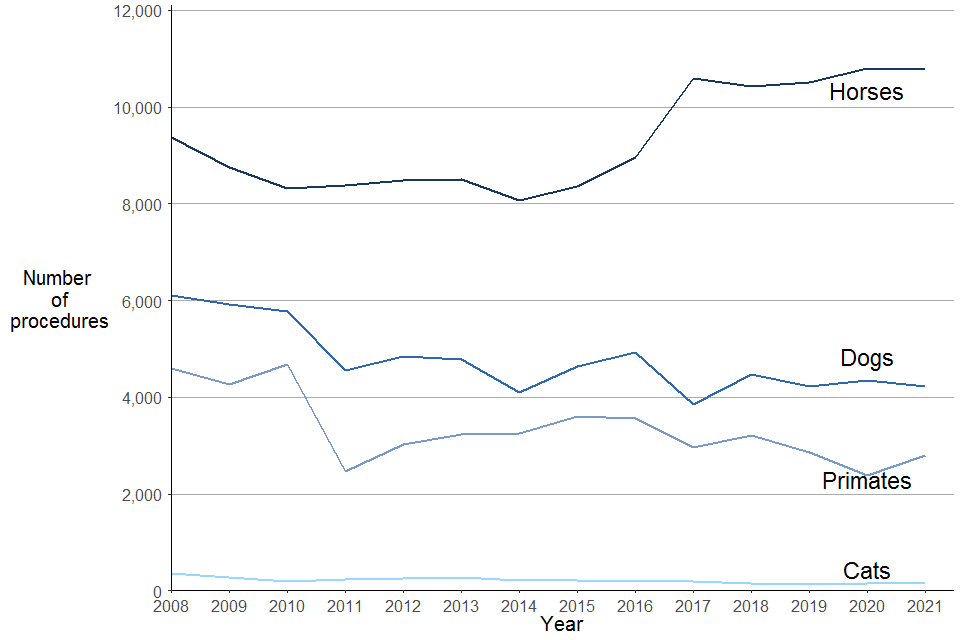
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables: Table 1.2 and Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2017: time series tables, Table 2.2.
The total number of procedures involving specially protected species has increased over the past decade from 16,000 in 2011 to 18,000 in 2021. Most notably the increase in the number of procedures using horses which have increased 29% and cats which have decreased 34% over the past decade. Procedures involving cats accounted for just 0.9% of all experimental procedures involving specially protected species.
The number of procedures involving horses had been decreasing from 2010 to 2014, with an increase in procedures between 2015 to 2017. Since 2017 the numbers have remained relatively steady. This year there was a decrease of 0.1% in procedures compared to 2020. In 2021, the majority (96%) of experimental procedures that used horses were for regulatory procedures. The main regulatory procedure carried out on horses was for the routine production of blood-based products, which are used for a variety of diagnostic purposes.
The number of experimental procedures that used cats have increased by 6% on last year. There were 160 experimental procedures that used cats in 2021, with around two thirds (66%) of procedures for basic research.
The species of primates that were used in experimental procedures in 2021 were cynomolgus monkeys (2,561 procedures), rhesus monkeys (118 procedures) and marmosets and tamarins (116 procedures). The total number of procedures (2,795 procedures) have increased by 17% on last year, although this is still 2% lower than the number of procedures involving non-human primates in 2019. The only primates used in regulatory procedures were cynomolgus monkeys.
In 2021, the use of dogs in procedures decreased by 3% in the last year, and have decreased by 7% over the last 10 years.
In 2021, the majority of experimental procedures that used primates and dogs were for regulatory procedures (82% and 67% respectively). These were mainly for testing the safety of products and devices for human medicine, dentistry and veterinary medicine.
Note: the figures in this section do not follow the rounding conventions as stated in the user guide.
Use of endangered species
Information was collected on whether any endangered species, as listed in Annex A of Council Regulation (EC) No 338/97, were used.
Of the 2,950 returns, 4 reported the use of endangered animals in 2021; specifically one species of eel was used for the research of the conservation of the species and the protection of the natural environment in the interests of the health or welfare of human beings or animals.
Place of birth of primates
Self-sustaining Colony
Marmosets, Tamarins, and other new-world primates A self-sustaining colony is a colony that contains no wild caught animals. Is kept in a way that ensures animals are used to humans and is sustained using animals from within or from other self-sustaining colonies.
Macaques and other old-world primates A self-sustaining colony is a colony that no longer sources animals from the wild (it may contain some existing wild caught animals) and is sustained using only captive bred animals.
Generation
- F0 - wild caught
- F1 - progeny of wild caught females
- F2 - progeny of captive bred females
Of the 2,204 primates used for the first time in experimental procedures in 2021, all marmosets, tamarins and rhesus monkeys were born in the UK at a licensed establishment, whereas 98% of cynomolgus monkeys were born in either Africa or Asia. All primates used for the first time in experimental procedures in 2021, were from self-sustaining colonies.
The place of birth of primates used in experimental procedures for the first time can be found in Table 2.2 of the data tables. The place of birth of all other species used in experimental procedures for the first time in each year since 2014 can be found in Table 2.1 of the data tables.
Of the 2,204 primates used for the first time in experimental procedures in 2021, 551 (25%) were F1 generation and 1,653 (75%) were F2 generation or greater. There were no f0 generation primates used in procedures in 2021.
Basic research: aims to expand our knowledge of the structure, functioning and behaviour of living organisms and the environment.
Applied research: attempts to address diseases through prevention and development of treatments. Within the data tables, this is shown as ‘Translational/Applied research’.
Regulatory testing: procedures carried out to satisfy legal requirements, including: ensuring substances are produced to legal specification; evaluating the safety or effectiveness of pharmaceuticals and other substances.
Note: the figures in this section do not follow the rounding conventions as stated in the user guide
Genetic status
Of the 1.73 million experimental procedures completed in 2021, 61% used animals that were not genetically altered.
Figure 6. Experimental procedures by genetic status, 2008 to 2021

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 4 and Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2017: time series tables, Table 3.2
As shown in Figure 6, and in line with the overall increase in experimental procedures in 2021, the number of experimental procedures involving non-GA animals has increased by 16% in the last year but decreased by 32% over the last decade.
The use of GA animals in experimental procedures had been relatively stable from 2015 to 2019, with a decrease in 2020 of 26%. In 2021 there was a 26% increase in procedures compared to 2020, with a 7% increase over the last decade. The increase in 2021 procedures, compared to last year, mirrors the overall increase in creation and breeding procedures, as shown in Figure 2. The decrease in 2020 is possibly related to reduced activity in some research sectors during the national lockdowns, see the Further information section.
Further information regarding the genetic status of GA animals used in experimental procedures in 2021 can be found in Table 4 of the data tables.
Purpose
As shown in Figure 7, around half (51%) of the experimental procedures carried out in 2021 were for basic research. A further 27% for applied research and the remainder were mostly conducted for regulatory testing purposes (21%).
Figure 7. Experimental procedures by purpose, 2021
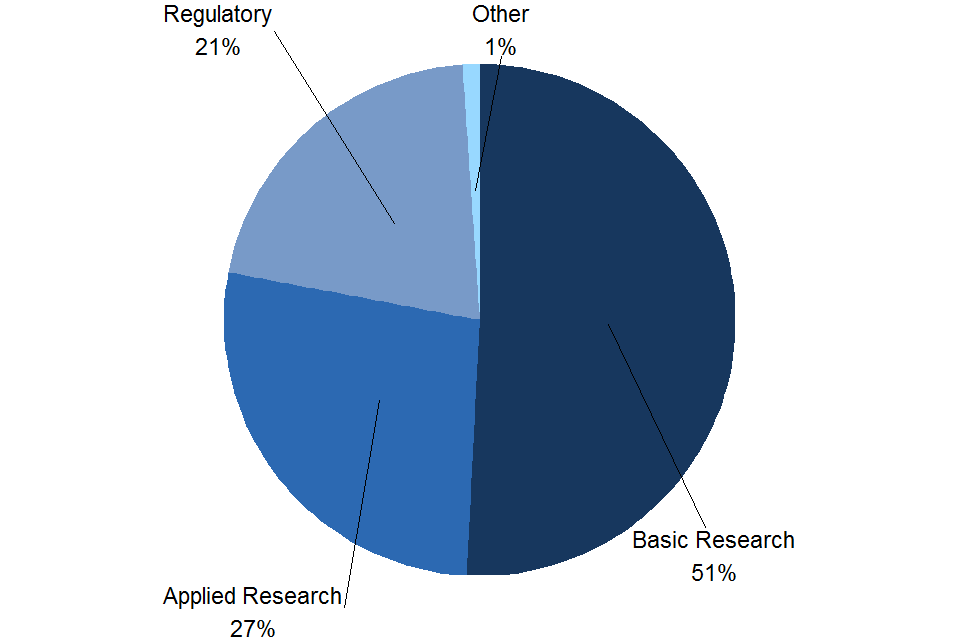
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 1.2
Notes:
- Other includes experimental procedures carried out for higher education or training, preservation of species and for the protection of the natural environment.
This year there has been a large increase in the proportion of applied research which has increased from 10% in 2020 to 27% in 2021. Whilst there was a large decrease in the proportion of regulatory procedures, which has decreased from 33% in 2020 to 21% in 2021. The rest of the purposes for experimental procedures have remained relatively stable since 2014.
Basic Research
In 2021, 877,000 experimental procedures were carried out for basic research purposes. The most common areas focused upon in this research, as shown in Figure 8, were: the nervous system (23%), immune system (20%) and oncology (12%).
Figure 8. Most common areas focused upon in experimental procedures for basic research, 2021

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 5
Notes:
- Research is classified as multisystemic when numerous body organs and systems are targeted.
The distribution of purposes for basic research has remained mostly similar since 2014. Studies into the immune system, the functioning and disease of the nervous system and cancer, including its development and control mechanisms (oncology) have been reported within the top five most common areas for basic research in each year since 2014.
For data on all purposes for basic research by species, see Table 5 of the data tables.
Applied research
There were 469,000 experimental procedures for applied research (27% of all experimental procedures) in 2021. Applied research attempts to address diseases through prevention and development of treatments, as shown in Figure 9. The most common areas of research were animal diseases and disorders (48%), human cancer (15%), and human infectious disorders (12%).
Almost all the experimental procedures for applied research focusing on animal diseases and disorders used fowl (96%); the remaining 4% involved mice, rats, fish, birds, ungulates (including pigs) and other mammals. Almost all (99%) experimental procedures for applied research focusing on human cancer used mice; the remaining 1% of procedures involved rats and pigs.
Figure 9. Most common areas focused upon in experimental procedures for applied research, 2021
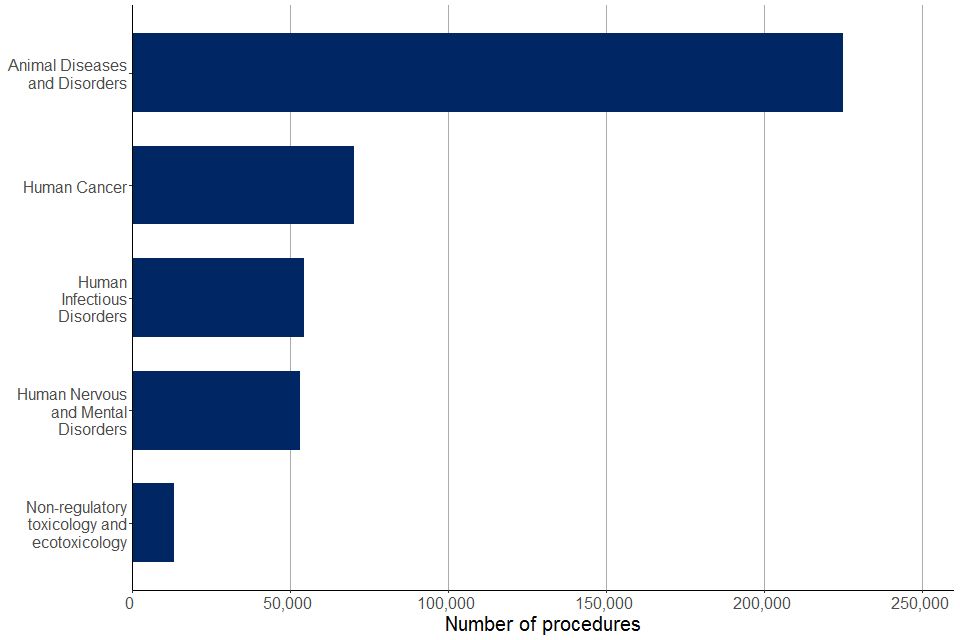
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 6
Notes:
- Non-regulatory toxicology and ecotoxicology covers toxicology, method development and investigations prior to regulatory studies.
This year there has been a large increase in proportion of animal diseases and disorders procedures, increasing from 6% in 2020 to 48% in 2021. With the majority of species used in animal diseases and disorders procedures being fowl (96%). Since 2014, human cancer, infectious disorders, and nervous and mental disorders have consistently being within the top five most common areas of applied research in each year.
For data on all purposes for applied research by species, see Table 6 of the data tables.
Regulatory
There were 362,000 procedures carried out for regulatory purposes in 2021 (21% of all experimental procedures). Regulatory procedures are carried out to satisfy the legal requirements necessary to enable materials, products, and devices to be licensed for use. Regulatory procedures are usually carried out during the final stages of research and development and focuses on safety and efficacy. The most common procedure in 2021 was toxicity and other safety testing (52%).
Of the 362,000 regulatory procedures in 2021, the most common legislative requirements were legislation on medicinal products for human (43%) and veterinary use (30%). No procedures were carried out for cosmetics testing.
The majority (90%) of regulatory procedures were undertaken to satisfy UK and/or EU legislation.
Routine production: covers studies carried out for manufacturing processes requiring regulatory approval.
Toxicity and other safety testing: studies for safety evaluation of products and devices for human medicine, dentistry, and veterinary medicine.
Quality control: the testing of quality control parameters of a product, and any controls carried out during the manufacturing process for registration purposes, to satisfy any other national or international requirements or to satisfy the in-house policy of the manufacturer.
Other efficacy and tolerance testing: efficacy testing of biocides and pesticides is covered under this category as well as the tolerance testing of additives in animal nutrition.
Figure 10 shows the proportion of each purpose of regulatory procedures carried out in 2021. With toxicity and other safety testing, quality control and routine production accounting for 52%, 26% and 18% respectively.
Figure 10. Experimental procedures for regulatory purposes by sub-purpose, 2021

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 7.1
The most common species used in regulatory procedures were rats (36%; 130,000). Of which, 97% were for toxicity and other safety testing including pharmacology. In contrast to their predominant use in experimental procedures for basic research and applied research, mice were used in around a third (32%) of all regulatory procedures, although they were still the second most commonly used species.
Techniques of special interest
Information was collected on whether any procedures were related to techniques of interest to the Home Office (i.e. areas related to Home Office policies). The areas of interest include testing of alcohol, tobacco, and household products.
In 2021, there were 24 experimental procedures which involved the testing of household product ingredients. There was no re-use of animals for these procedures, therefore these 24 procedures represent the use of 24 rats.
There were no experimental procedures which involved the testing of products containing alcohol or tobacco.
An additional area of interest is ascites methods of monoclonal antibody production because a non-animal alternative exists. No ascites methods of monoclonal antibody production were used in 2021.
Note: these figures do not follow the rounding conventions as stated in the user guide.
Rodenticide trials
Rodenticides are a category of pest control chemicals intended to kill rodents. Rodenticide trials are field trials of such chemicals and are occasionally undertaken by commercial companies that produce them to assess how safe and effective they are when used.
Of the 2,950 returns, 1 reported that rodenticide trials occurred in 2021. Home Office ask data suppliers only to indicate whether field trials of rodenticide substances occurred, as these trials are conducted in semi-field situations where the number of animals is not accurately known as the colonies are not intensively managed.
Severity
The severity (i.e. pain, distress or suffering) experienced by animals in procedures has been recorded since 2014. There are five severity assessments:
Sub-threshold: When a procedure was authorised under a project licence but did not actually cause suffering above the threshold of regulation, i.e. was less than the level of pain, suffering, distress or lasting harm that is caused by inserting a hypodermic needle according to good veterinary practice.
Non-recovery (under general anaesthesia): When the entire procedure was carried out under general anaesthesia from which the animal shall not recover consciousness. It includes unintended death of animals on recovery protocols while under anaesthesia, provided that no regulated procedure had been carried out prior to the induction of anaesthesia.
Mild: Any pain or suffering experienced by an animal was, at worst, only slight or transitory and minor so that the animal returns to its normal state within a short period of time.
Moderate: The procedure caused a significant and easily detectable disturbance to an animal’s normal state, but this was not life threatening. Most surgical procedures carried out under general anaesthesia and with good post-operative analgesia (i.e. pain relief) would be classed as moderate.
Severe: The procedure caused a major departure from the animal’s usual state of health and well-being. This would usually include long-term disease processes where assistance with normal activities such as feeding and drinking were required, or where significant deficits in behaviours/activities persist. It includes animals found dead unless an informed decision can be made that the animal did not suffer severely prior to death.
Severity assessments measure harms to an animal during a procedure and generally reflect the peak or cumulative severity of the entire procedure; they do not include harms caused to animals as a result of non-procedural events such as transport and housing.
Figure 11. Experimental procedures by severity, 2014 to 2021
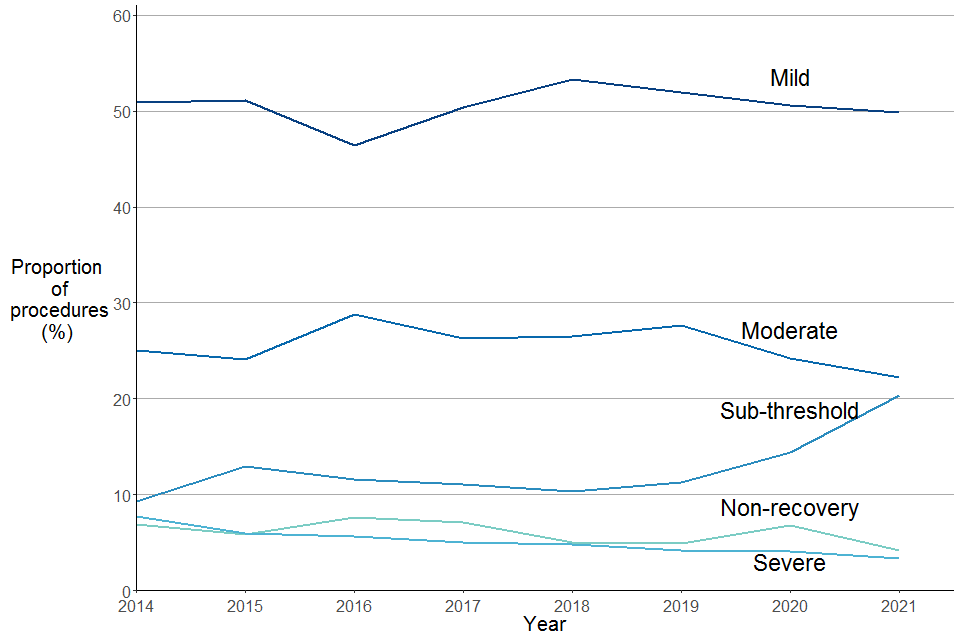
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 3.1
The proportions of severity assessments for procedures reported were relatively similar from 2014 to 2019 as shown in Figure 11. Since 2019 the proportion of procedures with a moderate severity has decreased, whilst the proportion of sub-threshold procedures has increased. Around half (50%) of experimental procedures in 2021 were mild. The proportion of severe, non-recovery and moderate severity assessments for procedures have decreased from last year.
Whilst the proportion of sub-threshold has increased from 14% to 20% since last year.
The severity assessment of experimental procedures varies according to the purpose. However, as shown in Figure 12, the most common severity assessment was mild for each purpose of experimental procedure.
Figure 12. Experimental procedures by severity and purpose, 2021
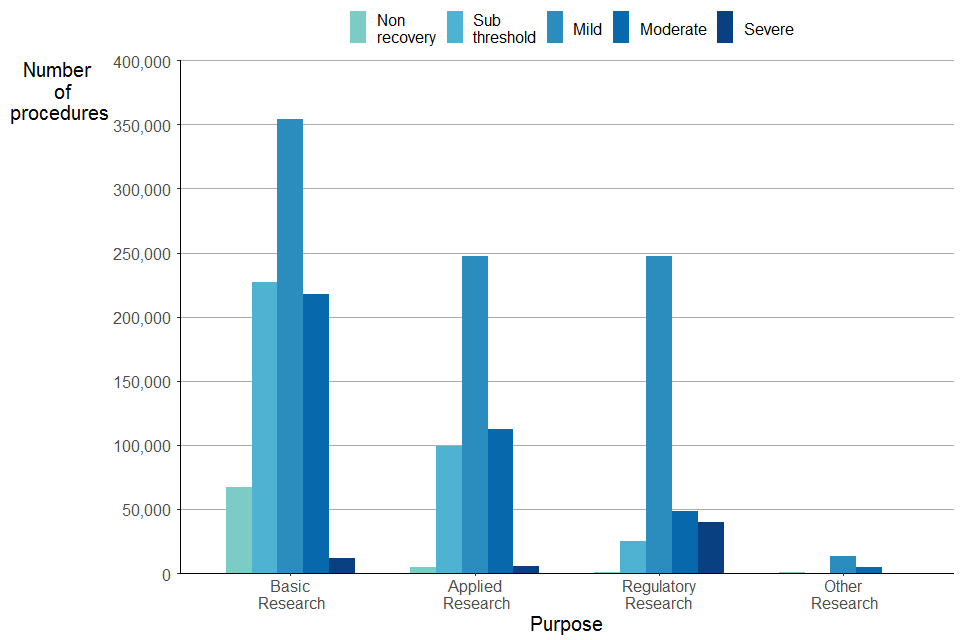
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 3.1
Neuromuscular blocking agents and anaesthesia
Neuromuscular blocking agents (NMBA) are used for muscle relaxation during some types of experimental procedure such as nerve stimulation under anaesthesia. NMBAs may be used only when given the authority to do so and with an appropriate level of anaesthesia and/or analgesia as determined in the project license.
The use of NMBA was recorded in 18 of the 2,950 returns. Of these, 14 returns reported that use of NMBA was whilst the animal was under general anaesthesia. Of the 4 returns that reported the use of NMBA whilst the animal was not under general anaesthesia, all involved fish larvae
Creation and breeding of genetically altered animals
This section covers only procedures performed for the purpose of creation and breeding of GA animals. That is, the breeding of animals whose genes have mutated or have been modified and have not been subsequently used in other procedures.
Species
Almost all (over 99%) of the procedures for the creation and breeding of GA animals involved mice (87%), fish (12%), or rats (0.7%). Other species used for creation and breeding of GA animals include; amphibians, ungulates (including pigs), and birds. Together they accounted for 0.3% of these procedures.
No specially protected species (horses, dogs, cats, or primates) were used in procedures counted under creation and breeding of GA animals.
Genetic status
Of the 1.33 million procedures for creation and breeding that used GA animals in 2021, 1.19 million (89%) used GA animals with no harmful phenotype (i.e. the animals did not appear or behave any differently from non-GA animals).
Figure 13. Creation and breeding of GA animals by type of genetic alteration, 2014 to 2021

Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 8
As shown in Figure 13, there has been an increase in the proportion of animals used for creation and breeding that are genetically altered without a harmful phenotype (increasing from 73% in 2014 to 89% in 2021).
There were some animals that were bred with the intention of producing GA animals, but resulted in non-GA animals being born (accounting for 4% of animals in this category in 2021). In addition, some animals used for the creation of a new genetic line will also have been genetically normal animals (e.g. those used for superovulation).
Purpose
Of the total 1.33 million procedures for the creation and breeding of GA animals, 90% were for the maintenance of already established GA lines, with the remainder for the creation of new lines.
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Tables 8, 9.1 and 10
Of the 132,000 procedures that were for the creation of new GA lines, the majority (91%) were to create new GA lines to be used in basic research. The most common areas within basic research were multisystemic research (32,000 breeding procedures), the nervous system (21,000 breeding procedures), oncology (12,000 breeding procedures) and developmental (9,000 breeding procedures).
Creation: includes the natural breeding of different strains to produce a new strain and procedures that use standard techniques such as vasectomy for the generation of novel transgenic or mutant lines of GA animals. The birth of a GA animal counts as creation when the line is new and before is it ‘established’ (i.e. stable and characterised).
Breeding: the production of GA animals of an established line that has been bred for at least two generations. Breeding procedures also include other techniques applied to the animal after birth e.g. genotyping but not any techniques applied as part of an experiment or study.
Severity
Animals in this type of procedure were not used in regulated experimental procedures. As such, the severity experienced by GA animals created and bred are assessed as follows:
-
the observable characteristics (phenotype) of the animals, e.g. development of congenital disease (i.e. diseases present at birth) or tumours
-
in the case of animals that have no harmful phenotype but that have been biopsied (taking a sample of tissue) specifically for genotyping to determine the genetic make-up of an animal, the biopsy procedures will generally be assessed as mild
-
the animals assessed as severe in this category are largely animals within breeding colonies that were found dead and where the death of the animal was either a result of its phenotype or, more commonly, unexplained (all animals found dead are reported as severe unless an informed decision can be made that the animal did not suffer severely prior to death)
-
a small number of the animals used to create new lines of GA animals will have been subjected to surgical procedures (classed as moderate) or the injection of drugs (classed as mild)
Figure 14. Creation and breeding of GA animals by severity, 2014 to 2021
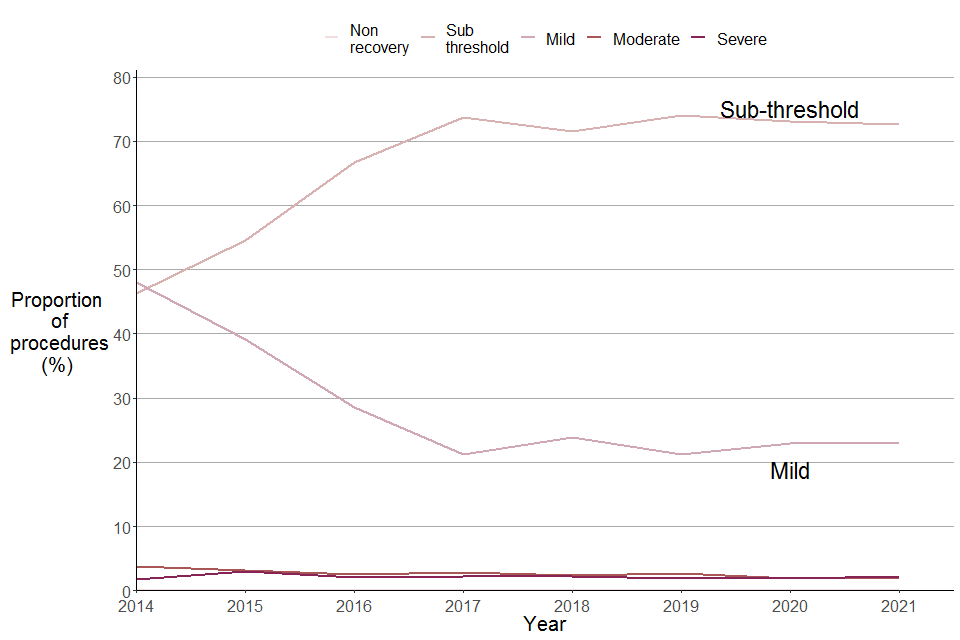
Source: Home Office, Annual Statistics of Scientific Procedures on Living Animals, Great Britain 2021: data tables, Table 8.
The severity assessments for creation and breeding in 2021 have remained stable since 2017, where sub-threshold procedures make up the majority (73%) and only 2% of creation and breeding procedures were assessed as severe. Mild procedures account for 23%, followed by moderate (2%) and non-recovery (0.1%).
As shown in Figure 14, up until 2017 there was an increase in proportion of sub-threshold and decrease in proportion of mild procedures. This change does not reflect a true change in the severity of creation/breeding procedures from 2014 to 2017. Home Office Inspectors believe that initially many creation/breeding procedures reported as ‘mild’ should have been reported as ‘sub-threshold’. Therefore the changing severity assessment profile reflects data suppliers improved familiarity and understanding of severity assessments. Since 2017, the proportions of sub-threshold and mild have remained stable.
Establishment and project licences
All projects and establishments seeking to conduct regulated procedures on living animals must be licensed under Animals (Scientific Procedures) Act 1986 (ASPA).
During 2021, there were 144 establishment licences, 7 of which did not have any active project licences and 2,950 project licences in force.
Information regarding establishment type is no longer collected in the return of procedures collection. Establishment type is not an indicator of the type of procedures carried out and often establishments could be categorised as more than one establishment type.
Further information
The data used in this release was extracted on the 7th of June 2022.
Frequency of release: Annually
Forthcoming release: Home Office statistics release calendar
Home Office responsible statistician: Amy Baxter
This report contains statistics on regulated scientific procedures performed using living animals under the Animals (Scientific Procedures) Act 1986 (ASPA).
Accompanying user guide
See the accompanying user guide for information including:
-
background information on the data collection
-
uses of the statistics, and links to related statistics
-
details on methodology and data quality issues
COVID-19 and animal procedures
The figures presented in this release relate to the number of scientific procedures on animals during the period January 2021 to the end of December 2021. In response to the coronavirus pandemic, restrictions in England and Wales started from 12 March 2020 and lockdown was applied on 23 March 2020, which imposed strict limits on daily life. In 2020 there was another lockdown applied on the 5 November 2020. Whilst in 2021 there was one lockdown applied on the 12 April 2021. This meant activity at establishments may have been affected. No extra data was collected in relation to the pandemic on its effect on the establishments.
Data quality
The UK Statistics Authority has designated these statistics as National Statistics, in accordance with the Statistics and Registration Service Act 2007, signifying compliance with the Code of Practice for Statistics.
National Statistics Status
National Statistics status means that our statistics meet the highest standards of trustworthiness, quality, and public value, and it is our responsibility to maintain compliance with these standards.
The designation of these statistics as National Statistics was confirmed in 2007 following a compliance check by the Office for Statistics Regulation. The statistics last underwent a full assessment of compliance against the Code of Practice in 2012.
Since the latest review by the Office for Statistics Regulation, Home Office statisticians have continued to comply with the Code of Practice for Statistics, and have made the following improvements:
-
Transitioned to a new online data collection system, with data validation to prevent invalid combinations of data being entered.
-
Created a reproducible analytical pipeline (RAP) to automate quality assurance processes and the production of statistical outputs, in line with RAP principles.
-
Each year, Home Office statisticians consult with colleagues in the Animals in Science Regulation Unit to ensure the collection remains suitable for its purpose. The publication is now being published in html format on gov.uk to improve accessibility of the publication. Home Office statisticians have also included breakdowns by country for tables 1.1 and 1.2, going back to 2014.
Revisions
It is standard practice across all Home Office statistical releases to incorporate revisions to previous years’ data in the latest release. Corrections and revisions follow the Home Office’s statement of compliance with the Code of Practice.
The time series data tables published in the 2021 statistical report include any revisions that have been made to previously published data for the years 2014 to 2020. Since the 2020 publication the following has been revised:
| What has changed | Number of procedures affected |
|---|---|
| Purposes changed from Protection of the natural environment in the interests of the health or welfare of human being or animals to Basic research | 237 |
| Species changed from sheep to cattle | 24 |
| Number of cattle used decreased | - 327 |
| Number of mice used increased | + 1536 |
| Xenopus and sheep were previously not included in the total for table 9.3 | + 374 |
| For 2014-2016, figures in table 10 included all creation and breeding procedures; procedures of new genetic lines have now been removed in these years | |
| 2014 | -353,924 |
| 2015 | -303,498 |
| 2016 | -226,307 |
Changes in legislation and definitions
Prior to 1986, figures were recorded for the number of ‘experiments’ on living animals, under the Cruelty to Animals Act 1876. In 1986, the Animals (Scientific Procedures) Act was introduced, and required all ‘scientific procedures’ to be recorded. This new, broader term largely explains the increase in figures directly after 1986 (see Figure 1).
At the beginning of 2013, an EU Directive (2010/63/EU) came into effect, and as a result changed the way in which the data was collected under UK law from 2014 onwards. All figures for procedures (1986 onwards) are comparable as the definition of a procedure is unchanged. As a result of the change in methodology, the 2014 data is subject to data quality issues (see the user guide for further information).
Additional statistics for animal use in Great Britain
The annual statistics release covers regulated procedures on living animals, under the Animals (Scientific Procedures) Act (ASPA) 1986. This comprises of procedures carried out using animals for experimental purposes, and procedures counted under creation/breeding of genetically altered (GA) animals (i.e. the use of GA animals to create offspring for use in experimental procedures). The use of non-GA animals for breeding, to produce non-GA offspring for use in experimental procedures, is covered under the 1986 Act but is not included in the annual statistics. The annual statistics also do not include the use of other animals ‘used’ specifically in the support of the production and use of animals in experimental procedures or e.g. sentinel animals for the monitoring of disease within the facilities. This data on breeding and genotyping of animals for 2017 was published by the Home Office in November 2018 on GOV.UK.
Feedback and enquiries
Home Office statisticians welcome feedback on the annual statistics release. If you have any feedback or enquiries about this publication, please contact the Statistical Transformation Team, the Home Office Unit which produced the statistics.
Public enquiries: HOAIStatisticalTransformation@homeoffice.gov.uk
Press enquiries: pressoffice@homeoffice.gov.uk
Telephone: 0300 123 3535
